ABSTRACT
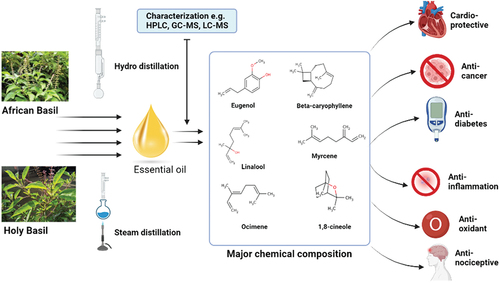
Ocimum species, including African basil (Ocimum gratissimum) and Holy basil (Ocimum sanctum), are valued for their diverse therapeutic properties particularly in the form of essential oils (EOs). This review provides a comprehensive analysis of the health and therapeutic potentials of Ocimum essential oils, specifically focusing on African and Holy basil. It encompasses various aspects, such as isolation methods, phytochemical characterization, bioactivities, bioavailability, and future directions. Various studies have highlighted the bioactive compounds within these EOs, such as eugenol, camphor, and α-pinene, attributing to their wide-ranging health benefits. Notably, Ocimum EOs exhibit potent antimicrobial, antioxidant, anti-inflammatory, antidiabetic, and anticancer properties, making them promising candidates for alternative medicine and pharmaceutical applications. However, the rapid absorption and poor bioavailability of EOs pose challenges, mitigated through nanoencapsulation techniques enhancing their efficacy. Future research directions include mechanistic studies utilizing specialized cell cultures and animal models, alongside clinical trials to validate reported bioactivities. Optimization of delivery systems and exploration of novel nano-formulations are also essential for optimizing the complete therapeutic potential of Ocimum essential oils. Lastly, efforts to enhance EO yield and augment bioactivity through genetic strategies and elicitation techniques have been suggested.
1. Introduction
Plants have been one of nature’s multi-purpose solutions to humanity’s significant problems- fostering humankind’s good health and wellness, providing raw materials for vast industrial purposes, and ensuring a sustainable and unpolluted environment (Citation1). The genus Ocimum has been very popular recently, comprising over 68 globally distributed species indigenous to several countries and continents. Different species of Ocimum have been popularized due to their inherent medicinal, perfumery, ceremonial, and culinary benefits (Citation2). Ocimum gratissimum and Ocimum sanctum (synonymous with Ocimum tenuiflorum) are exciting member species of this genus, possessing diverse bioactive compounds and phytochemicals with proven therapeutic benefits and health-boosting potential for man. Moreover, both are primarily known for their characteristic essential oils, promising therapeutic and industrial usefulness (Citation3).
Ocimum gratissimum (OG), commonly called African basil or clove basil, is indigenous to mainly Africa. However, they have been found in other tropical and subtropical parts like Southern Asia and America (Citation4). The plant is called different names in different societies. Although in Nigeria and some West African countries, it is popularly known as ‘Scent leave’. Many tribes of different countries have a vernacular name specific to the plant. For instance, the Igbo calls it ‘Nchuanwu or Ahuji’, the Yoruba refers to it as ‘Efirin’, and Hausa as ‘Daidoya’ (Citation5). Other African countries have a different name for the African basil, such as the Ugandan popularly call it ”Mujaaja”. African basil is a perennial herb belonging to the Lamiaceae family with an average height of less than 1 m and an erect plumy structure with many branches (Citation4). The plant is propagated through seed planting and stem cutting and is well-known for its aromatic nature, hence its name, ‘scent leave’ (Citation6).
Conversely, the Ocimum sanctum (OS) is popularly known as Holy basil or Tulsi. It is indigenous to the Indo-pacific region, with a significant concentration in the Himalayas up to an altitude of 6000 ft (Citation7). The name ‘Holy basil’ is possibly given to the plant due to the belief in the Himalayas mountain’s sacredness where the plant thrives. Holy basil is also an aromatic scrub with two cultivars that can be differentiated by their leave colors – the Rama Tulsi with green leaves and the Krishna Tulsi with purple leaves (Citation8).
Despite the global popularity and awareness of African and Holy basil, especially for traditional medicine, there have been limited studies on their essential oils (EOs). Like other aromatic and medical plants, most of the aromatic compounds of African and Holy basil are concentrated in the EO fraction of the plants, and this can be isolated majorly by hydro and steam distillation from the whole plant, their leaves, flower, root, stems, and seeds (Citation9). However, recent studies have tried assisted systems such as microwave-assisted, ultrasound-assisted, and supercritical fluid extraction to improve EO yields (Citation10). Compounds like eugenol, cis-ocimene, y-muurolene, and thymol are prevalent and abundant in the EO fraction of African and Holy basil. Many studies have identified them using different analytical approaches such as gas or liquid chromatography-tandem to mass spectrometry, nuclear magnetic resonance (NMR), GC-FID, and many more (Citation9). The bioactivities and therapeutic potential of both African and Holy Basil's EO have been reported in several studies. Among their vast bioactivities, we present an up-to-date review of their antimalarial, anti-diarrheal, hematoprotective, antioxidant/free radical scavenging, antimicrobial/anti-parasitic, anticancer, antidiabetic, anti-inflammatory, and cardio-protective and hepato-protective activities (Citation5,Citation11,Citation12). Furthermore, we adopted a comparative approach to understanding the differences in the isolation methods, purification strategies, analytical techniques for identifying compounds, and experimental methods for examining bioactivities and toxicities of the essential oil from African and Holy basil. Finally, we presented a perspective for future studies from the understanding gained from the comparative studies and how compounds from this oil can progress to a clinical trial for modern medicinal purposes.
2. Methodology
For this study, relevant and focused literature on the EOs of African and Holy Basil deposited on PubMed until 1 May 2022 was retrieved and used for this comparative review study. PubMed is our primary search engine, a curated repository of biomedical literature from MEDLINE, life sciences journals, and online books. At the same time, Scopus, ScienceDirect, and Google Scholar repositories were adopted as secondary search engines. We used concise and straightforward keywords such as ‘essential oil’, ‘Ocimum gratissimum’, and ”Ocimum sanctum .‘Ocimum tenuiflorum’, ‘African basil’, ‘Holy basil’, and together with other keywords specific to bioactivities. We use the Boolean connected ‘AND’ or ‘OR’ to join the keywords to obtain focus and specific literature.
From our PubMed search 29,091 search results output was obtained for ‘essential oil’, 308 for’Ocimum gratissimum,‘600 for’Ocimum sanctum,‘ and 129 for’Ocimum tenuiflorum.“Finally, 110 reports were retrieved for studies specific to African basil EO by searching ‘essential oil’ AND ‘Ocimum gratissimum’; while only 69 reports for Holy basil EO after a search with ‘essential oil’ AND (‘Ocimum gratissimum’ OR ‘Ocimum tenuiflorum’). Finally, our major inclusion was that the study must focus on either African or Holy basil; hence, we excluded other non-essential oil studies such as aqueous fraction, ethanol fraction, n-hexane fraction, and others.
3. Isolation of Essential Oils from African and Holy Basil
The most widely used conventional method for isolating EO from Ocimum species is steam distillation (SD) and hydro distillation (HD) (Citation13). In SD, the plant material is heated in a distillation flask exposed to steam from an outside source. The steam promotes the opening of glands enclosing the EO. Then, the EO in the steam rises and enters the narrow tubing to the condenser. The separation of the EO from the condensed steam in the Florentine flask is based on their density (Citation14). Several factors, including the plant material’s temperature, type, quality, distillation time, and operating pressure, determine EO quality and yield (Citation14). According to Abed et al. (Citation14), an increase in temperature enhances the solubility of the oil in the solvent and the diffusion coefficient, thereby improving the isolation. Several research works have employed these techniques to isolate EOs from different parts of African and Holy basil, as shown in . Although SD minimizes loss of polar compounds and modification of compounds if refluxing is controlled and saves time and energy, the major drawback of its usage is that it requires high temperature, takes a prolonged time to accomplish, and results in low yield (Citation21). In a study by Chimnoi et al. (Citation17) to isolate EO from 1.0 kg of African basil, using SD, only 1.25 g (0.125%) of EO was obtained, Kobenan et al. (Citation18) obtained a 1.26% yield from Côte d’Ivoire African basil EO isolated with SD. A similar amount (1.20%) was isolated from flowering aerial parts of the Holy Basil EO cultivated in Belgium using SD (Citation22). However, SD remains the primary isolation technique for basil oil mainly due to its simplicity and economic feasibility (Citation13)
Table 1. Isolation Techniques, Condition, and Yield of African and Holy Basil Essential Oil.
