ABSTRACT
Adenoviruses (AdVs) have a significant impact in both medical and environmental contexts. The objective of this study was to investigate the prevalence of AdV in different water types, such as untreated and treated wastewater, surface water, groundwater, drinking water, and other water matrices. A total of 239 articles were included in this meta-analysis. Adenoviruses were detected in various waters worldwide. The overall prevalence in water was found to be 59.2%, with the highest prevalence in untreated wastewater (83.1%) and treated wastewater (75.3%), followed by “other water matrices” (53.4%), surface water (49.5%) drinking water (22.7%), and groundwater (18.5%). Most of the studies did not assess the viability of the viruses, leading to weak links between water contamination and risk. Both human and animal AdV were found in water environments. The findings suggest that water, including drinking water, could be a significant route of AdV transmission in both developed and developing economies.
KEYWORDS:
Introduction
The Adenoviridae family cause a range of important diseases across many diverse animal species and is divided into six genera infecting vertebrates from humans to fish (Mastadenovirus, Aviadenovirus, Atadenovirus, Siadenovirus, Ichtadenovirus, Testadenovirus) (Benkő et al. Citation2022). Human adenovirus belong to the genus Mastadenovirus. There are 7 species of Human AdV (HAdV A-G) within which up to 113 types can be counted according to the Human Adenovirus Working Group (http://hadvwg.gmu.edu/).
HAdV is associated with a wide range of clinical symptoms, including jaundice, fever, nausea, sore throat, hemorrhagic cystitis, meningitis, pharyngitis, tonsillitis, cough, pneumonia, and bronchiolitis (Echavarría Citation2008; Lynch and Kajon Citation2016; La Rosa and Suffredini Citation2018; Rabaan et al. Citation2022). However, many infections are asymptomatic. Despite the consequences of HAdV infections are generally mild, severe disease can occurr in newborns, immunocompromised, and those with pre-existing conditions, including respiratory or cardiac disease (Zhang et al. Citation2016; Khanal et al. Citation2018; MacNeil et al. Citation2023). Mortality rates can exceed 50% for untreated severe HAdV pneumonia (Lynch and Kajon Citation2016). It has also been reported that HAdV type 36 is involved in the occurrence of metabolic disorders, including obesity in children, adults and adolescents (da Silva Fernandes et al. Citation2021).
HAdV have a worldwide distribution and infections occur at any age but most commonly in paediatric population. Nearly all individuals are infected with at least one type of HAdV by 6 years of age. HAdV is one of the main causes of respiratory infections in children, accounting for up to 10% of cases (Sandkovsky et al. Citation2014; Finianos et al. Citation2016; Xie et al. Citation2019), while it is responsible for 2–31% of childhood diarrhea cases in developed and developing countries (Hassou et al. Citation2020).
HAdV outbreaks can occur throughout the year with no seasonality in closed environments, such as hospitals, long-term care facilities, children’s camps, college dormitories, and military barracks (Kajon et al. Citation2019). Several waterborne outbreaks caused by HAdV have been documented, associated with recreational (mostly swimming pools) waters, some also with drinking water (Mena and Gerba Citation2009; Bonadonna and La Rosa Citation2019).
Adenoviruses on the whole have been detected in various waters worldwide including wastewater, river water, drinking water, seawaters, and swimming pools. Evidence has shown that adenoviruses survive longer in water than other enteric viruses, such as enteroviruses or hepatitis A virus. Once in water, they can be transmitted directly through ingestion of contaminated food or poorly treated water, by contact with recreational water (Graciaa et al. Citation2018; Federigi et al. Citation2020), or indirectly through hand-water and/or hand-mouth contact (Mattioli et al. Citation2015; Fuhrimann et al. Citation2016). It can also be transmitted via aerosol that can be generated during sewage treatments (Carducci et al. Citation2018).
Several systematic reviews and meta-analyses have been conducted on the presence of AdV in water. For instance, Van Abel and Taylor studied the incidence of HAdV in water in Sub-Saharan Africa (Van Abel and Taylor Citation2018). A recent review by Gholipour and colleagues found that AdV is commonly detected in sewage sludge globally (Gholipour et al. Citation2022). Previous systematic reviews have focused on the decline of AdV in surface waters and its disinfection (Boehm et al. Citation2019; Kokkinos et al. Citation2021). However, no study has examined the overall contamination of AdV in various water matrices. To fill this gap of knowledge, this study aimed at evaluating the overall prevalence of AdV in water environments, including raw and treated wastewater, surface water, groundwater, drinking water, and other water sources.
Material and methods
Protocol and registration
This study was conducted between May and November 2022. The review protocol was registered on International Prospective Register of Systematic Reviews (PROSPERO, no. CRD42022332153). This systematic review and meta-analysis was performed according to the Preferred Reporting Items for Systematic Reviews and Meta-Analysis (PRISMA) standard guidelines (Moher et al. Citation2009). The reporting items for systematic reviews and meta-analyses checklist is shown in Table S11.
Data sources and search strategy
The searches were performed in four databases: PubMed, Excerpta Medica Database (Embase), Web of Science, and Global Index Medicus, to identify all relevant articles containing data on the prevalence of all AdV (human and animal) in water environments. The full search strategy is presented in Table S1. A manual search was also conducted in others databases to identify any additional articles missed by the online search.
