ABSTRACT
Background: Obesity constitutes a risk factor for cognitive impairment. In rodent models, long-term exposure to obesogenic diets leads to hippocampal taurine accumulation. Since taurine has putative cyto-protective effects, hippocampal taurine accumulation in obese and diabetic models might constitute a counteracting response to metabolic stress. Objective: We tested the hypothesis that treatment with taurine or with N-acetylcysteine (NAC), which provides cysteine for the synthesis of taurine and glutathione, prevent high-fat diet (HFD)-associated hippocampal alterations and memory impairment. Methods: Female mice were fed either a regular diet or HFD. Some mice had access to 3%(w/v) taurine or 3%(w/v) NAC in the drinking water. After 2 months, magnetic resonance spectroscopy (MRS) was used to measure metabolite profiles. Memory was assessed in novel object and novel location recognition tests. Results: HFD feeding caused memory impairment in both tests, and reduced concentration of lactate, phosphocreatine-to-creatine ratio, and the neuronal marker N-acetylaspartate in the hippocampus. Taurine and NAC prevented HFD-induced memory impairment and N-acetylaspartate reduction. NAC, but not taurine, prevented the reduction of lactate and phosphocreatine-to-creatine ratio. MRS revealed NAC/taurine-induced increase of hippocampal glutamate and GABA levels. Conclusion: NAC and taurine can prevent memory impairment, while only NAC prevents alterations of metabolite concentrations in HFD-exposed female mice.
Introduction
Dementia represents a group of disorders characterized by cognitive decline that affects daily living activities, and its prevalence is expected to nearly triple in the next 30 years, likely due to increased longevity and unhealthy lifestyles [Citation1]. Obesity has been proposed as a risk factor for developing dementia [Citation2,Citation3], since it is associated with hypertension, cardiovascular disease, metabolic syndrome and type 2 diabetes (T2D), which are modifiable risk factors for cognitive impairment [Citation4]. Moreover, these vascular and metabolic factors might modulate genetic susceptibility for neurodegenerative disorders, namely Alzheimer’s disease [Citation5].
Obesogenic diets rich in fat and sugar are commonly employed to experimentally induce metabolic syndrome that progresses into T2D, and lead are known to cause memory impairment [Citation6]. Mice fed a high-fat diet (HFD) for 6 months show altered concentrations of metabolites measured in vivo using magnetic resonance spectroscopy (MRS), including prominent increase of taurine levels in the hippocampus [Citation7]. Notably, increased concentration of taurine was also reported in the hippocampus of non-obese T2D Goto-Kakizaki rats [Citation8] and streptozotocin-induced type 1 diabetic rats [Citation9,Citation10]. In a recent study, we measured hippocampal metabolite concentrations in a longitudinal manner during 6 months of high-fat and high-sucrose diet (HFHSD) feeding in mice [Citation11]. Among the MRS-measured metabolites, concentrations of taurine increased in the hippocampus after 8 weeks of HFHSD exposure, remained elevated for at least 4 months, and could be recovered to control levels by diet normalization. Interestingly, while the accumulation of taurine in the hippocampus of HFHSD-fed mice only appeared after glucose intolerance and insulin resistance were installed [Citation11], memory impairment has been reported within 1 week of HFD feeding [Citation12,Citation13]. Altogether, these findings suggest that brain taurine accumulation is unlikely to be related to the development of memory impairment in obesity and diabetes models, and might be a consequence of metabolic syndrome severity.
In contrast to these observations in rodent models of obesity and diabetes, mouse models for Alzheimer’s disease (AD) show reduced taurine concentration in the hippocampus and cortex, relative to controls [Citation14–17]. Interestingly, Takado and colleagues demonstrated an inverse relationship between absolute taurine concentration and tau protein accumulation in rTg4510 mice [Citation15], and Aytan and colleagues showed an inverse correlation between the ratio of taurine-to-creatine and a brain GFAP levels in the 5xFAD model of AD [Citation14], suggesting that taurine depletion is associated with features of neurodegeneration and astrogliosis. Furthermore, taurine administration has been proposed to prevent neurodegeneration and improve memory performance [Citation18,Citation19].
Given this inverse relation between taurine levels and neurodegeneration, and the reported benefits of taurine administration, we propose that the accumulation of taurine in the hippocampus of obesity and diabetes models is a counteracting beneficial response to metabolic syndrome aggravation. Thus, the present study aimed at testing whether dietary taurine supplementation prevents HFD-induced memory impairment and metabolite profile alterations in the hippocampus. N-acetylcysteine (NAC) can act as a donor of cysteine for the synthesis of taurine as well as glutathione, and NAC-treated new-born mice show a transient increase in cortical taurine levels [Citation20]. While, main biological effects of NAC are through increasing cysteine availability, it can also act as antioxidant, and interacts with disulfide bonds and thus modulate thiolated proteins [Citation21]. Accordingly, NAC is a well-tolerated drug that is suggested to exert beneficial effects on certain neurological and psychiatric disorders [Citation22]. Thus, we tested putative beneficial effects of stimulating of endogenous taurine and glutathione synthesis by NAC treatment, in parallel with taurine supplementation.
Obesogenic diets trigger a distinct metabolic syndrome in male and female mice, but both sexes develop memory impairment [Citation11]. An important sex difference is the lack of hyperinsulinemia emergence in females. By focusing on females, this study investigates the impact of obesity-associated metabolic syndrome, without the chronic insulin receptor stimulation in the hippocampus that is expected under hyperinsulinemia.
Materials and methods
Animals
Animal experiments were performed according to EU Directive 2010/63/EU under approval by the Malmö/Lund Committee for Animal Experiment Ethics (permit number 5123/2021), and are reported following the ARRIVE guidelines (Animal Research: Reporting In Vivo Experiments, NC3Rs initiative, UK). Sample size was estimated from previous MRS experiments [Citation11]. Female C57BL/6J mice (8-weeks old) purchased from Taconic Biosciences (Köln, Germany) were housed in groups of 5 on a 12 h light–dark cycle with lights on at 07:00, room temperature of 21–23°C and humidity at 55–60%. Cages were furnished with a climbing structure, cylinder, wood toys and nesting material. Mice were habituated to the facility for 2 weeks upon arrival. Cages were randomly assigned to one of the following 6 experimental groups (n = 10/group) using the RANDBETWEEN function in Excel (Microsoft, Redmond, WA-USA): control diet (CD), a HFD, CD and HFD supplemented with 3%(w/v) taurine (> = 98%, #W381306, Sigma-Aldrich, Germany) in drinking water (CD-T or HFD-T), and CD and HFD supplemented with 3%(w/v) N-acetylcysteine (99.9%, #A-2805, AG Scientific, San Diego, CA-USA) in the drinking water (CD-NAC or HFD-NAC). Dietary intervention and taurine/NAC supplementation started at 10 weeks of age and during 2 months ((a)). Food and water were provided ad libitum. Diets were acquired from Research Diets (New Brunswick, NJ-USA): a lard-based diet with 60% kcal of fat (D12492) and a control diet containing 10% kcal of fat (D12450J), with total energy of 5.21 and 3.82 kcal/g, respectively, and gross caloric intake was measured as the difference in weight between the food put into the cage and that remaining at the end of the week. Researchers knew group allocation during experiments, but not during sample processing and data analysis. This allowed to shuffle the sequence of mouse handling, thus minimizing potential confounders.