Hydrodistillation involves immersion and boiling of the plant material in a Clevenger apparatus. The principle of HD involves the hydro diffusion of EO from the plant samples placed in a chamber containing hot water (Citation23). The water is heated to produce the steam to break down the leaf structure. The steam carries the EO to a condensation chamber. The hydrophobic EO floats on top after condensation and is separated by decantation (Citation14). HD is inexpensive and easy, but it is not easy to constantly control heat transfer. It requires high temperature, thereby increasing operational costs and hydrolysis of some EO constituents (Citation21). This might cause odor, thermal degradation, loss of volatile compounds under prolonged heating, and low yield (Citation21). Thus, a yield of 1.66% (w/w), 0.18%, and 1.8% from HD of African Basil EO by Melo et al. (Citation24), Mohr et al. (Citation25), and Koba (Citation26), respectively.
Besides SD and HD, other newer techniques are currently employed in isolating EO from African and Holy basil, such as supercritical fluid extraction (SFE) and solvent-free microwave extraction (SFME). Supercritical fluid extraction (SFE) is a clean, innovative, and environmentally friendly technology that requires less temperature, leading to a high yield and quick EO isolation from aromatic herbs (Citation14). Additionally, SPE systems give the appropriate solvents or a mixture to target particular plant fractions and shorten the isolation time (Citation23). The commonly used isolated fluid in SFE is CO2 which is non-toxic, widely available, and easy to remove. The plant material is added inside a pocket and placed in an extraction vessel (EV). Supercritical fluid (CO2) is loaded from the bottom of the EV. The EO isolated by the SF flows through a depressurization valve to be separated into a separator (Citation14). The E isolation is affected by pressure, temperature, flow rate of fluids fractionation, and sample size. The major drawback of its usage in EO isolation is the high cost of operation.
Another newer technique is solvent-free microwave extraction (SFME), patented in 2004 (Citation27). SFME is assisted by microwaves, without any water or solvent, at atmospheric pressure. SFME operational procedure involves heating the plant sample in a microwave. The technique combines dry distillation and microwave heating at atmospheric temperature. The microwave heat energy reduces distillation time compared to other methods. This method can effectively isolate EO from samples with high water content due to the strong interaction between the microwave, salt, and nutrients containing physiological water. The amount of EOs isolated using SFME depends on the dipole moment of the compounds present in the EO since studies have shown that a high dipole moment favors organic compounds’ interaction with microwaves, making them more easily isolated, unlike aromatic compounds, which have low dipolar moments (Citation27). This method has been used to isolate EOs from basils, thyme, and mints. The advantages of using microwave energy are correlated to its faster energy transfer and effective heating, being environmentally friendly, and leading to a high yield of EO compared with SD and HD (Citation27).
The yield of EOs from African and Holy basil is determined by several factors, including the EO isolation techniques, distillation time, and developmental stage of the plant, as shown in . For instance, Shiwatoti et al. (Citation13) obtained a higher EO content (0.68 ± 0.05%) from Holy basil isolated with SD compared with 0.33 ± 0.02 recovered with HD under the same time interval (60 minutes). The high yield of EO by SD compared to HD is due to the direct contact of the plant with heat, which easily ruptures the oil gland leading to fast elution of the volatile compounds (Citation13). In addition, distillation time is a key factor to consider in isolating EOs because longer distillation time requires higher energy consumption which could trigger hydrolysis and thermal degradation of some compounds in the EO (Citation13). In a study conducted by Ibeh et al. (Citation19) to compare SD, HD, and SFME, they realized that the SFME required less distillation time and gave the highest yield (52 mins; 1.84%), followed by SD (102 mins; 1.33%) and HD (165 mins; 0.91%). The faster elution seen in SFME and SD relative to HD is due to the higher heat transfer index in both methods, resulting in the fast elution of volatiles. Moreso, the yield of EO could also depend on the developmental stage of the basil. In an experiment designed to isolate EO from Holy basil under different developmental stages (complete flowering stages, vegetative, and floral budding) of Holy basil by Saharkhiz et al. (Citation20), the yield obtained was 1.1% 0.98%, and 0.92% for the complete flowering stages, vegetative, and floral budding, respectively. Therefore, it is important to indicate the isolation techniques, the condition, and the plant part used to explain the variations in EO yield in different studies (Citation25).
4. Nature and phytochemical composition of African and Holy basil essential oil
An overview of the phyto-compounds and their chemotypes present in both plants (African and Holy basil) was reported in a study conducted by Joshi (Citation22). From the study, EOs isolated from African basil contained 31 identifiable compounds belonging to phenyl derivatives (76.0%), monoterpene hydrocarbons (15.5%), sesquiterpene hydrocarbons (6.1%), oxygenated monoterpenes (1.6%), and oxygenated sesquiterpene (0.1%) Chemotypes, with the compound – eugenol (75.1%) being the most abundant. However, the EOs isolated from Holy basil was constituted of 25 phytocompounds, of which 3 were phenyl derivatives (94.9%), 9 sesquiterpene hydrocarbons (2.6%), 5 oxygenated sesquiterpenes (0.9%), 5 monoterpene hydrocarbons (0.3%), and 3 oxygenated monoterpenes (0.2%). Methyl eugenol from the library of 25 compounds was the most abundant (92.4%) in Holy Basil EOs. Taken together, phenyl derivatives are the most abundant class in the EOs from both plants, whereas the composition of other chemotypes varies in the two species.
The nature and composition of EO from different basil species are strongly affected by the distillation techniques, distillation time, cultivars type, drying method, ecotypes, and chemotypes (Citation13). Other important but salient factors affecting the EOs composition include climate, ecological zone, vegetative stage, harvesting time, genetics, water, and nutrient availability (Citation15,Citation28). Many studies on African and Holy basil EOs revealed variations in the oil obtained using different distillation techniques. Using steam distillation (SD), 37 constituents were identified in the EO isolated from African basil leaves, and the major compounds (with high relative abundance) were eugenol (55.6%), cis-ocimene (13.9%), γ-muurolene (11.6%), (Z, E)-α-farnesene (5.6%), α-trans-bergamotene (4.1%), and caryophyllene (2.7%) (Citation17). On the other hand, another study by Melo et al. (Citation15). adopted the hydrodistillation (HD) protocol for EO isolation from the same plant – African basil leaves and identified 19 compounds belonging to several chemotypes. Based on the relative abundance, the chemotype classes of African basil EOs (which were isolated from HD) were phenylpropanoid (74.83%), oxygenated monoterpenes (16.09%), hydrocarbon sesquiterpenes (6.96%), minor amounts of hydrocarbon monoterpenes (0.92%) and oxygenated sesquiterpenes (0.62%). The phytochemicals identified consisted majorly of eugenol (74.83%), 1,8-Cineole (15.16%), β-Selinene (2.82%) and (E)-Caryophyllene (2.20%). In the same vein, Mohr et al. (Citation16). isolated oxygenated monoterpenes (72.30%), sesquiterpene hydrocarbons (10.16%), phenylpropanoids (7.42%), oxygenated sesquiterpenes (5.18%) and monoterpene hydrocarbons (4.82%) through HD of African basil leaves. Similarly, isolation of EO from African basil grown in Togo using HD recorded monoterpene hydrocarbons (56.21%), oxygenated monoterpenes (37.85% and sesquiterpene hydrocarbons (3.80% with Thymol (31.79%), p-cymene (15.57%) and γ-terpinene (12.34%), myrcene (6.94%) and α-thujene (6.11%) as the major components (Citation26) ().
Table 2. Phytochemicals of African Basil Essential Oil (OGEO) with Abundance > 1% (Adapted from Chukwuma Et Al. 12).
Based on the isolation techniques adopted, the composition of EO from Holy basil was predominantly oxygenated monoterpenes (75% in HD; 67% in SD), sesquiterpene hydrocarbon (17% in HD; 27% in SD), and a trace amount of monoterpene hydrocarbons (Citation13). Specifically, methyl eugenol (68% in HD; 59% in SD), caryophyllene (8% in HD; 13% in SD), and eugenol (7% in HD; 9% in SD) were the major identified compounds in Holy basil EO (Citation13) (). Some other studies have demonstrated that EOs compositions from both plants also vary depending on the vegetative stage before the isolation process (Citation15). Saharkhiz and his colleagues (Citation20) reported the variations of phytochemical constituents of EOs isolated from Holy basil at different stages of growth and development. From their study, eugenol (37.15%), 1.8-cineole (26.45%), and β-Bisabolen (20.99%) were the most abundant compounds in floral budding (emergence of flower buds), full flowering, and vegetative stages respectively.
Table 3. Phytochemicals of Holy Basil Essential Oil with Relative Abundance > 0.5% (Adapted from Chukwuma Et Al. 12).