Study selection
We included studies that met the following criteria: studies which contained data about the prevalence of AdV in water matrices. The following studies were excluded: (a) systematic review, meta-analysis, comment papers, case reports, and case series, research news, (b) no water matrix, (c) no extractable data, (d) duplicate data, (e) Sample size < or = 10, and (f) studies not written in English (Table S2).
Data extraction and management
Two reviewers screened the titles and abstracts of all the articles on Rayan platform, in case of discrepancies a third reviewer intervened as a referee. After the preliminary screening, the data of selected studies were extracted using a pre-designed Google data abstraction form. The following study characteristics were extracted: name of the first author, year of publication, sampling period, sampling approach (probabilistic/non-probabilistic), number of sites (multicenter, monocenter or nationally representative), setting (urban/rural), timing of sample collection (prospectively/retrospectively). Based on the data uploaded on the respective websites, we assigned countries to WHO region, United Nations Statistics Division (UNSD) region (UN Citation2022), and country income level (World Bank Citation2022).
Water matrices were categorized into five main groups: untreated wastewater, treated wastewater, surface water, drinking water, and groundwater. Any water matrix that did not fit into these categories was classified as “other water matrix,” which included studies that used combinations of categories for which we could not obtain individual prevalence data. We also collected methods used for AdV detection and characterization, such as molecular methods (including viral target genes) or culture-based methods. Additionally, we recorded the total number of samples tested, the number of AdV-positive samples, the type of adenovirus (human or animal strains), and the viral loads in the water matrix, when available. We reported data resulting in the highest detection rate when multiple detection methods or viral target genes were used for the same samples. For samples tested before and after viral concentration, we recorded data for the samples with the highest prevalence. To ensure accuracy, two reviewers screened all included studies.
Quality assessment
To evaluate the quality of the studies, we adapted a tool created by (Hoy et al. Citation2012) for prevalence studies. This tool allowed us to assess the risk of bias in the included studies and included nine criteria: representation of the study’s target population, representation of the sample, form of random selection, clear definition of the water matrix, validity and reliability of the assay detection method, mode of data collection, length of the study period, and reporting of numerators and denominators for AdV prevalence. By using this tool, we were able to evaluate the risk of bias in the studies and categorize them as low risk (7–9), moderate risk (4–6), or high risk (0–3) of bias. Additionally, this allowed us to assess their rigor and transparency, and the results of this evaluation are presented in Table S3.
Statistical analysis
We used the random-effects meta-analysis model developed by (DerSimonian and Laird Citation1986) to combine the study-specific estimates. To assess the heterogeneity among the studies, we conducted the Cochrane Q statistical test and reported I2 values. I2 values of 25%, 50%, and 75% were used to indicate low, moderate, and high heterogeneity, respectively (Higgins et al. Citation2003). We also evaluated publication bias using Egger’s test and a funnel plot (Egger et al. Citation1997). To further investigate the potential sources of heterogeneity, we conducted subgroup analyses based on country, WHO and UNSD regions, country income level, water matrices, and AdV detection assay. A p-value of less than 0.05 was considered to indicate a significant difference. All statistical analyses were conducted using R software version 4.1.0 (Schwarzer Citation2007; Borenstein et al. Citation2010).
We performed sensitivity analyses on studies with low risk of bias, as assessed by the Hoy et al evaluation tool. Additionally, we identified studies that included an internal process control virus (such as Murine norovirus, bacteriophage PP7, or adenoviruses) in all or some of the samples or water prior to the concentration step, in order to monitor and control all the steps of the procedure (concentration, extraction, and identification using molecular methods).
Results
Study selection
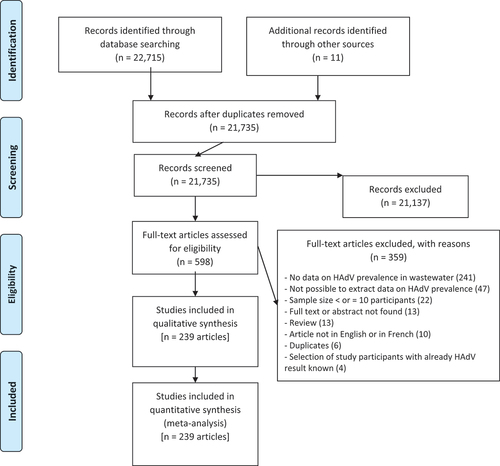
After searching multiple databases, we obtained 22,715 articles and 11 additional articles from other sources (). We removed 991 duplicates and 21,137 articles with irrelevant titles or abstracts. We identified 598 eligible articles, of which 359 were excluded for reasons specified in Table S2. Following the application of the eligibility criteria, 239 articles were included in the qualitative and quantitative analysis of this study (Table S5), which correspond to 343 prevalence data.
Overall study characteristics
Tables S4 and S5 display the characteristics of the included studies. These studies were published between 1981 and 2022, with sample collection periods ranging from 1979 to 2021. All the studies collected samples prospectively, with the majority being non-probabilistic (97.5%). The most frequently represented UNSD region was Northern America (19.3%), followed by South America (18.8%) and Eastern Asia (14.2%). In terms of WHO regions, the most represented WHO Regions were America (38.1%) and Europe (25.9%). The countries with the most representation were Brazil (16.7%) and the United States of America (15.5%).