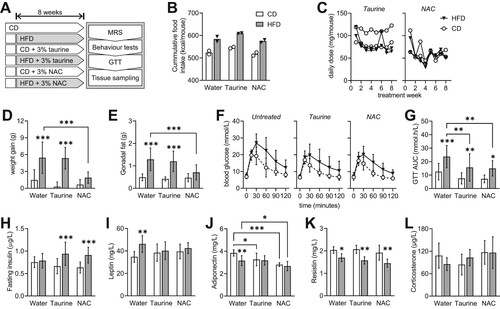
Figure 1. Study design and metabolic phenotype of female mice exposed to HFD and treated with either taurine or NAC. (a) After 8 weeks of treatment, all mice went through a behavioral testing, MRS and GTT before tissue collection. (b) Cages with HFD fed mice showed higher caloric intake than controls. (c) Estimated mean daily doses of taurine and NAC across the 8 treatment weeks was 85 ± 20 and 58 ± 15 mg/mouse. (d) Weight gain during treatment and (e) gonadal fat weight was larger in HFD-fed mice than controls, and prevented by NAC but not taurine treatment. (f) Glucose clearance in GTT was reduced by HFD feeding. (g) The area under the curve (AUC) of the GTT shows some effect of taurine and NAC on Glucose clearance. (h) Fasting insulin concentration in plasma was not affected by HFD, but was increased in HFD-fed mice treated with both taurine and NAC. Fed plasma concentrations of leptin (i), adiponectin (j), resistin (k) and corticosterone (l) were also impacted by either HFD or treatments. Data are shown as individual values of each cage in B-C, and as mean ± SD of n = 10 mice in D-H and of n = 6 in I-L. Open and filled bars/symbols represent CD- and HFD-fed mice, respectively. * P < 0.05, ** P < 0.01, *** P < 0.001 for comparison between HFD and CD within each treatment or as indicated, based on Fischer's LSD post-hoc testing following a significant ANOVA effect of diet, supplementation or interaction.
Magnetic resonance spectroscopy (MRS)
MRS was performed at 8 weeks under isoflurane anesthesia as detailed in Garcia-Serrano et al. [Citation11]. Briefly, isoflurane (Vetflurane, Virbac, Carros, France) was delivered through a nose cone using 1:1 (v/v) O2:air as carrier gas at variable rate of 1–2% for maintaining stable respiration between 60 and 100 breaths per minute. Warm water circulation was used to keep body temperature at 36–37°C. MRS was acquired on a preclinical 9.4 T Bruker BioSpec AV III (Bruker, Ettlingen, Germany) equipped with 1H quadrature transmit/receive cryoprobe, using STimulated Echo Acquisition Mode (STEAM) with repetition time of 4 s, echo time of 3 ms, mixing time of 20 ms, and spectral width of 4401.41 Hz. Water-suppressed spectra were acquired in 20 blocks of 16 scans from a volume of interest (VOI) placed in the dorsal hippocampus (1.8 mm × 1.2 mm × 1.5 mm). An unsuppressed water spectrum from the same VOI was acquired in one block of 16 averages. After block alignment and summation in MATLAB (MathWorks, Natick, MA), metabolite concentrations were determined with LCModel v.6.3-1A (Stephen Provencher Inc., Oakville, Ontario-Canada; RRID:SCR_014455), including a macromolecule (Mac) spectrum in the database and using the unsuppressed water signal as internal reference [Citation23]. LCModel analysis included alanine (Ala), ascorbate (Asc), aspartate (Asp), β-hydroxybutyrate, creatine (Cr), γ-aminobutyrate (GABA), glutamine (Gln), glutamate (Glu), glutathione (GSH), glycine (Gly), glycerophosphorylcholine (GPC), glucose (Glc), lactate (Lac), myo-inositol (Ins), N-acetylaspartate (NAA), phosphorylethanolamine (PE), phosphorylcholine (PCho), phosphocreatine (PCr), scyllo-inositol, and taurine (Tau). Cramér–Rao lower bound (CRLB) from the LCModel fit served to assess quantification reliability, and metabolites with CRLB larger than 30% were disregarded. Namely, β-hydroxybutyrate, N-acetylaspartylglutamate, glucose and scyllo-inositol were not further analysed, and phosphorylcholine and glycerophosphorylcholine were analysed as total choline (PCho + GPC).
Behavioral tests
Experiments were performed from 9:00 to 18:00, at an illuminance of 15 lx. After acclimatizing to the room for at least 1 h, open field test and object recognition tasks for testing novel object recognition (NOR) or novel location recognition (NLR) were conducted as detailed in Garcia-Serrano et al. [Citation11]. Exploration was observed in a cubic arena with a side length of 50 cm. Mice were first habituated to the empty arena for 8 min. Arena exploration was tracked and analysed with Any-maze 6.0 (Dublin, Ireland). Total walk distance, number of crossings between arena quadrants and immobility time, as well as exploration of the arena center at 6 cm from the walls were tracked. Thereafter, NLR was assessed by placing the mice in the arena with two identical objects, and allowed to explore them for 5 min (familiarization phase). Mice were then removed from the arena for 1 h (retention phase), and reintroduced for 5 min but with one object relocated to a different quadrant in the arena (recognition phase). For NOR, two new identical objects were used in the familiarization phase, and one of them was replaced by a novel object during the recognition phase. Time exploring each object was measured.
Glucose tolerance test (GTT)
Food was removed for 6 h starting at 08:00. After, blood from the vena saphena was collected to determine plasma insulin by ELISA (#10-1247-10, Mercodia, Uppsala, Sweden; RRID:AB_2783837), and a GTT with 2 g/kg glucose i.p. was carried out as before [Citation11].