Finally, besides the variation due to the distillation techniques adopted, it is worth noting that from several studies, eugenol and thymol-rich compounds as the most abundant constituents of the EOs from African and Holy basil (Citation17). For instance, 55.55 and 74.83% of eugenol were obtained in African basil EO by Chimnoi et al. (Citation17) and Melo et al. (Citation24), respectively. In comparison, 37.15 and 61.30% of eugenol were identified in Holy basil EO by Kumar et al. (Citation29) and Saharkhiz et al. (Citation20), respectively. Moreover, Prabhu et al. (Citation30) obtained a high abundance of eugenol (54.0, 34.6, and 73.1%) using SD, HD, and SFE, respectively. However, the second and third most abundant compounds in both African and Holy basil EO may generally be 1,8-Cineole (Citation15,Citation16), cis-ocimene (Citation17), thymol-p-cymene (Citation31) and b-caryophyllene (Citation29) (thymol-rich compounds). Thus, the EOs variation based on isolation techniques as well as the vegetative plant growth stages, calls for more study to understand the roles of other variables (such as cultivars type, preparation methods, climates, nutrient availability, genetics, and others) in the EOs composition, which possibly affects their biological activities and health-promoting potentials. As of current knowledge, eugenol being the most abundant in African and Holy basil EOs has demonstrated antimicrobial and antioxidant potency and thus can control oxidation of unsaturated lipids and free radical scavenging, thereby enhancing the shelf life of food products (Citation29).
5. Biological activities and therapeutic potential of Essential oils from African and Holy basil
Several natural products are currently used as alternative therapy for preventing, managing, and treating. Interestingly, EO from basils has been implicated with numerous bioactivities with potential therapeutic applications such as antimicrobial activities against human pathogenic bacteria and fungi, food spoilage microbes, and even plant pathogens (Citation5,Citation7,Citation32). Moreover, their EO is a rich source of antioxidants to subvert human metabolic diseases such as cancers, diabetes, and pro-inflammatory conditions (Citation32). This section reviewed recent findings on the bioactivities of African and Holy basil EOs, with the potential for promoting health vitality and combating human disease conditions ().
5.1 Antidiabetic activity
Diabetes mellitus is a metabolic disorder of diverse etiology with a steadily rising global prevalence. The condition is characterized by chronic and sustained hyperglycemia, distorting the primary metabolic pathways (carbohydrate, protein, and fat). Management of diabetic conditions is quite challenging and has never been straightforward (Citation33). Essential oil fractions from African and Holy basil have been demonstrated to have antidiabetic properties, such as reducing blood glucose levels, altering glycation activities, improving the serum insulin level, and many more (). These EOs contain a number of bioactive compounds which perform antidiabetic functions. In a study by Singh et al. (Citation34), the EOs of three different species of Ocimum (including O. gratissimum and O. tenuiflorum (O. sanctum) were purified, characterized, and evaluated for anti-glycation activity. The compounds identified include but are not limited to eucalyptol, camphor, eugenol, eugenol methyl ether (EME), ocimene, α-pinene, terpinolene, β-caryophyllene. Of all the metabolites, in vitro inhibition of advanced glycation end products (AGEs) by eugenol was the highest (Citation34). The highest inhibition of glycation was observed by the EO of O. gratissimu, which also has the highest eugenol contents. However, O. tenuiflorum, rich in EME, showed the least inhibition of glycation (only about 10%). Hence, eugenol’s excellent results and anti-glycation potential fostered further investigation by other in vivo studies (Citation34).
Table 4. Antidiabetic, Anti-Inflammatory, Antinociceptive/Anesthetic Activities of African and Holy Basil Essential Oils.
Moreover, an older study determined the eugenol treatment effect on blood glucose, HbA1c, and insulin in streptozocin-induced (STZ) diabetic mice. It was observed that mice in the control and vehicle groups did not significantly decrease blood glucose (396 mg/dL and 353 mg/dL, respectively). In comparison, mice in the eugenol-treated group showed a 38% decrease in blood glucose level from 420 mg/dL to 262 mg/dL (two-tailed p-value: 0.0042) (Citation35). A decrease in blood glucose could result from the inhibition of α-glucosidase activity, which will consequently help reduce the formation of AGEs; this decreases (Citation35). It was reported that 90 mg L−1 EO of O. gratissimum improved plasma glucose level immediately after anesthesia induction and 1 hr after recovery. Hence, further validating the glucose-lowering capacity of African basil EO (Citation36).
Conversely, antidiabetic studies with Holy basil EO are sparse. However, several studies have identified valuable anti-hyperglycemic compounds in other solvent extracts such as methanol, hydroalcoholic, and other organic solvents (Citation37,Citation53). One interesting study by Nivetha et al. (Citation36) showed the activities of Holy basil EO as a potent antidiabetic agent. Although the EO used for the study were procured and were not characterized by GC/MS, they showed about 41–61% inhibition of α-amylase and 45–66% inhibition of α-glucosidase at a dosage of 50–150 µg/ml. Moreover, the glucose uptake rate by yeast cells under the EO concentrations of 50, 100, and 150 µg/ml was linear. Compared to the control without the EO, Holy basil oil improved the percentage glucose uptake by 34.38% and 28.6% for 5 and 10 mM glucose concentration, respectively (Citation38). Future studies on the antidiabetic potentials of Holy basil EO using cell lines or animal models may pave the way for more understanding.
5.2 Anti-inflammatory activity
Inflammation is a double-edged sword in biology, with both positive and negative consequences. Primary systemic/diseased health conditions such as cancers, leukemia, ischemia, diabetes, and many more are dominated by excessive inflammatory responses (Citation5). Non-steroidal anti-inflammatory drugs are commonly used to combat excess inflammatory response; however, there are countless reports of their toxic consequences (Citation54). EO from Africa and Holy basils have been reported in a few studies to have anti-inflammatory activity. EO from African basil leaves within a concentration between 5–20 μg/ml was administered to challenged human gingival fibroblasts, causing about a 3–46% decrease in PGE2 concentration. However, the baseline concentration of other inflammatory markers, such as IL-6 and IL-8, was not significantly altered (Citation39).
Conversely, uncharacterized EO isolated via HD from the leaves and flowers of Holy Basil was recently shown to exhibit anti-inflammatory activity. Administering a dose of 100 µg/mL of the EO to the THP-1 cells resulted in about 99% inhibition of PGE2 concentration and 40% inhibition of COX-2 (Citation44). In another study, the anti-inflammatory activity of Holy basil EO was reported to have a higher concentration (above 250 µg/mL) to cause 100% inhibition of matrix metalloproteinase-9 (MMP-9) activity in lipopolysaccharide (LPS)-induced inflammatory cells. Moreover, a dose-dependent downregulation of MMP-9 expression was also observed (Citation45). A similar trend of inhibiting the production of inflammation markers by EO from Holy Basil has been reported in older studies (Citation55).
5.3 Anaesthetic and antinociceptive activities (inhibition of pain sensation)
Anesthesia is referred to as induced numbness of sensation to prevent pain and discomfort. Anesthetics are usually called antinociceptive substances because they inhibit a special pain receptor called the nociceptor. Nociceptors are wired for pain stimuli and send sensations that prevent humans from danger (Citation56). Apart from concerns arising for other side effects of synthetic anesthetics, the prolonged duration, which may arise from increased dosage, may lead to permanent numbness or total loss of sensation. One important criterion to judge an anesthetic is the time it can sustain numbness (anesthesia induction time) and the time taken for recovery (recovery time) (Citation57). The hot plate and formalin test are popular experimental systems investigating antinociceptive activity. In contrast, the Von Frey test is standard for animal models checking for allodynia or neuropathic pains (Citation58).
Natural products such as anesthetics are better alternatives due to minor toxicity concerns, although lesser antinociceptive effects can be observed. EOs from African and Holy Basil have been a valuable source for antinociceptive products, as demonstrated in several recent scientific reports (). Paula-Freire and his colleagues, in some studies, have reported the potential of EO from African basil and its major phytochemical components (Eugenol, Myrcene, and Trans-caryophyllene) to counter nociceptive and neuropathic pains in rat and murine models (Citation41–43). At a concentration of 40 mg/kg of EO and above, the experimental animals were insensitive to pain (did not lick their excited paw or engage in a frequent jump) for about 10 to 30 minutes for both the hot plate test and formalin test. Moreover, purified eugenol and myrcene showed similar effects, with a reduced concentration of 10 mg/kg (Citation41,Citation42).