More than half of the studies, 146 out of 239 (61.1%), were conducted in high-income countries. The water matrices were categorized into 6 groups, with the most represented being surface water (56.1%), followed by untreated wastewater (33.9%) and treated wastewater (24.3%). The majority of the data, 226 out of 239 (94.6%), was considered to be at a moderate 3.2 Overall study characteristics (Table S6).
The most commonly reported detection methods are based on molecular approach, particularly quantitative (59.4%) and qualitative (33.5%) Polymerase Chain Reaction (PCR). Cultural methods were used in 6.7% of the articles, and only 1 study (0.4%) considered an enzyme-linked immunosorbent assay (ELISA). The analysis of molecular data revealed that AdV concentrations in positive samples varied widely across different types of water sources, as shown in Table S7, with high concentration found in untreated wastewater (up to 6.2 × 108 genome copies/L). Treated water samples exhibited a range of viral loads from 1.0 × 10° to 2.8 × 107 genome copies/L. The range of concentrations in surface water varied widely, with the lowest concentration recorded at 1.0 × 10^2 genome copies/L and the highest at 2.0 × 10^9 genome copies/L. In drinking water, viral loads ranged from 1.1 × 101 to 5.7 × 107 genome copies/L, while in groundwater, viral loads ranged from 4.0 × 101 to 2.7 × 104 genome copies/L.
Human adenovirus genotyping results
Seventy-six studies, published between 1983 and 2022 and comprising of 89 sets of data, reported the genotyping of human strains of Adenovirus in wastewater, collected between 1980 and 2019 (Table S8). These strains were reported in all WHO regions, but primarily in America and Europe. The majority of the strains were found in surface water (37 studies) followed by untreated (20 studies) and treated wastewater (16 studies). The most commonly identified strains were HAdV-F41 (n = 494) and HAdV-F40 (n = 39). Other strains identified were HAdV-C (n = 286) (types HAdV-C1, HAdV-C2, HAdV-C5, HAdV-C6, HAdV-C10, HAdV-C12, and HAdV-C19), HAdV-A (n = 90) (types HAdV-A2, HAdV-A12, and HAdV-A31), HAdV-B (N = 84) (types HAdV-B3, HAdV-B6, HAdV-B7, HAdV-B11, HAdV-B14, HAdV-B34, HAdV-B35, and HAdV-B72), HAdV-D (n = 67) (types HAdV-D8, HAdV-D45, and HAdV-D56), and HAdV-E (n = 7) (types HAdV-E4 and HAdV-E47).
Animal adenovirus genotyping results
Eight studies published between 2015 and 2022 reported the genotyping of animal strains of Adenovirus in wastewater samples collected between 2012 and 2017 (Table S8). The studies were conducted primarily in China (6 studies) and one each in Brazil and Bangladesh. Human strains of Adenovirus were also found in these water samples. The majority of the studies reported the presence of animal strains in surface water (7 studies), with one study reporting their presence in untreated wastewater. Detection of animal strains was conducted using PCR-based methods and identified strains such as Bovine AdV, Murine AdV, Pigeon AdV, and mainly Porcine AdV (54 strains of AdV-C5 and one strain of AdV-C3). The reported viral concentration in these studies ranged from 6.1 × 102 to 2.0 × 109 genome copies/L.
Detection of human adenoviruses by cell culture
A total of thirty-nine studies, comprising of 53 sets of data, were conducted between 1979 and 2021 to isolate human strains of adenovirus from various water sources using culture-based methods. Among these studies, 21 were focused on surface water sources, 15 on untreated wastewater, 6 on treated wastewater, 4 on drinking water and groundwater each, and 3 on other water matrices (as detailed in Table S9). The most commonly used cells for isolation attempts were Human lung carcinoma epithelial cells (A549) (28 studies), followed by Buffalo green monkey kidney cells (BGMK) (18 studies), Epidermoid carcinoma of the larynx cell lines (HEp-2) (7 studies), and others. The other cells lines included African rhesus monkey kidney cells (MA104) (2 studies), Human epithelial cell line(Borrie) (2 studies), HeLa-R cells (2 studies), PLC/PRF/5 hepatoma (2 studies), Primary cynomolgus monkey kidney cells (2 studies), Rhabdomyosarcoma cells (RD) (2 studies), Verda reno cells (VERO) (2 studies), Colorectal adenocarcinoma cells (Caco-2) (1 study), FRhK-4/R (1 studies), Human embryonic kidney cells (HEK 293A) (1 study), Monkey kidney (1 study), Human embryonic fibroblast (1 study), and Primary vervet monkey kidney (1 study).
Thirty-five studies (46 dataset) successfully isolated human Adenovirus strains using cell culture, 14 from untreated wastewater, 6 from treated wastewater, 18 from surface water, 4 from groundwater, 3 from drinking water, and 2 for other water matrices (Table S9).