Tissue collection
At the end of the study, mice were anesthetized with isoflurane, and blood was drawn from the portal vein into heparinized tubes kept on ice. Mice were decapitated after cardiac perfusion with 20 mL of cold phosphate-buffered saline (PBS; in mmol/L: 137 NaCl, 2.7 KCl, 1.5 KH2PO4, 8.1 Na2HPO4, pH 7.4). Hippocampi were dissected and quickly frozen in N2 (l), and then stored at −80°C. Collected blood samples were centrifuged at 21,000×g and 4°C for 15 min, and plasma was frozen at −80°C.
Plasma hormones
ELISA kits from Abcam (Cambridge, UK) were used to determine plasma leptin (#ab100718, RRID:AB_2889903), adiponectin (#ab108785, RRID:AB_2891131), resistin (#ab205574, RRID:AB_2891132) and corticosterone (#ab108821, RRID:AB_2889904) in samples collected upon mouse killing.
Plasma taurine determination
Metabolites were extracted by mixing 300 μL of methanol with 70 μL of plasma, followed by sonication on ice for 30 min, and centrifugation at 13,000×g and 4°C for 30 min. Supernatants were dried using a Savant SpeedVac Concentrator, and then resuspended in 100 mM sodium phosphate buffer pH 7.4 prepared in 2H2O (>99.9%, Sigma-Aldrich), containing 0.01% NaN3. Sodium fumarate (5 µmol/L) was included as internal standard for quantification by 1H nuclear magnetic resonance (NMR) spectroscopy [Citation24]. Spectra were acquired at 25°C on an 11.7 T Varian Inova spectrometer equipped with a 5 mm broadband probe (Agilent technologies, Santa Clara, CA, USA), and using a Carr-Purcell-Meiboom-Gill sequence with spectral width of 8012.8 Hz, acquisition time of 2.045 s, relaxation delay of 10 s, and 520 acquisitions. Taurine concentration was determined by measuring peak areas using NUTS (Acorn NMR, Fremont, USA).
Real-time polymerase chain reaction (RT–PCR)
RNA was isolated from the hippocampus with Trizol (#15596026, Invitrogen), and then 1 µg of total RNA was reverse transcribed with random hexamer primers using the qScript cDNA SuperMix (#95048, Quantabio, England), according to the manufacturers’ instructions. The resulting cDNA was used for quantitative RT–PCR as detailed previously [Citation11] using PerfeCTa SYBRgreen SuperMix (#95054, Quantabio, England) and the primers listed in Table S1. Gene expression was determined using the comparative cycle threshold (CT) method (ΔΔCT) with normalization to the average expression of L14 and GAPDH.
Immunoblotting
Western blotting of hippocampal protein extracts was carried out as detailed previously [Citation7] with antibodies against the taurine transporter TauT (SLC6a6, #PA5-37460, Invitrogen, Life Technologies Europe, Stockholm, Sweden; RRID:AB_2554074), GABA transporter GAT-2 (SLC6a13, #PA5-113493, Invitrogen; RRID:AB_2868226) and GAPDH (#ab9485, Abcam; RRID:AB_307275).
Statistical analysis
Results are presented as mean ± SD. All statistical analyses were carried out in Prism 9.3.0 (GraphPad, San Diego, CA-US; RRID:SCR_002798). After confirming that data showed no serious deviation from normality (Kolmogorov–Smirnov and Shapiro–Wilk tests), data were analysed by two-way ANOVA with diet and treatment as factors. Upon significant effects of diet, supplementation or their interaction, the Fisher’s least significant difference (LSD) test was used for independent comparisons between HFD and control groups within each treatment, or between treated and untreated groups within each diet. Partial least-squares (PLS) regression with three components was applied on z-scores of metabolic phenotype parameters and hippocampal metabolite concentrations using MATLAB 2019a (MathWorks, Natick, MA, USA). The PLS model was fit to a composite variable of memory performance (product of NOR and NLR scores). The variable importance in projection (VIP) was calculated for each independent parameter.
Results
Food intake and metabolic phenotype
Total caloric intake over 2 months was higher for cages with HFD-fed mice than controls, with minimal effects across treatments ((b)). Estimated daily doses of taurine and NAC were on average 78 (range 38–122) and 67 (range 30–120) mg/mouse ((c)). After 2 months, HFD-fed mice showed higher weight gain than controls, which was prevented by NAC but not taurine treatment ((d); diet F(1,54) = 60.4, P < 0.001; treatment F(2,54) = 8.72, P < 0.001, interaction F(2,54) = 6.69, P = 0.003). When compared to the respective controls, gonadal fat was increased in HFD and taurine-treated HFD but not in NAC-treated HFD ((e); diet F(1,54) = 51.7, P < 0.001; treatment F(2,54) = 4.52, P = 0.015, interaction F(2,54) = 4.73, P = 0.013).
Glycemia after a 6-hour fasting was similar across all groups ((f); diet F(1,54) = 0.744, P = 0.392; treatment F(2,54) = 0.864, P = 0.428, interaction F(2,54) = 0.518, P = 0.599). After glucose injection, HFD-fed mice in any group showed slower glucose clearance than the respective controls ((f)). However, the area under the curve of the GTT indicates that both taurine and NAC improved glucose clearance, especially in HFD-fed mice ((g); diet F(1,54) = 27.4, P < 0.001; treatment F(2,54) = 6.92, P = 0.002, interaction F(2,54) = 0.392, P = 0.678).
In contrast to males, female mice are resistant to develop insulin resistance upon exposure to obesogenic diets [Citation11]. Accordingly, HFD did not elicit fasting hyperinsulinaemia, but treatments with taurine and NAC caused a small increase in circulating insulin in HFD-fed but not CD-fed mice ((h); diet F(1,54) = 18.6, P < 0.001; treatment F(2,54) = 0.211, P = 0.810, interaction F(2,54) = 3.22, P = 0.048). We further analysed levels of circulating hormones that act on the brain, namely adipokines and corticosterone. Leptin was increased in HFD-fed mice versus controls but not upon Taurine or NAC treatment ((i); diet F(1,54) = 5.86, P = 0.022; treatment F(2,54) = 0.204, P = 0.816, interaction F(2,54) = 1.88, P = 0.170). Adiponectin was reduced by HFD-feeding and further reduced by Taurine and NAC treatments ((j); diet F(1,54) = 4.88, P = 0.035; treatment F(2,54) = 11.0, P < 0.001, interaction F(2,54) = 2.10, P = 0.140). Resistin was lowered by HFD-feeding with no effect of treatments ((k); diet F(1,54) = 38.6, P < 0.001; treatment F(2,54) = 2.46, P = 0.103, interaction F(2,54) = 0.440, P = 0.648). Corticosterone levels showed no effects of diet or treatment ((l); diet F(1,54) = 0.0266, P = 0.872; treatment F(2,54) = 2.04, P = 0.148, interaction F(2,54) = 1.40, P = 0.262).