Anesthetics have also gained profound relevance in aquaculture, where they can be applied to relieve fish from stress and unpleasant environmental conditions. From experimental studies, total anesthesia is achieved when a complete loss of sensation to external stimuli and swimming balance for fish. Conversely, recovery is achieved when there is regain of swimming balance and sensitivity to external stimuli (Citation59). A digital chronometer is commonly adopted to measure the anesthesia induction and recovery time, as well as estimate the ventilator frequencies and opercular movements per minute (Citation48). Ferreira et al. (Citation36) investigated the antinociceptive activities of EO of O. gratissimum L. at a concentration between 10–300 mg/L for anesthesia of Oreochromis niloticus fish. It was recorded that the minimum concentration for Holy basil EO anesthesia activity was 215 mg/L at an induction time of 38.79 s. Moreover, depending on the EO concentration, the animals recovered within 149.55 to 568.1 seconds. Also, the ventricular frequency at anesthesia induction showed a quadratic pattern with a minimum practical concentration/value of 208.33 mg L−1 (11.27 beats min−1) (Citation36). The study also investigated changes in blood hematology and oxidative tension immediately after anesthesia induction and 1 h after recovery for possible adverse effects on animal physiology that may have resulted from the use of Holy Basil EO as an antinociceptive. The EO did not adversely affect the hematological parameters and antioxidant enzymes during induction and after recovery (Citation36).
In similar research, a different concentration of EO of O. gratissimum L. (10, 20, 30, 40, 50, 60, 70, 150, and 300 mg L−1) was administered to juvenile silver catfish (Rhamdia quelen) to determine the duration for anesthetic induction and recovery, which was ascertained by its potential mechanism of action via GABAergic transmission and the development of tolerance (Citation40). The experiment determined the anesthetic effect of the EO in association with benzodiazepine (BDZ). In the same vein, the GABAergic-like action and development of tolerance were assessed in fish exposed to BDZ (diazepam 150 µM), EO (40 mg L−1), or both EO and BDZ (EO+BDZ) at the same concentrations. At the end of the experiment, it was observed that the EO of O. gratissimum had an effective and safe anesthetic for silver catfish, interacting with the GABAA-benzodiazepine receptor as its mechanism (Citation40). Other studies on analgesic and antinociceptive activities of African and Holy Basil EOs are summarized in .
5.4 Cardio-protective (Hypocholesterolemic, Anti-obesity, and anti-hypertension)
Cardiovascular disease is one of the leading causes of death in the world. Preventing and managing cardiovascular conditions are a significant priority and concern individuals and national and global health institutions (Citation60). EO from basil has shown potential in a few studies to improve cardiac functions and prevent cardiovascular diseases. Results from these few studies are fantastic and will be summarized in this section to draw the attention of the general scientific community to the need for more research efforts to harness its underexplored potential ().
Table 5. Antioxidant and Cardioprotective Activities of African and Holy Basil Essential Oils.
In a study by Pires et al. (Citation63), African basil EO at a concentration of 3–300 µg/mL caused an endothelium-dependent vasorelaxation to the aorta mesenteric vascular beds of rats which were excited about contraction with 0.1 µmol/L phenylephrine. Moreover, the EO of African basil successfully caused a reduction in the perfusion pressure induced by noradrenaline (6 µmol/L). In comparison, the contraction effects were only partially eliminated by well-known nitric oxide antagonists (Nitro-L-arginine methyl ester (100 µmol/L), K+ channel hyper polarizers, tetraethylammonium (5 mmol/L), and prostacyclin (PGI2) synthesis, indomethacin (10 µmol/L) (Citation63).
An older study by Lahlou et al. (Citation61). investigated the effects of African basil EO on some cardiovascular indices such as mean aortic pressure (MAP or hypotension) and heart rate (HR or bradycardia) in rats. The findings from the study corroborate the cardio-protective activity of EOs from Ocimum species. Intravenous bolus injection of EO of African basil (1–20 mg/kg) caused a sharp and dose-dependent decrease in MAP and HR in both anesthetized and conscious rats. More so, eugenol, the principal constituent of African basil EO, gave similar effects at a concentration of 1–10 mg/kg. The hypotension and bradycardia effects on both conscious and anesthetized rats suggest that the cardiac effects of the EO may be due to its vasodilatory effect on vascular smooth muscle. A similar study by Leal et al. (Citation62) reported the cardio-protective effects of intravenous treatment using EO of O. gratissimum and its major compound, eugenol, on deoxycorticosterone acetate (DOCA-salt)-hypertensive rats. The study reported that intravenous treatment with EOOG (1–20 mg/kg) or eugenol (1–10 mg/kg) had a dose-dependent decrease in blood pressure of DOCA-salt hypertensive rats was also attributed to an improved vascular smooth relaxation by the African basil EO.
Similarly, a few studies established that EO from Holy basil had cardioprotective activity. The anti-hyperlipidemic effect/activity of EO from O. sanctum leaves in high-cholesterol diet-fed rats was investigated and documented by Suanarunsawat et al. (Citation64). The EO isolated by HD and characterized with GC-MS was used to treat hypercholesterolemic induced rats at a dosage of 80 µl/kg BW/day for 7 weeks. The hypercholesterolemic condition was induced with cholesterol powder (2.5 g% (w/w) and 6% (v/w) palm oil added to rat feed. The study examined the concentration of lipid peroxidases through the thiobarbituric acid reactive substances (TBARS) assay and other antioxidant enzyme concentrations in the liver and heart tissues. The serum cholesterol, triacylglycerol, HDL-C, and LDL-C were also determined. The administration of EO from the O. sanctum significantly (p < 0.05) decreased the serum lipid profile and lipid peroxidation in the heart while improving the cardiac antioxidant enzymes. This suggests that EO treatment successfully prevented atherosclerosis and protected against hypercholesterolemia. Also, Gupta et al. (Citation65) reported a significant decrease in serum cholesterol, triacylglycerol, LDL + VLDL in cholesterol-fed (100 mg/kg body weight/day) rabbits treated with O. sanctum L. seed oil (0.8 gm/kg body weight/day) for four weeks when compared with untreated cholesterol-fed group suggesting that O. sanctum L. possess hypo-cholesterolemic activity.
5.5 Antioxidant activity
Essential oils of African and Holy basil are potent antioxidants against oxidative stress and reactive radicals, unavoidably generated in the biological system due to diverse factors (). Some predisposing factors to oxidative stress are disease state, stress, immunological reaction, and others (Citation73–77). Moreover, adverse effects of prolonged oxidative stress may result in inflammation, cancer, and other metabolic distortions (Citation73,Citation78,Citation79). This section presents recent (2018 –2022) studies on the antioxidant activity of African and Holy basil EO (). Among the numerous assay for examining antioxidant capacity, most studies adopted the 2,2-diphenyl-1-picrylhydrazyl radical scavenging assays (DPPH), the 2,2′-azino-bis(3-ethylbenzothiazoline-6-sulfonate) radical cation scavenging assay (ABTS), Nitric oxide scavenging assay (NO assay) and lipid peroxidation inhibition assay. Moreover, a few studies estimated the total phenolic contents (TPC) and upregulation of other in vivo antioxidant enzymes such as Glutathione Peroxidase (GPx) and Superoxide dismutase (SOD) (Citation80).
In a recent study by Joshi et al. (Citation66), the effects of seasonal variation on the yield, phytochemical constituents, and antioxidant capacity of EO from African basil were evaluated (Citation66). Due to seasonal variation, the recorded EO yield is between 0.17 to 1.07% (w/w), with eugenol as the principal constituent (65.65 ± 0.57 to 85.71 ± 0.55%), and variations in the antioxidant capacity. The antioxidant activity of different oil fractions based on seasons (January to December) was assayed for their DPPH radical scavenging activities. The result was reported as the concentration that gave a 50% inhibition (IC50), ranging from 2.04 ± 0.04 to 3.02 ± 0.03 μg/ml (Citation66). Similarly, de Castro et al. (Citation68) recorded about 68.83–73.85% scavenging of DPPH radical by EO of African basil harvested in different seasons, with significant constituents of p-Cymene (3.4–22.5%), γ-Terpinene (21–37.0%) and Thymol (33.2–449.7%). Finally, another study by Araujo et al. (Citation67) recorded about 60% ABTS radical scavenging activity for a nano-formulation of EOs from the leaves of African basil. The contents of the EO were majorly eugenol (51.19%), 1,8-cineole (29.55%), and β-selinene (5.63%) (Citation67).