Adenovirus prevalence in water matrices
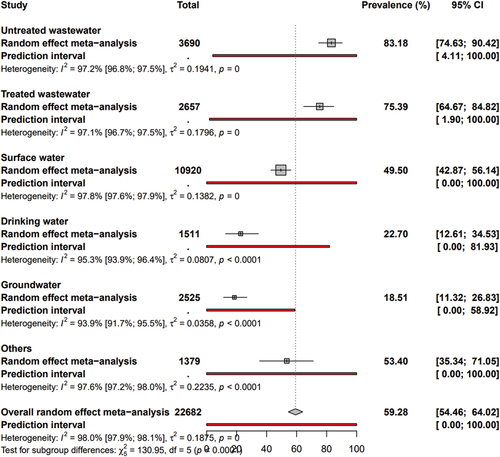
and S1 show adenovirus prevalence in water matrices. The “other” category includes a combination of five main categories from 16 studies where individual category prevalence data was not available (16 out 29 studies). It also includes samples of irrigation water (3 studies), swimming pool water (2 studies), gray water (2 studies), dairy farm water (1 study), distilled water (1 study), finished water (1 study), reclaimed water (1 study), slaughterhouse sewage (1 study), and water tanker samples (1 study).
The global prevalence of AdV in water was 59.28% (95% CI: 54.46–64.02). The prevalence varied across different water matrices, with the highest prevalence in untreated water at 83.18% (95% CI: 74.63–90.42), followed by treated wastewater at 75.39% (95% CI: 64.67–84.82), other water matrices at 53.40% (95% CI: 35.34–71.05), surface water at 49.50% (95% CI: 42.87–56.14), drinking water at 22.70% (95% CI: 12.61–34.53), and groundwater at 18.51% (95% CI: 11.32–26.83).
Heterogeneity and publication bias
The degrees of heterogeneity and publication bias were presented in and Fig S2. The significant and high heterogeneity (H ˃ 1 and I2 ˃ 75%) and the presence of publication bias (P < 0.05 for Egger’s test) were associated with the estimation of prevalence data in the different groups of water matrices. The publication bias results obtained by Egger’s test were confirmed by the funnel plot (Fig S2).
Table 1. Summary of global meta-analysis results for prevalence of Adenovirus in different water matrices divided by risk of bias and process control.
Subgroup analyses
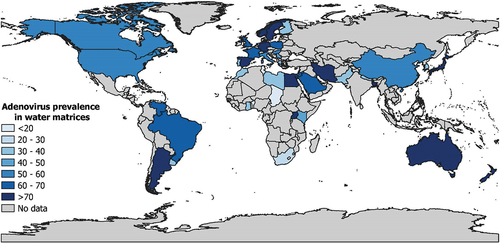
Table S10 presents the subgroup analysis. The global prevalence () was significantly different according to countries (p < 0.001) with higher prevalence in Uganda (94.3%, 95% CI: 68.0–100, 4 prevalence data), followed by Sweden (90.7, 95% CI: 61.5–100, 4 prevalence data), and Germany (89.8%, 95% CI: 68–100, 13 prevalence data). According to WHO region (p = 0.001), significantly higher prevalence was found in Europe (67.1%, 95% CI: 59.1–74.7, 112 prevalence data), followed by America (59.9%, 95% CI: 50.8–68.6, 115 prevalence data) and Western Pacific (58.0%, 95% CI: 47.9–67.9, 62 prevalence data). For UNSD region (p < 0. 001), higher prevalence was in Eastern Europe (75.2%, 95% CI: 41.5–97.8, 3 prevalence data), followed by Northern Europe (74.8%, 95% CI: 60.1–87.3, 17 prevalence data), Oceania (74.5%, 95% CI: 57.8–88.4, 18 prevalence data), Western Europe (72.1%, 95% CI: 54.0–87.3, 30 prevalence data), Southeastern Asia (71.1%, 95% CI: 40.9–93.9, 5 prevalence data), and Eastern Africa (70.8%, 95% CI: 31.5–98.0, 9 prevalence data).
The AdV prevalence in the water matrix did not show a statistically significant difference based on the income level of the country (p = 0.056).The AdV prevalence increased significantly over time (p = 0.001), [1979–2000] (31.6%, 95% CI: 19.0–45.6, 16 prevalence data), [2000–2010] (56.8%, 95% CI: 49.2–64.4, 130 prevalence data), and > 2010 (63.0%, 95% CI: 55.7–70.1, 149 prevalence data). Prevalences based on quantitative PCR were the highest (69.3%, 95% CI: 63.1–75.3, 217 prevalence data), p 0.001.Untreated wastewater (83.2%, 95% CI: 74.6–90.4, 81 prevalence data), treated wastewater (73.9%, 95% CI: 63–83.6, 58 prevalence data) and surface water (50%, 95% CI: 43.4–56.7, 134 prevalence data) showed significantly higher AdV prevalences than drinking water (22.7%, 95% CI: 12.6–34.5, 22 prevalence data) and groundwater (18.5%, 95% CI: 11.3–26.8, 19 prevalence data) (p < 0.001).
Discussion
The knowledge of adenovirus importance has increased significantly during the last decades, and adenovirus is now playing an important role in both clinical settings and in the environmental context (Allard and Vantarakis Citation2017). The frequency and extent of adenovirus disease outbreaks appear to increase, therefore, understanding and fighting disease emergence and transmission routes are essential (MacNeil et al. Citation2023).