Taurine homeostasis
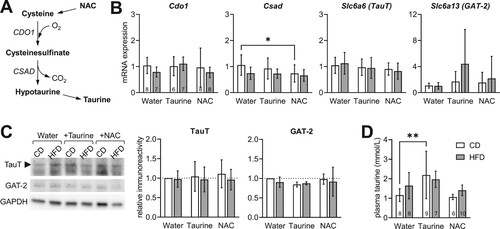
Taurine synthesis from cysteine occurs through oxygenation and decarboxylation reactions catalyzed by cysteine dioxygenase 1 (CDO1) and cysteine sulfinic acid decarboxylase (CSAD) ((a)). Brain taurine is not only synthesized locally, but it is also transported from the periphery via the taurine transporter TauT (Slc6a6) and the GABA transporter GAT-2 (Slc6a13) [Citation25]. HFD had no significant impact on the relative hippocampal expression of these four players in taurine homeostasis ((b)). NAC treatment decreased CSAD expression in CD-fed mice (P = 0.0421 vs. untreated CD), and no effects of Taurine were observed ((b); diet F(1,38) = 4.45, P = 0.042; treatment F(2,38) = 1.74, P = 0.189, interaction F(2,38) = 0.582, P = 0.564). Western blot analysis of TauT and GAT-2 did not reveal significantly altered density of either taurine carrier in the hippocampus ((c)). NMR spectroscopy analysis of plasma extracts showed that treatment with taurine increases its concentration in circulation ((d); diet F(1,40) = 1.09, P = 0.303; treatment F(2,40) = 7.37, P = 0.002, interaction F(2,40) = 1.27, P = 0.291). NAC had no effect on plasma taurine concentration. Similar alterations were observed for taurine concentration in the hippocampus (see below).
Figure 2. Expression of taurine metabolism enzymes in the hippocampus of mice treated with taurine and NAC. (a) Schematic representation of endogenous synthesis of taurine from cysteine of NAC origin through oxygenation and decarboxylation reactions catalyzed by cysteine dioxygenase 1 (CDO1) and cysteine sulfinic acid decarboxylase (CSAD). (b) Relative mRNA expression of Cdo1, Csad and taurine carriers TauT (Slc6a6) and GAT-2 (Slc6a13) in the hippocampus (n = 6–8 as noted in bars of the first graph). (c) Western blot analysis of hippocampal TauT and GAT-2 density (n = 4). (d) Concentration of taurine in plasma (n = 6–10 as noted in each bar). Data are shown as mean ± SD. Open and filled bars/symbols represent CD- and HFD-fed mice, respectively. * P < 0.05, ** P < 0.01, *** P < 0.001 based on Fischer's LSD post-hoc comparison after significant effect of diet, supplementation or interaction in ANOVA.
Taurine and NAC treatments prevent HFD-induced memory impairment
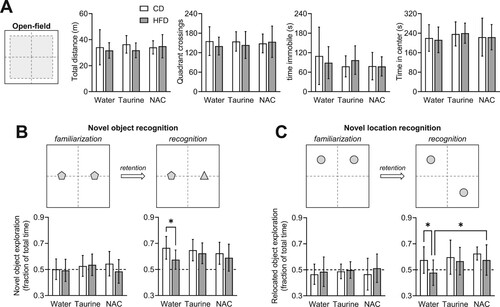
Behavior was assessed in object exploration tasks. Mice were firstly allowed to freely explore the empty area. Such open-field exploration was not impacted on HFD, taurine or NAC, as revealed by similar total walking distance, number of crossings between quadrants, total immobility time, fraction of time spent in arena center ((a)). These results suggest the absence of stress or anxiety-like behavior induced by any treatment, which could interfere with the following memory tests. Relative to controls, untreated HFD-fed mice showed impaired memory as depicted by reduced preference for novelty in NOR and NRL (respectively, P = 0.025 and P = 0.041 vs. CD). On the other hand, memory performance was preserved in HFD-fed mice treated with either taurine or NAC in the NOR task ((b); diet F(1,53) = 4.95, P = 0.030; treatment F(2,53) = 0.577, P = 0.565, interaction F(2,53) = 0.851, P = 0.433), as well as in the NLR task ((c); diet F(1,53) = 4.78, P = 0.033; treatment F(2,53) = 2.72, P = 0.075, interaction F(2,53) = 0.535, P = 0.589).
Figure 3. HFD-induced memory impairment was prevented by taurine and NAC treatments. (a) Open-field exploration was not impacted by HFD exposure or treatments with taurine or NAC, as revealed by similar total walking distance, number of crossings between quadrants, total immobility time, fraction of time spent in arena center. (b,c) Novel object recognition (NOR) and novel location recognition (NLR) tests showed identical object exploration in the familiarization phase for all groups (mean at ∼50% for each object; graphs on the left), while impaired novelty recognition was identified by both NOR (b) and NLR (c) in untreated HFD-fed mice. Open and filled bars represent mice fed CD and HFD, respectively. Data are expressed as mean ± SD of generally n = 10. Due to object exploration below 10 s, one non-supplemented CD-fed mouse was excluded from NOR in recognition phase (n = 9), and one taurine-supplemented HFD-fed mouse was excluded from NLR in recognition phase (n = 9). * P < 0.05 based on Fischer's LSD post-hoc comparison after significant effect of diet, supplementation or interaction in ANOVA.
Metabolite profile alterations in the hippocampus
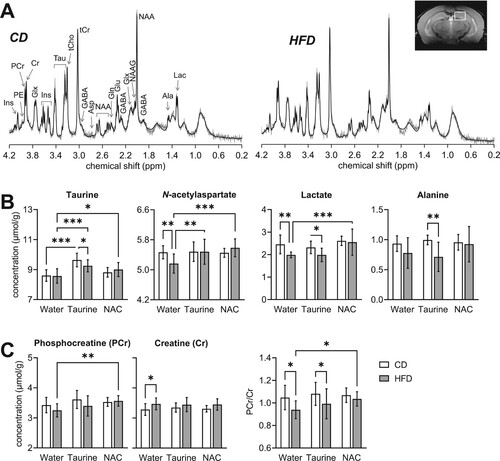
Metabolic alterations induced by long-term HFD exposure occur throughout the whole brain, but are particularly prominent in the hippocampus [Citation7,Citation11]. Therefore, we set to measure hippocampal metabolites using MRS ((a); Table S2). As expected, supplementation with taurine increased its concentration in the hippocampus of CD- and HFD-fed mice (P < 0.001 for both relative to the respective untreated group; (b)). NAC treatment had a smaller effect on hippocampal taurine, which was only significantly increased in HFD-fed mice (P = 0.027 vs. untreated HFD). N-acetylaspartate concentration, which is considered a neuronal health marker [Citation26], was decreased by HFD consumption (−6%, P = 0.007 vs. CD), but not upon supplementation with either taurine or NAC ((b)). Lactate concentration was also decreased by HFD feeding (−19%, P = 0.004 vs. CD), but not upon NAC treatment ((b)). A similar trend was observed for alanine. Small variations in phosphocreatine and creatine were observed in the hippocampus of HFD-fed mice ((c)). Most importantly, their ratio was significantly reduced by HFD (−10%, P = 0.014 vs. CD), a modification that was prevented by treatment with NAC but not taurine ((c); diet F(1,54) = 9.75, P = 0.003; treatment F(2,54) = 2.04, P = 0.140, interaction F(2,54) = 0.832, P = 0.441).