Holy basil EO also possesses remarkable antioxidant properties owing to reports from a few scientific investigations. A recent study by Kunihiro et al. (Citation69) reported the isolation and characterization of EO from Holy basil with major constituents including caryophyllene oxide (23.8%), β-caryophyllene (18.2%), germacrene D (14.6%), α-copaene (14.4%) and eugenol (10.0%) (Citation69). The study investigated the total antioxidant capacity of the EO using the DPPH scavenging assay, ABTS assays, Lipid peroxidase inhibition, and Total phenolic contents. From their result, the concentration at 50% radical scavenging activities (SC50) for DPPH radical was 41.8 ± 3.8 μg/Ml, while ABTS cation radical was 189.5 ± 1.4 μg/ml. Moreover, the total phenolic content was estimated to be 109.9 mg/g, while the inhibition of lipid peroxidation, assayed by the β-Carotene bleaching assay, at 200 μg/mL EO in ethanol gave about 61.1 ± 5.2% inhibition. Finally, it was reported that eugenol significantly contributed to the antioxidant capacity of the EO, as a dose-dependent decrease with decreasing eugenol concentration was observed (Citation69).
In another exciting study, Sallam et al. (Citation46). explored the protective role of Holy basil EO nano-emulsion against the oxidative damage and genotoxicity of Titanium Dioxide. The study showed that rats exposed to titanium nanoparticles (TiO2-NPs) of 28.0 nm and ξ-potential of −33.97 mV showed severe DNA damage, fragmentation, and breakages in their livers as the distorted increase of oxidative stress and liver marker enzymes (Citation46). Moreover, a corresponding decrease in antioxidant enzymes and other histological alterations were observed. Nano-formulation of the EO from Holy basil alleviates the toxic effects of TiO2-NPs. In the study, the Holy basil EO contained 55 compounds of major linalool (53.9%) and methyl chavicol (12.63%) and caused remarkable improvement in all histological and other tested parameters. In addition, administration of the EO fostered antioxidant enzymes such as GPx (from 12.02 to 30.82 U/g), CAT (from 2.87 to 5.54 mU/g), and SOD (from 14.24 to 21.45 U/g). Finally, Holy basil EO nano-emulsion prevented liver DNA fragmentation, damage, and breakage when co-administered with the toxicant TiO2-NPs (Citation46). Several other studies by Rattanamaneerusmee et al. (Citation80), Thokchom et al. (Citation66), and Soundararajan et al. (Citation68) on the antioxidant activity of Holy basils EO are summarized in .
5.6 Anticancer and cytotoxicity activities of African and Holy Basil essential oils
Cancer is one of the leading causes of death globally. Usually, it is caused by dysregulation and abnormal signaling affecting cell cycles which foster the uncontrollable growth of body cells. Drugs or substances that cause toxicity, hamper proliferation, foster apoptosis, and disrupt cellular activities are cytotoxic. Generally, many chemotherapeutic agents attack cancer cells through several cytotoxic mechanisms (Citation81). EOs from African and Holy basil have been shown to possess cytotoxic effects and potencies as an anticancer agent and may be a better alternative to destroy metastatic cells selectively. Based on available studies, cytotoxicity has been more pronounced with African basil EO during the induction of apoptosis. One recent study on African basil EO showed its cytotoxic effects on human gingival fibroblast cells. The study adopted the 3-(4, 5-dimethylthiazol-2-yl)-2, 5-diphenyl-tetrazolium bromide cytotoxic assay, reporting an IC50 of 36 ± 2 µg/mL. More so, the expression of pro-inflammatory markers such as IL-6, IL-8, and PGE2 was investigated with ELISA and EIA assays. Although there were no significant changes in the concentration of IL-6 and IL-8, the baseline concentration of PGE2 decreased by 43% at 20 μg/mL concentration (Citation39). The result from the study suggests that the EO may be cytotoxic to cancerous cells but regulates inflammation and the expression of inflammatory markers during the cytotoxic reaction.
On the other hand, Holy basil EO has been shown in several studies to foster apoptosis, especially in cancerous cell cultures. Boonyanugomol et al. (Citation69) recently reported the apoptosis-inducing activity of EO extracted from the leaves of Holy basil with main contents of caryophyllene (25.85%) and α-pinene (11.66%). In the study, the viability of the human gastric cancer cells was evaluated using the MTT assays, the inhibition of metastasis activities was determined by cell migration and invasion assays, and finally, the qRT-PCR was used to estimate the expression of apoptosis-related genes. The researchers reported a decrease in cancer cell viability following a dose-dependent manner with IC50 of 163.42 μg/mL. Moreover, the EO causes significant inhibition of cell migration and induction of chromatin condensation, fragmentation, and apoptotic cell death. Finally, apoptotic genes such as TP53, BAX, and BAK were upregulated, while anti-apoptotic genes (BCL-2 and BCL-xL) were downregulated (Citation82).
Similarly, the differentiation-inducing effects of Holy basil EO (with camphor, cineol, eugenol, limonene, and rosmarinic acid as major compounds) were assayed by estimating the ducts formation number sizes in human rectum adenocarcinoma cells (RCM-1). It was reported that the fractions of duct formation index were 4.03 ± 0.26, a function of the duct formation numbers and the size of ducts (Citation70). Although there are few studies on the anticancer effects of EO from either African or Holy basil, the older studies have been summarized in .
Table 6. Cytotoxicity and Anticancer Activities of African and Holy Basil Essentials Oils.
6. Bioavailability of African and Holy Basil essential oils
EOs are rapidly absorbed and cross the blood-brain barrier into the central nervous system, interacting with receptors and promoting their various biological functions (Citation86). However, the fast absorption of EOs and their volatile constituents in the stomach and the proximal small intestine results in poor bioavailability, causing a limited fraction of the oil to reach systemic circulation (Citation87). Although EOs are suitable penetrants, their poor bioavailability could be due to undesired environmental conditions such as light, heat, pressure, chemical, and high oxygen concentration (Citation86). These features are also true of African and Holy basil EOs.
Therefore, encapsulation of EO is an efficient technique necessary to preserve its efficacy. Encapsulation increases bioavailability and water solubility and controls the release of the EOs (Citation88). Nanoencapsulation increases the concentration of the EO bioactive compounds, enhances the utilization of lower doses, and reduces side effects (Citation89). Several studies have demonstrated the nanoencapsulation of African and Holy basil EO using polymeric substances (Citation89–92). Moreover, the nano-formulation of essential oils generally, such as that from African and Holy basil, are commonly presented as liquid forms (liquid solutions, emulsions, micelles), semi-solid forms (liposomes, gels) or solid forms (microcapsules or microcomposites) and aerosols (Citation87,Citation93,Citation94).
7. Prospects and Conclusion
EOs are one of the most valuable phytoconstituents of African and Holy basil plants, possessing several therapeutic and industrial advantages. Studies have shown that phytochemical compositions vary based on the plant varieties, planting season, harvest time, and isolation/processing methods. Due to the variation in composition, diverse bioactivities have been reported in the recent literature. This study presented an overview of the isolation processes or systems popularized for obtaining essential oil from African and Holy basil and a thorough review of recent findings on several bioactivities with potent therapeutic advantages such as antioxidant, anticancer, anti-inflammatory, anti-diabetes, and antinociceptive, cardioprotective activities. Some of these bioactivities have yet to be studied in depth from our literature surveys. Hence, we present the following recommendations as prospects for future research.
First, there is a need for more mechanistic studies on specialized cell culture models (ex vivo) and animal models (in vivo). Most studies reporting on the bioactivities of the EO from both plants were performed in vitro via inhibition studies, with only a few cell culture studies. Therefore, future research is rational to validate the findings on the bioactivities of these plant EO through more in-depth mechanistic studies and in vivo in animal models. For instance, anticancer bioactivities of these EO have been shown mainly through cytotoxicity potentials and an uprise in inflammatory markers. Studies on specific regulation of cancer cell genes and proteins are needed, and the point where this regulation occurs could be helpful to understand the state where the EO would be most practicably applied for cancer treatment and management. Similar kinds of studies would also be relevant for detailed investigation into the cardio, hepatic, and hemato-protective activities of EO. Moreover, research should investigate other reported ethnobotanical potencies of the EO, such as bone health-promoting, neuromodulatory, and reproduction/fertility activities (Citation75,Citation95–97).
There are no clinical trials on human subjects for the reported biological activities of African and Holy basil EO. Delving into clinical trial studies in future research would foster the transition of knowledge of these bioactivities from “bench to market .“In addition, effective delivery systems for these EOs can be optimized for the real-life therapeutic application of these EOs. Finally, studies on relevant composites such as nanoparticles and other nano-technological systems may foster improved EO bioactivities (Citation98).
Lastly, studies on systems to promote the overproduction of EOs fractions from these plants should be promoted. Future studies on the effects of biotic and abiotic elicitation strategies on EO yield, composition, and biological activities would be helpful. Using quantitative trait loci and other plant breeding approaches (Citation99), other genetic strategies could improve the yield of the isolated and bioactivities of isolated EOs.