Human Adenovirus infections can be spread through several pathways, with water being a major contributor. This can mainly occur through direct ingestion of contaminated water, consumption of food contaminated with water, or through recreational activities (Mattioli et al. Citation2015; Fuhrimann et al. Citation2016; Bonadonna and La Rosa Citation2019). This highlights the importance of understanding the prevalence and persistence of AdVs in water sources in order to control and prevent their transmission. To achieve this objective, we carried out a comprehensive analysis of the global prevalence of AdV in different water sources. Our review included 239 articles and 343 prevalence reports, spanning a 41-year period from 1981 to 2022. The results showed that the overall prevalence of AdV in water was 59.2%, which was significantly higher than other waterborne viruses such as Hepatitis A and E viruses, with prevalences of 16.7% and 9.8%, respectively (Takuissu et al. Citation2022, Citation2023). The prevalence of AdV was observed to vary significantly depending on the type and quality of the water matrix. As expected, untreated wastewater showed the highest contamination rate with a prevalence of 83.1%. This result is not surprising, since adenoviruses have a high prevalence in the human population (Katayama et al. Citation2008; Nordgren et al. Citation2009; Wong et al. Citation2012), either symptomatic or asymptomatic, with up to 90% of adults having been infected with at least one serotype of AdV in their lifetime. A considerably high prevalence was also observed in treated wastewater (76.96%). This could be attributed to the fact that HAdV, being a double-stranded DNA virus, is more stable and thus more resistant to different water treatment methods (Wei et al. Citation2009).
The high prevalence of AdV contamination in surface waters (49.5%) can be attributed to a number of factors. One contributing factor is the discharge of untreated or inadequately treated wastewater from urban areas into water sources. This is particularly problematic in areas where water treatment facilities are limited or inadequate.
Another contributing factor is the runoff from agricultural and rural areas, which can introduce AdVs from livestock and wildlife into water sources. In addition, heavy rains and flooding can lead to runoff of contaminated water from various sources into surface waters, further increasing the risk of AdV contamination (Silva et al. Citation2011; Wyn-Jones et al. Citation2011).
An AdV prevalence of 18.5% in groundwater was found in this study. The factors mentioned previously can also contribute to groundwater contamination with AdVs. In addition, because groundwater is often recharged by surface water, contamination of surface water sources can lead to contamination of groundwater sources as well. A prevalence of 22.7% was observed in drinking water, with HAdV being found in high concentrations (up to 5.7 × 107 copies/L) (Rigotto et al. Citation2010).; However, only three studies (3/22) have investigated infectivity using ICC-PCR techniques, and two of them detected infectious HAdV particles (Lee and Kim Citation2002; Lee et al. Citation2005). These studies found infectivity in up to 40% of the tap water samples, with sequencing revealing enteric HAdVs. These findings suggest a potential human health risk from the ingestion of contaminated drinking water, as enteric HAdV types are able to infect hosts at very low doses. A statistical analysis of data from multiple clinical trials demonstrated that ingestion of an average of 20 HAdV particles could result in a 1% probability of gastrointestinal illness (Teunis et al. Citation2016)., The infectivity of HAdV particles was investigated in a limited number of studies using ICC-PCR techniques, but the method did not precisely quantify infectious particles, so the health risk cannot be accurately assessed.
Regarding the prevalence in different countries around the world, Uganda, a low-income country characterized by inadequate sanitary conditions, had the highest prevalence of HAdV at 94.3%. This is consistent with the increased risk of waterborne pathogens, including water-borne viruses, in low-income countries (Romero-Sandoval et al. Citation2019).
However, the highest AdV prevalences were observed in Europe overall when analyzing the UNSD and WHO regions. This may be attributed to the use of more effective detection methods and higher levels of testing in the region. Therefore, it is possible that the actual prevalence in other regions could be underestimated.
The analysis found that HAdV-F is the most commonly detected human AdV type, which is consistent with previous studies indicating that HAdV-40 and HAdV-41 are the most prevalent types found in water sources (Ogorzaly et al. Citation2015). These types of HAdV are frequently associated with gastrointestinal symptoms, which is consistent with their high detection rates in patients with gastrointestinal disorders (Ouédraogo et al. Citation2016; Reis et al. Citation2016; Khanal et al. Citation2018). It is worth noting that Adenovirus species C have also been detected in water environments, which is particularly interesting as they are typically associated with respiratory infections.
This review also highlights the occurrence of animal-derived strains of Adenovirus in wastewater. The most commonly detected strain was porcine AdV-C5 (54 strains), with other strains such as Bovine AdV, Murine AdV, and Pigeon AdV also being reported. The concentration of the virus in the wastewater samples was highly variable, ranging from 6.1 × 102 to 2.0 × 109 genome copies/L. The presence of animal-derived adenoviruses in water environments such as rivers can be attributed to various factors. Infected animals can release the virus into the water through their feces or respiratory secretions, leading to contamination. Furthermore, human activities like agriculture and animal husbandry can also contribute to the dissemination of animal-derived adenoviruses in water sources through inadequate disposal of animal waste or runoff from livestock facilities.
Of the 39 studies using cell culture to detect infectious AdV, 35 successfully isolated human Adenovirus strains. However, it is important to note that certain types of HadV, specifically HAdV-F types, may not adapt well to the cell cultures that are commonly used for the laboratory detection of HAdV infectivity (Tiemessen and Kidd Citation1994), thus, limitation of the testing method should be considered for the interpretation of negative results.