Figure 4. HFD-induced alterations of metabolite concentrations in the hippocampus. (A) Typical spectra acquired with STEAM at 9.4 T (gray line) from the hippocampus of CD- and HFD-fed mice. Respective LCModel fitting result is represented by the overlaid solid lines. The VOI location in the dorsal hippocampus is represented in the inset anatomical image. From right to left: Ala, alanine; Lac, lactate; GABA, γ-aminobutyrate; NAA, N-acetylaspartate; NAAG, N-acetylaspartylglutamate; Glx, glutamate (Glu) + glutamine (Gln); Asp, aspartate; tCr, total creatine = creatine (Cr) + phosphocreatine (PCr); tCho, total choline = phosphorylcholine + glycerophosphorylcholine; Tau, taurine; Ins, myo-inositol; PE, phosphorylethanolamine. (B) Treatment with taurine and/or NAC increased hippocampal taurine concentration, and prevented the HFD-induced reduction in N-acetylaspartate and/or lactate levels. (C) NAC treatment prevented the HFD-induced reduction of phosphocreatine-to-creatine ratio. Open and filled bars represent mice fed CD and HFD, respectively. Data are expressed as mean ± SD of n = 10. *P < 0.05, **P < 0.01, ***P < 0.001 based on Fischer's LSD post-hoc comparison after significant effect of diet, supplementation or interaction in ANOVA.
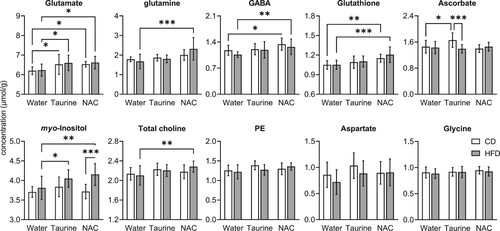
Other metabolites were not modified by HFD exposure per se, but their concentrations in the hippocampus were increased by taurine and/or NAC treatment (, Table S2). Both taurine and NAC increased the content of glutamate in both CD-fed and HFD-fed mice (all P < 0.05 relative to the respective untreated group), While taurine had no impact on glutamine, NAC increased glutamine concentration in the hippocampus of HFD-fed mice (P < 0.001 vs. untreated HFD; P = 0.034 vs. NAC-treated CD). Similarly, NAC but not taurine treatment increased total choline concentration in HFD-fed mice (P = 0.006 vs. untreated HFD). Furthermore, in the hippocampus of both CD and HFD-fed mice, NAC increased the concentration of GABA (for both, P < 0.05 compared to untreated groups) and glutathione (for both, P < 0.01 compared to untreated groups). On the other hand, taurine treatment increased ascorbate concentration in CD-fed mice (P = 0.010 compared to untreated group) but not HFD-fed mice. Finally, while myo-inositol was not affected by treatments in CD-fed mice, myo-inositol concentration increased in the hippocampus of HFD-fed mice upon treatments with taurine (P = 0.0291 vs. untreated HFD) and NAC (P = 0.002 vs. untreated HFD; P < 0.001 vs. NAC-treated CD).
Figure 5. Taurine and NAC treatments impacted metabolite concentrations in the hippocampus. Open and filled bars represent mice fed CD and HFD, respectively. Data are expressed as mean ± SD of n = 10. *P < 0.05, **P < 0.01, ***P < 0.001 based on Fischer's LSD post-hoc comparison after significant effect of diet, supplementation or interaction in ANOVA.
Determinants of memory impairment
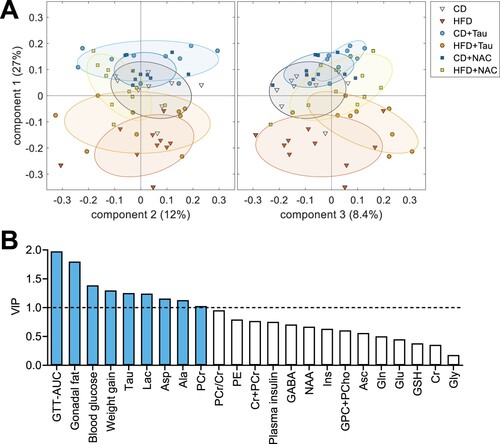
A partial least-squares (PLS) regression of a composite variable of memory performance with hippocampal metabolite concentrations and other independent metabolic parameters was used to identify key predictors of memory performance. Since hippocampal metabolites and metabolic syndrome parameters were also impacted on taurine and NAC treatments alone, the analysis with the 3-component PLS regression explained less than 50% of the observed variance. Nevertheless, it allowed good separation of CD and HFD mice, and some level of memory recovery by taurine and NAC treatments can be observed in the distribution of mice in the component space ((a)). VIPs calculated from the PLS model suggest that glucose clearance in GTT, gonadal fat weight, fasting blood glucose and weight gain are important determinants of memory impairment in female mice under HFD ((b)). From the metabolite profile measured in the hippocampus, five metabolites appear as important determinants of memory impairment (VIP > 1), namely taurine, lactate, aspartate, alanine and phosphocreatine.
Figure 6. PLS regression of the metabolite profile and metabolic syndrome parameters to a composite variable of memory performance (product of NLR and NOR scores). (a) Component space showing individual mice (symbols) and group SD (ellipsoids). (b) VIP scores calculated from the resulting PLS model.
Discussion
This study demonstrates that taurine and NAC treatments have beneficial effects on HFD-induced alterations of hippocampal metabolism, and prevent the development of memory impairment in female mice fed a HFD for 2 months. Accordingly, taurine has also been proposed to improve memory in animal models such as the APP/PS1 mouse model of Alzheimer’s disease [Citation19], aged mice [Citation27], or rats exposed to noise stress [Citation28]; and NAC was suggested to prevent memory impairment in an Alzheimer’s disease model of intra-hippocampal administration of amyloid-β [Citation29] or amyloid-β oligomers [Citation30].