In conclusion, while significant progress has been made in understanding the health and therapeutic potentials of African and Holy basil EOs, there remain numerous opportunities for future research to explore and optimize their efficacy, delivery, and production methods. By addressing these challenges, we can harness the full therapeutic benefits of these natural remedies and pave the way for their integration into mainstream healthcare practices.
Disclosure statement
No potential conflict of interest was reported by the author(s).
References
- A. Gleich, M. Große, W. Körner, M. Jones, K.G. Steinhäuser, A.V. Gleich, M.G. Ophoff and W. Körner, The Necessity of a Global Binding Framework for Sustainable Management of Chemicals and Materials—Interactions with Climate and Biodiversity. Sustainable Chemistry, 3(2), 205–237 (2022). doi: 10.3390/suschem3020014.
- S. Gupta, A. Srivastava, A.K. Shasany and A.K. Gupta, Genetics, Cytogenetics, and Genetic Diversity in the Genus. In: The Ocimum Genome. Compendium of Plant Genomes. Edits., A. Shasany and C. Kole, pp. 73–87, Springer, Cham (2018).
- E.M. Zahran, U.R. Abdelmohsen, H.E. Khalil, S.Y. Desoukey, M.A. Fouad and M.S. Kamel, Diversity, phytochemical and medicinal potential of the genus Ocimum L. (Lamiaceae), Phytochemistry Reviews, 19(4), 907–953 (2020). doi: 10.1007/s11101-020-09690-9.
- E.I. Nweze and E.E. Eze, Justification for the use of Ocimum gratissimum L in herbal medicine and its interaction with disc antibiotics. BMC Complementary and Alternative Medicine, 9(1), 37 (2009). doi: 10.1186/1472-6882-9-37.
- O.C. Ugbogu, O. Emmanuel, G.O. Agi, C. Ibe, C.N. Ekweogu, V.C. Ude, M.E. Uche, R.O. Nnanna and E.A. Ugbogu, A review on the traditional uses, phytochemistry, and pharmacological activities of clove basil (Ocimum gratissimum L.). Heliyon, 7, e08404 (2021). doi: 10.1016/j.heliyon.2021.e08404.
- C. Priyanka, S. Shivika and S. Vikas: A Review on Ethnomedicinal Properties, Phytochemical Constituents, and Pharmacological Profile, Biotechnological Approaches for Medicinal and Aromatic Plants: Conservation, Genetic Improvement and Utilization, 251–270, (2018).
- D. Singh and P.K. Chaudhuri, A review on phytochemical and pharmacological properties of holy basil (Ocimum sanctum L.). Industrial Crops and Products, 118, 367–382 (2018). doi: 10.1016/j.indcrop.2018.03.048.
- M.M. Cohen, Tulsi – Ocimum sanctum: a herb for all reasons. Journal of Ayurveda and Integrative Medicine, 5(4), 251 (2014). doi: 10.4103/0975-9476.146554.
- S. Shiwakoti, O. Saleh, S. Poudyal, A. Barka, Y. Qian and V.D. Zheljazkov, Yield, Composition and Antioxidant Capacity of the Essential Oil of Sweet Basil and Holy Basil as Influenced by Distillation Methods. Chemistry & Biodiversity. doi:10.1002/CBDV.201600417
- E. Da, S. Moura, L. Rita D’, A. Faroni, F. Fernandes Heleno, A. Aparecida, Z.A.A. Rodrigues, L. Henrique, F. Prates and M.E. Lopes Optimal Extraction of Ocimum basilicum Essential Oil by Association of Ultrasound and Hydrodistillation and Its Potential as a Biopesticide Against a Major Stored Grains Pest. Molecules, 25(12), 2781(2020).
- K. Sneha, A. Narayanankutty, J.T. Job, O.J. Olatunji, A. Alfarhan, A.C. Famurewa and V. Ramesh, Antimicrobial and Larvicidal Activities of Different Ocimum Essential Oils Extracted by Ultrasound-Assisted Hydrodistillation. Molecules, 27(5), 1456 (2022). doi: 10.3390/MOLECULES27051456.
- I.F. Chukwuma, N.O. Uchendu, R.O. Asomadu, W.F.C. Ezeorba and T.P.C. Ezeorba, African and Holy Basil – a review of ethnobotany, phytochemistry, and toxicity of their essential oil: Current trends and prospects for antimicrobial/anti-parasitic pharmacology. Arabian Journal of Chemistry, 16(7), 104870 (2023). doi: 10.1016/j.arabjc.2023.104870.
- S. Shiwakoti, O. Saleh, S. Poudyal, A. Barka, Y. Qian and V.D. Zheljazkov, Yield, Composition and Antioxidant Capacity of the Essential Oil of Sweet Basil and Holy Basil As Influenced by Distillation Methods, Chemical Diversity. doi:10.1111/ijlh.12426
- M. Yousefi, M. Rahimi-Nasrabadi, S.M. Pourmortazavi, M. Wysokowski, T. Jesionowski, H. Ehrlich and S. Mirsadeghi, Supercritical Fluid Extraction of Essential Oils. Trends in Analytical Chemistry. doi:10.1016/j.trac.2019.05.038
- R.S. Melo, Á. Maria, A. Azevedo, T. Helena, S. Rodrigues, I.L. Ponte and V.A. Carneiro, Chemical Composition and Antimicrobial Effectiveness of Ocimum gratissimum L. Essential Oil Against Multidrug-Resistant Isolates of Staphylococcus aureus and Escherichia coli. Molecules, 24(21), 3864–3880 (2019). doi: 10.3390/molecules24213864.
- F.B.M. Mohr, C. Lermen, Z.C. Gazim, J.E. Gonçalves and O. Alberton, Antifungal activity, yield, and composition of Ocimum gratissimum essential oil. Genetics and Molecular Research, doi: 10.4238/gmr16019542.
- N. Chimnoi, N. Reuk-Ngam, P. Chuysinuan, P. Khlaychan, N. Khunnawutmanotham, D. Chokchaichamnankit, W. Thamniyom, S. Klayraung, C. Mahidol and S. Techasakul, Characterization of essential oil from Ocimum gratissimum leaves: Antibacterial and mode of action against selected gastroenteritis pathogens. 118, 290–300 (2018). 10.1016/j.micpath.2018.03.041.
- K.C. Kobenan, K.K.N. Bini, M. Kouakou, K.I. Sinan, G. Zengin, G.E.C. Ochou, N.R. Boka, P. Menozzi, G. Ochou and A.E. Dick, Chemical Composition and Spectrum of Insecticidal Activity of the Essential Oils of Ocimum gratissimum L. and Cymbopogon citratus Stapf on the Main Insects of the Cotton Entomofauna in Côte d’Ivoire. Chemistry & Biodiversity, 18(11), e2100497 (2021). doi: 10.1002/cbdv.202100497.
- S.C. Ibeh, O.D. Akinlabi, I. Asmau, J. Audu and A.M. Muritala, Extraction of Ocimum Gratissimum Using Different Distillation Techniques. International Journal of Scientific & Technology, 2017, 6, 26–28.
- M.J. Saharkhiz, A.A. Kamyab, N.K. Kazerani, K. Zomorodian, M.J. Rhimi and M.J. Rahimi, Chemical Compositions and Antimicrobial Activities of Ocimum sanctum L. Essential Oils at Different Harvest Stages. Jundishapur Journal of Microbiology, 8(1), 1–7 (2015). doi: 10.5812/jjm.13720.
- S. Moura, L.R.D. Faroni, Z.A.A. Rodrigues, L.H.F. Prates and M.E.L.R. de Queiroz, Optimal Extraction of Ocimum basilicum Essential Oil by Association of Ultrasound and Hydrodistillation and Its Potential as a Biopesticide Against a Major Stored Grains Pest, Molecules. Molecules, 25(12), 2781–2796 (2020). doi: 10.3390/molecules25122781.
- R.K. Joshi, Chemical composition, in vitro Antimicrobial and antioxidant Activities of Essential Oils of Ocimum gratissimum, O santum and major Constituents. Indian Journal of Pharmaceutical Sciences, 75(4), 457–462 (2013). doi: 10.4103/0250-474X.119834.
- T.P.C. Ezeorba, K.I. Chukwudozie, C.A. Ezema, E.G. Anaduaka, E.J. Nweze and E.S. Okeke, Potentials for health and therapeutic benefits of garlic essential oils: Recent findings and future prospects. Pharmacological Research - Modern Chinese Medicine, 3(100075), 100075 (2022). doi: 10.1016/j.prmcm.2022.100075.