This systematic review highlights some limitations. Firstly, there is a high level of heterogeneity among the data (I2 ˃ 75%), with only 5% of the studies being conducted in low-income economies, where the prevalence of AdV is highest. This low representation of low-income economies significantly impacts the overall prevalence estimated. Secondly, only a small percentage (3%) of the studies included in the review have a low risk of bias indicating that there is a potential risk that the findings may be influenced by factors that affect the validity or reliability of the results. This raises concerns regarding the quality of the methodology employed in these studies. A significant publication bias was detected, indicating that the results of this systematic review or meta-analysis may be influenced by the selective publication of studies with positive or statistically significant findings. This may lead to an overestimation of the true effect size, as studies with negative or non-significant results may be less likely to be published or included in the review.
In conclusion, results of this study show a high overall prevalence of AdV in water matrices, especially in treated and untreated wastewater. Moreover, even if the presence of viable virus in drinking water was revealed only in few studies, this data suggested a possible risk of infection for consumers that is necessary to deep investigate. High frequence of HAdV-F type confirmed the role of fecal contamination as route of viral spreading in the environment underlining the necessity to improve the water treatment especially for wastewater.
Supplemental Material
Download MS Word (1.5 MB)Acknowledgements
This study is carried out within the European Union – NextGenerationEU as part of the National Recovery and Resilience Plan (NRRP) PE13 INF-ACT.
Disclosure statement
No potential conflict of interest was reported by the author(s).
Supplementary data
Supplemental data for this article can be accessed online at https://doi.org/10.1080/09603123.2023.2255559
Additional information
Funding
References
- Allard A, Vantarakis A. 2017. Adenoviruses. In: Rose JB, and Jiménez-Cisneros B, editors. Water and sanitation for the 21st century: health and microbiological aspects of excreta and wastewater management (Global Water Pathogen Project). E. Lansing (MI): Michigan State University, UNESCO. doi:10.14321/waterpathogens.11.
- Benkő M, Aoki K, Arnberg N, Davison AJ, Echavarría M, Hess M, Jones MS, Kaján GL, Kajon AE, Mittal SK, et al. 2022. ICTV Virus Taxonomy Profile: Adenoviridae 2022. J Gen Virol. 103(3):001721. doi: 10.1099/jgv.0.001721.
- Boehm AB, Silverman AI, Schriewer A, Goodwin K. 2019. Systematic review and meta-analysis of decay rates of waterborne mammalian viruses and coliphages in surface waters. Water Res. 164:114898. doi: 10.1016/j.watres.2019.114898.
- Bonadonna L, La Rosa G. 2019. A review and update on waterborne viral Diseases associated with swimming pools. Int J Environ Res Public Health. 16(2):166. doi: 10.3390/ijerph16020166.
- Borenstein M, Hedges LV, Higgins JP, Rothstein HR. 2010. A basic introduction to fixed-effect and random-effects models for meta-analysis. Res Synth Methods. 1(2):97–111. doi: 10.1002/jrsm.12.
- Carducci A, Donzelli G, Cioni L, Federigi I, Lombardi R, Verani M. 2018. Quantitative microbial risk assessment for workers exposed to bioaerosol in wastewater treatment plants aimed at the choice and setup of safety measures. Int J Environ Res Public Health. 15(7):15. doi: 10.3390/ijerph15071490.
- da Silva Fernandes J, Schuelter-Trevisol F, Cancelier ACL, Goncalves e Silva HC, de Sousa DG, Atkinson RL, Trevisol DJ. 2021. Adenovirus 36 prevalence and association with human obesity: a systematic review. Int J Obes. 45(6):1342–1356. doi: 10.1038/s41366-021-00805-6.
- DerSimonian R, Laird N. 1986. Meta-analysis in clinical trials. Control Clin Trials. 7(3):177–188. doi: 10.1016/0197-2456(86)90046-2.
- Echavarría M. 2008. Adenoviruses in immunocompromised hosts. Clin Microbiol Rev. 21(4):704–715. doi: 10.1128/CMR.00052-07.
- Egger M, Davey Smith G, Schneider M, Minder C. 1997. Bias in meta-analysis detected by a simple, graphical test. Bmj. 315(7109):629–634. doi: 10.1136/bmj.315.7109.629.
- Federigi I, Bonadonna L, Bonanno Ferraro G, Briancesco R, Cioni L, Coccia AM, Della Libera S, Ferretti E, Gramaccioni L, Iaconelli M, et al. 2020. Quantitative microbial risk assessment as support for bathing waters profiling. Mar Pollut Bull. 157:111318. doi: 10.1016/j.marpolbul.2020.111318.
- Finianos M, Issa R, Curran MD, Afif C, Rajab M, Irani J, Hakimeh N, Naous A, Hajj M-J, Hajj P, et al. 2016. Etiology, seasonality, and clinical characterization of viral respiratory infections among hospitalized children in Beirut, Lebanon. J Med Virol. 88(11):1874–1881. doi: 10.1002/jmv.24544.
- Fuhrimann S, Winkler MS, Stalder M, Niwagaba CB, Babu M, Kabatereine NB, Halage AA, Utzinger J, Cissé G, Nauta M, et al. 2016. Disease burden due to gastrointestinal pathogens in a wastewater system in Kampala, Uganda. Microb Risk Anal. 4:16–28. doi: 10.1016/j.mran.2016.11.003.
- Gholipour S, Ghalhari MR, Nikaeen M, Rabbani D, Pakzad P, Miranzadeh MB. 2022. Occurrence of viruses in sewage sludge: A systematic review. Sci Total Environ. 824:153886. doi: 10.1016/j.scitotenv.2022.153886.