HFD exposure for 2 months caused a marked reduction of N-acetylaspartate concentration, which is synthesized from mitochondrial acetyl-CoA and aspartate in neurons [Citation31], as thus it is generally taken as a biomarker of neuronal health [Citation26]. Moreover, HFD triggered a reduction of the energy indicator phosphocreatine-to-creatine ratio, and the concentration of lactate that is produced from the glycolytic end-product pyruvate. Taken together, these three metabolic alterations suggest an overall depression of energy metabolism in the hippocampus, including deactivation of glycolysis and reduced mitochondrial activity, which may have a detrimental impact on neuronal function [Citation32]. Notably, NAC supplementation was able to prevent these alterations. On the other hand, taurine was only able to prevent the HFD-induced reduction of N-acetylaspartate levels.
We had hypothesized that NAC stimulates endogenous taurine since both neurons and astrocytes can produce taurine from cysteine [Citation33,Citation34]. However, it is interesting to note that cultured astrocytes use exogenously cysteine to produce hypotaurine, taurine and glutathione at similar rates, neurons in culture produce glutathione at a much faster rate than any other metabolite [Citation34]. In our study, concentrations of taurine in both plasma ((d)) and hippocampus ((a)) increased with taurine supplementation but were unaltered by NAC treatment, suggesting that there is limited taurine synthesis from NAC-derived cysteine. Accordingly, CSAD expression was slightly reduced by NAC treatment. On the other hand, increased glutathione levels were observed in the hippocampus of NAC-treated mice, particularly prominent in HFD-fed mice (), which is in line with stimulation of glutathione synthesis. Thus, modes of action of the treatments are likely independent, with NAC effects being mediated via glutathione and not taurine metabolism. Through stimulation of the synthesis of the major antioxidant glutathione (via γ-glutamylcysteine ligase), NAC can prevent brain oxidative stress and neuroinflammation (e.g. [Citation35]). Accordingly, Choy et al. [Citation36] reduced glutathione brain levels in rats by treatment with 2-cyclohexene-1-one, and demonstrated that memory impairment is recoverable by NAC administration.
Further analysis of the metabolite profile of the hippocampus provides insight into putative mechanisms of action of taurine and NAC. Increased hippocampal taurine carries benefits per se, including promotion of neurogenesis and synaptogenesis [Citation37], which is in line with increased concentration of the neuronal marker N-acetylaspartate, as well as glutamate, which was increased by both taurine and NAC. Glutamate is mainly located in neurons, and interfaces energy metabolism and neuronal activity [Citation38]. Glutamate is key for memory performance, but excitatory glutamatergic neurons require adequate inhibitory control [Citation39]. NAC but not taurine induced an accumulation of the inhibitory neurotransmitter GABA in the hippocampus. In this way, NAC can increase the inhibitory tonus. On the other hand, taurine itself is known to act as neurotransmitter via GABAA, GABAB and glycine receptors [Citation40,Citation41]. In line with a role of NAC treatment on neurotransmitter metabolism, it also increased glutamine concentration in the hippocampus of HFD mice. Given that glutamine is exclusively synthesized in glia [Citation38], NAC treatment appears to be a modulator of the glutamate–glutamine cycle between neurons and astrocytes.
Taurine and NAC increased the concentration of myo-inositol in the hippocampus of HFD-fed mice but not controls. Increases in the content of myo-inositol are believed to represent astrogliosis [Citation26,Citation42], but such a relation has not been replicated in the hippocampus of diabetes models [Citation8,Citation9]. We speculate that taurine and NAC treatments are unlikely induce gliosis in the hippocampus. Similarly, the NAC-induced increase in total choline in HFD-fed mice relative to untreated HFD-fed mice is unlikely linked to neuroinflammation as proposed in other studies [Citation43], and simply represents the abundance of mobile metabolites resulting from enzymatic modification of phosphatidylcholine in cell membranes unrelated to gliosis [Citation44].
Taurine and NAC supplementation not only impacted the brain, but they also ameliorated metabolic syndrome upon HFD feeding. Female mice under HFD developed obesity and glucose intolerance but not substantial hyperglycemia or hyperinsulinemia, as observed upon HFHSD feeding [Citation11]. NAC but not taurine prevented obesity development. As observed by others, HFD-induced glucose intolerance was prevented by treatment with taurine [Citation45–47] and NAC [Citation48]. Both treatments modulated circulating levels of adipokines, in line with possible effects of NAC [Citation49] and taurine [Citation50] on adipose tissue.
A PLS regression of hippocampal metabolite concentrations and other independent metabolic parameters revealed that levels of taurine, lactate, aspartate, alanine and phosphocreatine in the hippocampus are linked to memory impairment. These metabolites are involved in energy metabolism pathways, and taurine is thought to improve mitochondrial health [Citation51], suggesting that ATP production for fueling memory might underlie memory dysfunction upon HFD exposure. Notably, the most important determinants of memory impairment in this analysis were not hippocampal metabolites, but parameters defining the metabolic syndrome, namely weight gain, fat deposition and glucose homeostasis (fasting glycemia and glucose tolerance), supporting the notion of obesity as risk factor for cognitive impairment [Citation2,Citation3].
The present study was limited to female mice. While most studies have been conducted on male rodents, our recent work suggests that the development of memory impairment promoted by HFHSD is similar in mice of either sex despite higher metabolic syndrome severity in males [Citation11]. In particular, in contrast to males, female mice under HFHSD did not develop hyperinsulinemia. By focusing on female mice in the present work, we were able to study obesity-associated effects in the absence of hyperinsulinemia, and thus increased insulin concentration in the brain.
In conclusion, memory impairment and metabolic alterations induced by HFD on the hippocampus of female mice are partially prevented by taurine supplementation, and fully recovered by treatment with NAC. NAC acts as a cysteine donor that could stimulate taurine synthesis, but it mainly resulted in accumulation of glutathione in the hippocampus, likely providing an enhanced antioxidant defence. Mechanisms by which these treatments exert neuroprotection and prevent memory impairments likely involve energy metabolism, and synthesis of glutamate and/or GABA.
Brain MRS studies in obese individuals and diabetes patients are limited, have been generally conducted at low magnetic field, and taurine concentrations in the human brain are much lower than in rodents [Citation26]. Therefore, it is challenging to observe taurine concentration changes in the living human brain, and confirm the taurine accumulation observe in rodent models. Nevertheless, our findings suggest that cognitive impairment risk in obesity and diabetes might be reduced by augmenting dietary availability of taurine and enhancing endogenous glutathione synthesis.