- R.S. Melo, Á.M.A. Azevedo, A.M.G. Pereira, R.R. Rocha, R.M.B. Cavalcante, M.N.C. Matos, P.H.R. Lopes, G.A. Gomes, T.H.S. Rodrigues, H.S. Dos Santos, I.L. Ponte, R.A. Costa, G.S. Brito, F.E.A. Catunda and V.A. Carneiro, Chemical Composition and Antimicrobial Effectiveness of Ocimum gratissimum L. Essential Oil Against Multidrug-Resistant Isolates of Staphylococcus aureus and Escherichia coli, Molecules 2019. Molecules, 24(21), 3864 (2019). doi: 10.3390/molecules24213864.
- F.B.M. Mohr, C. Lermen, Z.C. Gazim, J.E. Gonçalves and O. Alberton, Antifungal Activity, Yield, and Composition of Ocimum Gratissimum Essential oil, Genetics and Molecular Research. GMR. doi:10.4238/GMR16019542.
- K. Koba, P.W. Poutouli, C. Raynaud and K. Sanda, Antifungal Activity of the Essential Oils from Ocimum gratissimum L. Grown in Togo. Journal of Scientific Research, 1, 164–171 (2009). doi: 10.3329/jsr.v1i1.1131.
- M. Chenni, D. El Abed, N. Rakotomanomana, X. Fernandez and F. Chemat, Comparative study of essential oils extracted from Egyptian basil leaves (Ocimum basilicum L.) using hydro-distillation and solvent-free microwave extraction. Molecules, 21(1), 113–128 (2016). doi: 10.3390/molecules21010113.
- R.S. Verma, P.S. Bisht, R.C. Padalia, D. Saikia and A. Chauhan, Chemical composition and antibacterial activity of essential oil from two Ocimum spp grown in sub-tropical India during spring-summer cropping season. Asian Journal of Traditional Medicines, 2011, 6, 211–217.
- A. Kumar, R. Shukla, P. Singh and N.K. Dubey, Chemical composition, antifungal and antiaflatoxigenic activities of Ocimum sanctum L. essential oil and its safety assessment as plant based antimicrobial. Food and Chemical Toxicology, 48(2), 539–543 (2010). doi: 10.1016/j.fct.2009.11.028.
- K.S. Prabhu, R. Lobo, A.A. Shirwaikar and A. Shirwaikar, Ocimum gratissimum: a review of its chemical, pharmacological and ethnomedicinal properties. The Open Complementary Medicine Journal, 1(1), 1–15 (2009). doi: 10.2174/1876391X00901010001.
- K.B.G.H. Kpoviessi, S. D.E. Kpoviessi, Y. Ladekan, F. Gbaguidi, M. Frédérich, G.C. Moudachirou, M. Quetin-Leclercq, J. Accrombessi and J. Bero, In vitro antitrypanosomal and antiplasmodial activities of crude extracts and essential oils of Ocimum gratissimum Linn from Benin and influence of vegetative stage. Journal of Ethnopharmacology, 155(3), 1417–1423 (2014). doi: 10.1016/j.jep.2014.07.014.
- M. Zagoto, G.F.E. Cardia, E.M.T. da Rocha, K.S.M. Mourão, V. Janeiro, R.K.N. Cuman, A.A. Pinto, R.L. Contiero and P.S.L. de Freitas, Biological activities of basil essential oil: a review of the current evidence. Research, Society and Development, 10(12), e363101220409–e363101220409 (2021). doi: 10.33448/rsd-v10i12.20409.
- C. Egbuna, C.G. Awuchi, G. Kushwaha, M. Rudrapal, K.C. Patrick-Iwuanyanwu, O. Singh, U.E. Odoh, J. Khan, J. Jeevanandam, S. Kumarasamy, V.O. Chukwube, M. Narayanan, S. Palai, M.-A. Găman, C.Z. Uche, D.S. Ogaji, N.J. Ezeofor, A.G. Mtewa, C.C. Patrick-Iwuanyanwu, S.S. Kesh, C. Shivamallu, K. Saravanan, H. Tijjani, M. Akram, J.C. Ifemeje, M.C. Olisah and C.J. Chikwendu, Bioactive Compounds Effective Against Type 2 Diabetes Mellitus: A Systematic Review. Current Topics in Medicinal Chemistry, 21(12), 1067–1095 (2021). doi: 10.2174/18734294MTE1ENjAgx.
- P. Singh, R.H. Jayaramaiah, S.B. Agawane, G. Vannuruswamy, A.M. Korwar, A. Anand, V.S. Dhaygude, M.L. Shaikh, R.S. Joshi, R. Boppana, M.J. Kulkarni, H.V. Thulasiram and A.P. Giri, Potential Dual Role of Eugenol in Inhibiting Advanced Glycation End Products in Diabetes: Proteomic and Mechanistic Insights. Scientific Reports. 10.1038/SREP18798.
- P. Singh, R.H. Jayaramaiah, S.B. Agawane, G. Vannuruswamy, A.M. Korwar, A. Anand, V.S. Dhaygude, M.L. Shaikh, R.S. Joshi, R. Boppana, M.J. Kulkarni, H.V. Thulasiram and A.P. Giri, Potential Dual Role of Eugenol in Inhibiting Advanced Glycation End Products in Diabetes: Proteomic and Mechanistic Insights OPEN. Nature Publishing Group. 10.1038/srep18798.
- A.L. Ferreira, G.C. Favero, T.P. Boaventura, C. de Freitas Souza, N.S. Ferreira, S.N. Descovi, B. Baldisserotto, B.M. Heinzmann and R.K. Luz, Essential oil of Ocimum gratissimum (Linnaeus, 1753): efficacy for anesthesia and transport of Oreochromis niloticus. Fish Physiology and Biochemistry, 47(1), 135–152 (2021). doi: 10.1007/s10695-020-00900-x.
- T. Suanarunsawat, G. Anantasomboon and C. Piewbang, Anti-diabetic and anti-oxidative activity of fixed oil extracted from Ocimum sanctum L. leaves in diabetic rats. Experimental and Therapeutic Medicine, 11(3), 832–840 (2016). doi: 10.3892/etm.2016.2991.
- G. Nivetha, V. Vishnupriya and R. Gayathri, Comparative Evaluation of Anti-Diabetic Activity of Lemon Grass Oil and Tulasi Oil. International Journal of Pharmaceutical Sciences Review and Research, 2016, 39, 221–225.
- F. Ocheng, F. Bwanga, E.A. Boström, M. Joloba, A.K. Borg-Karlson, T. Yucel-Lindberg, C. Obua and A. Gustafsson: Essential Oils from Ugandan Medicinal Plants: In Vitro Cytotoxicity and Effects on IL-1 β-Induced Proinflammatory Mediators by Human Gingival Fibroblasts, Evidence-based Complementary and Alternative Medicine, doi:10.1155/2016/5357689.
- L. Silva, T.V. Parodi, P. Reckziegel, V. Garcia, M.E. Bürger, B. Baldisserotto, C.A. Malmann, A.M.S. Pereira and B.M. Heinzmann, Essential oil of Ocimum gratissimum L.: Anesthetic effects, mechanism of action and tolerance in silver catfish, Rhamdia quelen, Aquaculture. Aquaculture, 350–353, 91–97 (2012). doi: 10.1016/j.aquaculture.2012.04.012.
- L.I.G. Paula-Freire, G.R. Molska, M.L. Andersen and E.L.D.A. Carlini, Ocimum gratissimum Essential Oil and Its Isolated Compounds (Eugenol and Myrcene) Reduce Neuropathic Pain in Mice. Planta medica, 82(3), 211–216 (2016). doi: 10.1055/s-0035-1558165.
- L.I.G. Paula-Freire, M.L. Andersen, G.R. Molska, D.O. Köhn and E.L.A. Carlini, Evaluation of the antinociceptive activity of Ocimum gratissimum L. (Lamiaceae) essential oil and its isolated active principles in mice. Phytotherapy Research: PTR, 27(8), 1220–1224 (2013). doi: 10.1002/ptr.4845.
- L.I.G. Paula-Freire, M.L. Andersen, V.S. Gama, G.R. Molska and E.L.A. Carlini, The oral administration of trans-caryophyllene attenuates acute and chronic pain in mice. Phytomedicine, 21(3), 356–362 (2014). doi: 10.1016/j.phymed.2013.08.006.
- N. Homnan, S. Thongpraditchote, M. Chomnawang and K. Thirapanmethee, In vitro Anti-inflammatory effects of Thai herb essential oils. Pharmaceutical Sciences Asia, 47(2), 153–163 (2020). doi: 10.29090/psa.2020.02.019.0020.