- Graciaa DS, Cope JR, Roberts VA, Cikesh BL, Kahler AM, Vigar M, Hilborn ED, Wade TJ, Backer LC, Montgomery SP, et al. 2018. Outbreaks associated with untreated recreational water — United States, 2000–2014. MMWR Morb Mortal Wkly Rep. 67(25):701–706. doi: 10.15585/mmwr.mm6725a1.
- Hassou N, Bouseettine R, Abouchoaib N, Ennaji MM. 2020. Enteric adenoviruses: emerging of a Public Health Threat. In: Ennaji MM, editor. Emerging and Reemerging viral pathogens. Academic Press; pp. 879–905. doi: 10.1016/B978-0-12-819400-3.00039-9.
- Higgins JP, Thompson SG, Deeks JJ, Altman DG. 2003. Measuring inconsistency in meta-analyses. Bmj. 327(7414):557–560. doi: 10.1136/bmj.327.7414.557.
- Hoy D, Brooks P, Woolf A, Blyth F, March L, Bain C, Baker P, Smith E, Buchbinder R. 2012. Assessing risk of bias in prevalence studies: modification of an existing tool and evidence of interrater agreement. J Clin Epidemiol. 65(9):934–939. doi: 10.1016/j.jclinepi.2011.11.014.
- Kajon AE, Lamson DM, George SK. 2019. Emergence and re-emergence of respiratory adenoviruses in the United States. Curr Opin Virol. 34:63–69. doi: 10.1016/j.coviro.2018.12.004.
- Katayama H, Haramoto E, Oguma K, Yamashita H, Tajima A, Nakajima H, Ohgaki S. 2008. One-year monthly quantitative survey of noroviruses, enteroviruses, and adenoviruses in wastewater collected from six plants in Japan. Water Res. 42(6–7):1441–1448. doi: 10.1016/j.watres.2007.10.029.
- Khanal S, Ghimire P, Dhamoon AS. 2018. The repertoire of adenovirus in human disease: the innocuous to the deadly. Biomedicines. 6(1):30. doi: 10.3390/biomedicines6010030.
- Kokkinos P, Venieri D, Mantzavinos D. 2021. Advanced oxidation processes for water and wastewater viral disinfection. A systematic review. Food Environ Virol. 13(3):283–302. doi: 10.1007/s12560-021-09481-1.
- La Rosa G, Suffredini E. 2018. Adenovirus. In Handbook of foodborne diseases. CRC Press; pp. 13–24. doi: 10.1201/b22030-2.
- Lee SH, Kim SJ. 2002. Detection of infectious enteroviruses and adenoviruses in tap water in urban areas in Korea. Water Res. 36(1):248–256. doi: 10.1016/S0043-1354(01)00199-3.
- Lee SH, Lee C, Lee KW, Cho HB, Kim SJ. 2005. The simultaneous detection of both enteroviruses and adenoviruses in environmental water samples including tap water with an integrated cell culture-multiplex-nested PCR procedure. J Appl Microbiol. 98(5):1020–1029. doi: 10.1111/j.1365-2672.2004.02496.x.
- Lynch JP III, Kajon AE. 2016. Adenovirus: epidemiology, global spread of novel serotypes, and advances in treatment and prevention. Semin Respir Crit Care Med. 37(4):586–602. doi: 10.1055/s-0036-1584923.
- MacNeil KM, Dodge MJ, Evans AM, Tessier TM, Weinberg JB, Mymryk JS. 2023. Adenoviruses in medicine: innocuous pathogen, predator, or partner. Trends Mol Med. 29(1):4–19. doi: 10.1016/j.molmed.2022.10.001.
- Mattioli MCM, Davis J, Boehm AB. 2015. Hand-to-mouth contacts result in greater ingestion of feces than dietary water consumption in Tanzania: a quantitative fecal exposure assessment model. Environ Sci Technol Lett. 49(3):1912–1920. doi: 10.1021/es505555f.
- Mena KD, Gerba CP. 2009. Waterborne Adenovirus. In: Whitacre D, editor. Reviews of environmental contamination and toxicology. New York, NY: Springer; pp. 133–167.
- Moher D, Liberati A, Tetzlaff J, Altman DG. 2009. Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. Bmj. 339(jul21 1):b2535. doi: 10.1136/bmj.b2535.
- Nordgren J, Matussek A, Mattsson A, Svensson L, Lindgren P-E. 2009. Prevalence of norovirus and factors influencing virus concentrations during one year in a full-scale wastewater treatment plant. Water Res. 43(4):1117–1125. doi: 10.1016/j.watres.2008.11.053.
- Ogorzaly L, Walczak C, Galloux M, Etienne S, Gassilloud B, Cauchie H-M. 2015. Human adenovirus diversity in water samples using a next-generation amplicon sequencing approach. Food Environ Virol. 7(2):112–121. doi: 10.1007/s12560-015-9194-4.
- Ouédraogo N, Kaplon J, Bonkoungou IJO, Traoré AS, Pothier P, Barro N, Ambert- Balay K. 2016. Prevalence and genetic diversity of enteric viruses in children with diarrhea in Ouagadougou, Burkina Faso. PloS One. 11(4):e0153652. doi: 10.1371/journal.pone.0153652.