Author contributions
JMND designed the study and analysed data. AMGS, JPPV and VF performed experiments and analysed data. JMND and AMGS wrote the manuscript. All authors revised the manuscript.
Supplemental Material
Download MS Word (15.1 KB)Acknowledgements
The authors thank Dr. Vladimir Denisov for access to NMR spectrometer. The Lund University Bioimaging Centre is acknowledged for providing access to 9.4 T MRI scanner.
Disclosure statement
No potential conflict of interest was reported by the authors.
Data availability
All data are contained within the manuscript, and can be shared upon request to the corresponding author.
Additional information
Funding
References
- GBD 2019 Dementia Forecasting Collaborators. Estimation of the global prevalence of dementia in 2019 and forecasted prevalence in 2050: an analysis for the global burden of disease study 2019. Lancet Public Health. 2022;S2468-2667(21):00249–8.
- Qizilbash N, Gregson J, Johnson ME, Pearce N, Douglas I, Wing K, et al. BMI and risk of dementia in two million people over two decades: a retrospective cohort study. Lancet Diabetes Endocrinol. 2015;3(6):431–436.
- Albanese E, Launer LJ, Egger M, Prince MJ, Giannakopoulos P, Wolters FJ, et al. Body mass index in midlife and dementia: systematic review and meta-regression analysis of 589,649 men and women followed in longitudinal studies. Alzheimers Dement (Amst). 2017;8:165–178.
- Livingston G, Huntley J, Sommerlad A, Ames D, Ballard C, Banerjee S, et al. Dementia prevention, intervention, and care: 2020 report of the lancet commission. Lancet. 2020;396(10248):413–446.
- Guerreiro RJ, Gustafson DR, Hardy J. The genetic architecture of Alzheimer's disease: beyond APP, PSENs and APOE. Neurobiol Aging. 2012;33(3):437–56.
- Garcia-Serrano AM, Duarte JMN. Brain metabolism alterations in type 2 diabetes: what did we learn from diet-induced diabetes models? Front Neurosci. 2020;14:229.
- Lizarbe B, Soares AF, Larsson S, Lizarbe B, Soares AF, Larsson S, et al. Neurochemical modifications in the hippocampus, cortex and hypothalamus of mice exposed to long-term high-fat diet. Front Neurosci. 2019;12:985.
- Duarte JMN, Skoug C, Silva HB, Carvalho RA, Gruetter R, Cunha RA. Impact of caffeine consumption on type 2 diabetes-induced spatial memory impairment and neurochemical alterations in the hippocampus. Front Neurosci. 2019;12:1015.
- Duarte JMN, Carvalho RA, Cunha RA, Gruetter R, et al. Caffeine consumption attenuates neurochemical modifications in the hippocampus of streptozotocin-induced diabetic rats. J Neurochem. 2009;111(2):368–79.
- Zhang H, Huang M, Gao L, Lei H. Region-specific cerebral metabolic alterations in streptozotocin-induced type 1 diabetic rats: an in vivo proton magnetic resonance spectroscopy study. J Cereb Blood Flow Metab. 2015;35(11):1738–45.
- Garcia-Serrano AM, Mohr AA, Philippe J, Skoug C, Spégel P, Duarte JMN. Cognitive impairment and metabolite profile alterations in the hippocampus and cortex of male and female mice exposed to a fat and sugar-rich diet are normalized by diet reversal. Aging Dis. 2022;13(1):267–283.
- McLean FH, Campbell FM, Sergi D, Grant C, Morris AC, Hay EA, et al. Early and reversible changes to the hippocampal proteome in mice on a high-fat diet. Nutr Metab (Lond). 2019;16:57.
- de Paula GC, Brunetta HS, Engel DF, Gaspar JM, Velloso LA, Engblom D, et al. Hippocampal function is impaired by a short-term high-fat diet in mice: increased blood-brain barrier permeability and neuroinflammation as triggering events. Front Neurosci. 2021;15:734158.
- Aytan N, Choi JK, Carreras I, Brinkmann V, Kowall NW, Jenkins BG, et al. Fingolimod modulates multiple neuroinflammatory markers in a mouse model of Alzheimer's disease. Sci Rep. 2016;6:24939.
- Takado Y, Takuwa H, Takuya U, et al. Correlations between brain metabolites and tau protein accumulation assessed by 1H-MRS and tau PET in Alzheimer’s disease model mice. Proc Intl Soc Mag Reson Med. 2018;26:4976.
- Chiquita S, Ribeiro M, Castelhano J, Oliveira F, Sereno J, Batista M, et al. A longitudinal multimodal in vivo molecular imaging study of the 3xTg-AD mouse model shows progressive early hippocampal and taurine loss. Hum Mol Genet. 2019;28(13):2174–2188.
- Tondo M, Wasek B, Escola-Gil JC, de Gonzalo-Calvo D, Harmon C, Arning E, et al. Altered brain metabolome is associated with memory impairment in the rTg4510 mouse model of tauopathy. Metabolites. 2020;10(2):69.
- Louzada PR, Paula Lima AC, Mendonca-Silva DL, Mendonca-Silva DL, Noël F, De Mello FG, et al. Taurine prevents the neurotoxicity of beta-amyloid and glutamate receptor agonists: activation of GABA receptors and possible implications for Alzheimer's disease and other neurological disorders. FASEB J. 2004;18(3):511–8.
- Kim HY, Kim HV, Yoon JH, Kang BR, Cho SM, Lee S, et al. Taurine in drinking water recovers learning and memory in the adult APP/PS1 mouse model of Alzheimer's disease. Sci Rep. 2014;4:7467.
- Duarte JMN, Kulak A, Gholam-Razaee MM, das Neves Duarte JM, Cuenod M, Gruetter R, et al. N-acetylcysteine normalizes neurochemical changes in the glutathione-deficient schizophrenia mouse model during development. Biol Psychiatry. 2012;71(11):1006–14.
- Aldini G, Altomare A, Baron G, Vistoli G, Carini M, Borsani L, et al. N-Acetylcysteine as an antioxidant and disulphide breaking agent: the reasons why. Free Radic Res. 2018;52(7):751–762. doi:10.1080/10715762.2018.1468564.
- Deepmala SJ, Kumar N, Slattery J, Delhey L, Berk M, et al. Clinical trials of N-acetylcysteine in psychiatry and neurology: a systematic review. Neurosci Biobehav Rev. 2015;55:294–321.
- Duarte JMN, Do KQ, Gruetter R. Longitudinal neurochemical modifications in the aging mouse brain measured in vivo by 1H magnetic resonance spectroscopy. Neurobiol Aging. 2014;35(7):1660–8. doi:10.1016/j.neurobiolaging.2014.01.135.
- Duarte JMN, Cunha RA, Carvalho RA. Different metabolism of glutamatergic and GABAergic compartments in superfused hippocampal slices characterized by nuclear magnetic resonance spectroscopy. Neurosci. 2007;144:1305–1313.
- Zhou Y, Holmseth S, Guo C, Hassel B, Höfner G, Huitfeldt HS, et al. Deletion of the γ-aminobutyric acid transporter 2 (GAT2 and SLC6A13) gene in mice leads to changes in liver and brain taurine contents. J Biol Chem. 2012;287(42):35733–35746.
- Duarte JMN, Lei H, Mlynárik V, Gruetter R. The neurochemical profile quantified by in vivo 1H NMR spectroscopy. Neuroimage. 2012;61(2):342–62.
- El Idrissi A. Taurine improves learning and retention in aged mice. Neurosci Lett. 2008;436(1):19–22.
- Haider S, Sajid I, Batool Z, Madiha S, Sadir S, Kamil N, et al. Supplementation of taurine insulates against oxidative stress, confers neuroprotection and attenuates memory impairment in noise stress exposed male wistar rats. Neurochem Res. 2020;45(11):2762–2774.
- Shahidi S, Zargooshnia S, Asl SS, Komaki A, Sarihi A, et al. Influence of N-acetyl cysteine on beta-amyloid-induced Alzheimer's disease in a rat model: a behavioral and electrophysiological study. Brain Res Bull. 2017;131:142–149.
- More J, Galusso N, Veloso P, Montecinos L, Finkelstein JP, Sanchez G, et al. N-Acetylcysteine prevents the spatial memory deficits and the redox-dependent RyR2 decrease displayed by an Alzheimer's disease rat model. Front Aging Neurosci. 2018;10:399.
- Baslow MH. N-acetylaspartate in the vertebrate brain: metabolism and function. Neurochem Res. 2003;28(6):941–53.
- Belenguer P, Duarte JMN, Schuck PF, Ferreira GC. Mitochondria and the brain: bioenergetics and beyond. Neurotox Res. 2019;36(2):219–238.
- Brand A, Leibfritz D, Hamprecht B, Dringen R. Metabolism of cysteine in astroglial cells: synthesis of hypotaurine and taurine. J Neurochem. 1998;71(2):827–32.
- Vitvitsky V, Garg SK, Banerjee R. Taurine biosynthesis by neurons and astrocytes. J Biol Chem. 2011;286(37):32002–10.
- Dwir D, Cabungcal JH, Xin L, Giangreco B, Parietti E, Cleusix M, et al. Timely N-acetyl-cysteine and environmental enrichment rescue oxidative stress-induced parvalbumin interneuron impairments via MMP9/RAGE pathway: a translational approach for early intervention in psychosis. Schizophr Bull. 2021;47(6):1782–1794.
- Choy KH, Dean O, Berk M, Bush AI, van den Buuse M, et al. Effects of N-acetyl-cysteine treatment on glutathione depletion and a short-term spatial memory deficit in 2-cyclohexene-1-one-treated rats. Eur J Pharmacol. 2010;649(1-3):224–8.
- Shivaraj MC, Marcy G, Low G, Ryu JR, Zhao X, Rosales FJ, et al. Taurine induces proliferation of neural stem cells and synapse development in the developing mouse brain. PLoS One. 2012;7(8):e42935.
- Sonnay S, Gruetter R, Duarte JMN. How energy metabolism supports cerebral function: insights from 13C magnetic resonance studies In vivo. Front Neurosci. 2017;11:288.
- Duarte JMN, Xin L. Magnetic resonance spectroscopy in schizophrenia: evidence for glutamatergic dysfunction and impaired energy metabolism. Neurochem Res. 2019;44(1):102–116.
- Lynch JW. Molecular structure and function of the glycine receptor chloride channel. Physiol Rev. 2004;84(4):1051–1095.
- Albrecht J, Schousboe A. Taurine interaction with neurotransmitter receptors in the CNS: an update. Neurochem Res. 2005;30(12):1615–21.
- Harris JL, Choi IY, Brooks WM. Probing astrocyte metabolism in vivo: proton magnetic resonance spectroscopy in the injured and aging brain. Front Aging Neurosci. 2015;7:202.
- Yasmin A, Pitkänen A, Jokivarsi K, Poutiainen P, Gröhn O, Immonen R, et al. MRS reveals chronic inflammation in T2w MRI-negative perilesional cortex – a 6-months multimodal imaging follow-Up study. Front Neurosci. 2019;13:863.
- Iorio E, Podo F, Leach MO, Koutcher J, Blankenberg FG, Norfray JF. A novel roadmap connecting the 1H-MRS total choline resonance to all hallmarks of cancer following targeted therapy. Eur Radiol Exp. 2021;5(1):5.
- Ribeiro RA, Santos-Silva JC, Vettorazzi JF, Cotrim BB, Mobiolli DDM, Boschero AC, et al. Taurine supplementation prevents morpho-physiological alterations in high-fat diet mice pancreatic β-cells. Amino Acids. 2012;43(4):1791–801.
- Camargo RL, Batista TM, Ribeiro RA, Velloso LA, Boschero AC, Carneiro EM, et al. Effects of taurine supplementation upon food intake and central insulin signaling in malnourished mice fed on a high-fat diet. Adv Exp Med Biol. 2013;776:93–103.
- Figueroa AL, Figueiredo H, Rebuffat SA, Vieira E, Gomis R. Taurine treatment modulates circadian rhythms in mice fed a high fat diet. Sci Rep. 2016;6:36801.
- Falach-Malik A, Rozenfeld H, Chetboun M, et al. N-Acetyl-L-Cysteine inhibits the development of glucose intolerance and hepatic steatosis in diabetes-prone mice. Am J Transl Res. 2016;8(9):3744–3756.
- Shen FC, Weng SW, Tsao CF, Chang C-S, Lian W-S, Chuang J-H, et al. Early intervention of N-acetylcysteine better improves insulin resistance in diet-induced obesity mice. Free Radic Res. 2018;52(11-12):1296–1310.
- Kim KS, Jang MJ, Fang S, Yoon SG, Seong JK, Yang H-I, et al. Anti-obesity effect of taurine through inhibition of adipogenesis in white fat tissue but not in brown fat tissue in a high-fat diet-induced obese mouse model. Amino Acids. 2019;51(2):245–254.
- Rafiee Z, García-Serrano AM, Duarte JMN. Taurine supplementation as a neuroprotective strategy upon brain dysfunction in metabolic syndrome and diabetes. Nutrients. 2022;14(6):1292.