- Rabaan AA, Bakhrebah MA, Nassar MS, Natto ZS, Al Mutair A, Alhumaid S, Aljeldah M, Garout M, Alfouzan WA, Alshahrani FS, et al. 2022. Suspected Adenovirus Causing an emerging HEPATITIS among children below 10 years: a review. Pathogens. 11(7):712. doi: 10.3390/pathogens11070712.
- Reis TAV, Assis ASF, Valle D, Barletta VH, Carvalho I, Rose TL, Portes SAR, Leite JPG, da Rosa e Silva ML. 2016. The role of human adenoviruses type 41 in acute diarrheal disease in Minas Gerais after rotavirus vaccination. Braz J Microbiol. 47(1):243–250. doi: 10.1016/j.bjm.2015.11.011.
- Rigotto C, Victoria M, Moresco V, Kolesnikovas CK, Corrêa AA, Souza DS, Miagostovich MP, Simões CMO, Barardi CRM. 2010. Assessment of adenovirus, hepatitis a virus and rotavirus presence in environmental samples in Florianopolis, South Brazil. J Appl Microbiol. 109(6):1979–1987. doi: 10.1111/j.1365-2672.2010.04827.x.
- Romero-Sandoval N, Cifuentes L, León G, Lecaro P, Ortiz-Rico C, Cooper P, Martín M. 2019. High rates of exposures to waterborne pathogens in indigenous communities in the Amazon region of Ecuador. Am J Trop Med Hyg. 101(1):45. doi: 10.4269/ajtmh.18-0970.
- Sandkovsky U, Vargas L, Florescu DF. 2014. Adenovirus: current epidemiology and emerging approaches to prevention and treatment. Curr Infect Dis Rep. 16(8):1–8. doi: 10.1007/s11908-014-0416-y.
- Schwarzer G. 2007. Meta: an R package for meta-analysis. R News. 7(3):40–45.
- Silva HD, García-Zapata MT, Anunciação CE. 2011. Why the use of adenoviruses as water quality virologic marker? Food Environ Virol. 3(3–4):138–140. doi: 10.1007/s12560-011-9069-2.
- Takuissu GR, Kenmoe S, Ebogo-Belobo JT, Kengne-Ndé C, Mbaga DS, Bowo-Ngandji A, Ndzie Ondigui JL, Kenfack-Momo R, Tchatchouang S, Kenfack-Zanguim J, et al. 2023. Occurrence of hepatitis a virus in water matrices: a systematic review and meta-analysis. Int J Environ Res Public Health. 20(2):1054. doi: 10.3390/ijerph20021054.
- Takuissu G, Kenmoe S, Ndip L, Ebogo-Belobo J, Kengne-Ndé C, Mbaga D, Bowo-Ngandji A, Oyono MG, Kenfack-Momo R, Tchatchouang S, et al. 2022. Hepatitis E virus in water environments: a systematic review and meta-analysis. Food Environ Virol. 14(3):1–13. doi: 10.1007/s12560-022-09530-3.
- Teunis P, Schijven J, Rutjes S. 2016. A generalized dose-response relationship for adenovirus infection and illness by exposure pathway. Epidemiol Infect. 144(16):3461–3473. doi: 10.1017/S0950268816001862.
- Tiemessen CT, Kidd AH. 1994. Adenovirus type 40 and 41 growth in vitro: host range diversity reflected by differences in patterns of DNA replication. J Virol. 68(2):1239–1244. doi: 10.1128/jvi.68.2.1239-1244.1994.
- UN. 2022. UNSD — Methodology.
- Van Abel N, Taylor MB. 2018. The use of quantitative microbial risk assessment to estimate the health risk from viral water exposures in sub-Saharan Africa: a review. Microb Risk Anal. 8:32–49. doi: 10.1016/j.mran.2017.12.001.
- Wei J, Jin Y, Sims T, Kniel KE. 2009. Survival of human adenovirus 41 in land-applied manure and biosolids. Food Environ Virol. 1(3–4):148–154. doi: 10.1007/s12560-009-9021-x.
- Wong K, Fong T-T, Bibby K, Molina M. 2012. Application of enteric viruses for fecal pollution source tracking in environmental waters. Environ Int. 45:151–164. doi: 10.1016/j.envint.2012.02.009.
- World Bank. 2022. World Bank country and Lending groups – World Bank data Help Desk.
- Wyn-Jones AP, Carducci A, Cook N, D’Agostino M, Divizia M, Fleischer J, Gantzer C, Gawler A, Girones R, Höller C, et al. 2011. Surveillance of adenoviruses and noroviruses in European recreational waters. Water Res. 45(3):1025–1038. doi: 10.1016/j.watres.2010.10.015.
- Xie L, Zhang B, Xiao N, Zhang F, Zhao X, Liu Q, Xie Z, Gao H, Duan Z, Zhong L, et al. 2019. Epidemiology of human adenovirus infection in children hospitalized with lower respiratory tract infections in Hunan, China. J Med Virol. 91(3):392–400. doi: 10.1002/jmv.25333.
- Zhang S-Y, Luo Y-P, Huang D-D, Fan H, Lu Q-B, Wo Y, Chen G, Zhang X-A, Li Y, Tong Y-G, et al. 2016. Fatal pneumonia cases caused by human adenovirus 55 in immunocompetent adults. Infect Dis. 48(1):40–47. doi: 10.3109/23744235.2015.1055585.