- T. Manaharan, R. Thirugnanasampandan, R. Jayakumar, G. Ramya, G. Ramnath and M.S. Kanthimathi, Antimetastatic and anti-inflammatory potentials of essential oil from edible Ocimum sanctum leaves. Scientific World Journal. doi:10.1155/2014/239508
- M.F. Sallam, H.M.S. Ahmed, A.A. El-Nekeety, K.A. Diab, S.H. Abdel-Aziem, H.A. Sharaf and M.A. Abdel-Wahhab, Assessment of the Oxidative Damage and Genotoxicity of Titanium Dioxide Nanoparticles and Exploring the Protective Role of Holy Basil Oil Nanoemulsions in Rats. Biological Trace Element Research, 1–16(3), 1301–1316 (2022). doi: 10.1007/s12011-022-03228-0.
- C. de Lima Boijink, C.A. Queiroz, E.C. Chagas, F.C.M. Chaves and L.A.K.A. Inoue, Anesthetic and anthelminthic effects of clove basil (Ocimum gratissimum) essential oil for tambaqui (Colossoma macropomum). Aquaculture, 457, 24–28 (2016). doi: 10.1016/j.aquaculture.2016.02.010.
- A.J. Becker, L. Jensen Vaz, L. De Oliveira Garcia, W. Wasielesky, B.M. Heinzmann and B. Baldisserotto, Anesthetic potential of different essential oils for two shrimp species, Farfantepenaeus paulensis and Litopenaeus vannamei (Decapoda, Crustacea). Animal production. doi:10.1590/0103-8478cr20200793
- A. Soares Ribeiro, E. dos Santos Batista, J. Koiji Dairiki, F. Célio Maia Chaves and L. Antônio Kioshi Aoki Inoue, Anesthetic properties of Ocimum gratissimum essential oil for juvenile matrinxã. Animal Sciences Maringá, 38, 1–7 (2016). doi: 10.4025/actascianimsci.v38i1.28787.
- L.L. Silva, K. Garlet and B. Oliveira, Effects of anesthesia with the essential oil of Ocimum gratissimum L. in parameters of fish stress. Revista Brasileira de Plantas Medicinais, 17(2), 215–223 (2015). doi: 10.1590/1983-084X/13_034.
- T.P. Boaventura, C.F. Souza, A.L. Ferreira, G.C. Favero, M.D. Baldissera, B.M. Heinzmann, B. Baldisserotto and R.K. Luz, Essential oil of Ocimum gratissimum (Linnaeus, 1753) as anesthetic for Lophiosilurus alexandri: Induction, recovery, hematology, biochemistry and oxidative stress. Aquaculture, 529(735676), 735676 (2020). doi: 10.1016/j.aquaculture.2020.735676.
- L.A. da Silva, M.A. Martins, F.E. Santo, F.C. Oliveira, F.C.M. Chaves, E.C. Chagas, M.L. Martins and C.M. de Campos, Essential oils of Ocimum gratissimum and Zingiber officinale as anesthetics for the South American catfish Pseudoplatystoma reticulatum, Aquaculture. Aquaculture, 528(735595), 735595 (2020). doi: 10.1016/j.aquaculture.2020.735595.
- S. Parasuraman, S. Balamurugan, P.V. Christapher, R.R. Petchi, W.Y. Yeng, J. Sujithra and C. Vijaya, Evaluation of Antidiabetic and Antihyperlipidemic Effects of Hydroalcoholic Extract of Leaves of Ocimum tenuiflorum (Lamiaceae) and Prediction of Biological Activity of its Phytoconstituents. Pharmacognosy Research, 7, 156 (2015). doi: 10.4103/0974-8490.151457.
- P. Patrignani and C. Patrono, Cyclooxygenase inhibitors: From pharmacology to clinical read-outs. Biochimica Et Biophysica Acta (BBA) - Molecular and Cell Biology of Lipids, 1851(4), 422–432 (2015). doi: 10.1016/j.bbalip.2014.09.016.
- M.A. Kelm, M.G. Nair, G.M. Strasburg and D.L. DeWitt, Antioxidant and cyclooxygenase inhibitory phenolic compounds from Ocimum sanctum Linn. Phytomedicine: International Journal of Phytotherapy and Phytopharmacology, 7(1), 7–13 (2000). doi: 10.1016/S0944-7113(00)80015-X.
- A. Taylor and G. McLeod, Basic pharmacology of local anaesthetics. BJA Education, 20(2), 34–41 (2020). doi: 10.1016/j.bjae.2019.10.002.
- U.S. Misal, S.A. Joshi and M.M. Shaikh, Delayed recovery from anesthesia: a postgraduate educational review. Anesthesia, Essays and Researches, 10(2), 164 (2016). doi: 10.4103/0259-1162.165506.
- J.R. Deuis, L.S. Dvorakova and I. Vetter, Methods used to evaluate pain behaviors in rodents. Frontiers in Molecular Neuroscience. doi:10.3389/FNMOL.2017.00284/PDF
- M.W. Skår, G.T. Haugland, M.D. Powell, H.I. Wergeland, O.B. Samuelsen and C. Garcia de Leaniz, Development of anaesthetic protocols for lumpfish (cyclopterus lumpus L.): effect of anaesthetic concentrations, sea water temperature and body weight. Public Library of Science ONE, 12(7), e0179344 (2017). doi: 10.1371/journal.pone.0179344.
- G. O’donoghue, C. Peiris, N. Aerts, S. Anthierens, P. Van Bogaert, L. Peremans and H. Bastiaens, Prevention of Cardiovascular Diseases in Community Settings and Primary Health Care: A Pre-Implementation Contextual Analysis Using the Consolidated Framework for Implementation Research. International Journal of Environmental Research and Public Health, 19(14), 8467 (2022). doi: 10.3390/ijerph19148467.
- S. Lahlou, L.D.F. Leal Interaminense, J.H. Leal-Cardoso, S.M. Morais and G.P. Duarte, Cardiovascular effects of the essential oil of Ocimum gratissimum leaves in rats: role of the autonomic nervous system. Clinical and Experimental Pharmacology & Physiology, 31(4), 219–225 (2004). doi: 10.1111/j.1440-1681.2004.03976.x.
- L.F. Leal Interaminense, J.H. Leal-Cardoso, P.J. Caldas Magalhães, G. Pinto Duarte and S. Lahlou, Enhanced hypotensive effects of the essential oil of Ocimum gratissimum leaves and its main constituent, eugenol, in DOCA-salt hypertensive conscious rats. Planta medica, 71(4), 376–378 (2005). doi: 10.1055/s-2005-864109.
- F. Pires Alana, S.V.F. Madeira, P.M.G. Soares, C.M. Montenegro, E.P. Souza, A.P. Recende, R.S. de Moura, A.A.S. Assreuy and D.N. Criddle, The role of endothelium in the vasorelaxant effects of the essential oil of Ocimum gratissimum in aorta and mesenteric vascular bed of rats. Canadian Journal of Physiology and Pharmacology, 90(10), 1380–1385 (2012). doi: 10.1139/y2012-095.
- T. Suanarunsawat, W.D. Na Ayutthaya, T. Songsak, S. Thirawarapan and S. Poungshompoo, Antioxidant Activity and Lipid-Lowering Effect of Essential Oils Extracted from Ocimum sanctum L. Leaves in Rats Fed with a High Cholesterol Diet. Journal of Clinical Biochemistry and Nutrition, 46(1), 52–59 (2010). doi: 10.3164/jcbn.09-52.
- S. Gupta, P.K. Mediratta, S. Singh, K.K. Sharma and R. Shukla, Antidiabetic, antihypercholesterolaemic and antioxidant effect of Ocimum sanctum (Linn) seed oil. Indian Journal of Experimental Biology, 2006, 44(4), 300–304.
- R.K. Joshi, Antioxidant activity influenced by seasonal variation of essential oil constituents of Ocimum gratissimum L. ACS Food Science and Technology, 1(9), 1661–1669 (2021). doi: 10.1021/acsfoodscitech.1c00192.
- T.D.S. Araujo, J.M.A.R. da Costa, F. de Oliveira Silva Ribeiro, A.C. de Jesus Oliveira, J. Do Nascimento Dias, A.R. de Araujo, A.B. Barros, M. da Paixão Brito, T.M. de Oliveirade Oliveira, M.P. de Almeida, K.N. de Carvalho Castro, F.H. dos Santos Fogaça, D.A. da Silva and B.W.S. de Souza, Nanoemulsion of cashew gum and clove essential oil (Ocimum gratissimum Linn) potentiating antioxidant and antimicrobial activity. International Journal of Biological Macromolecules, 193, 100–108 (2021). doi: 10.1016/j.ijbiomac.2021.09.195.
- J.A.M. de Castro, O.S. Monteiro, D.F. Coutinho, A.A.C. Rodrigues, J.K.R. da Silva and J.G.S. Maia, Seasonal and Circadian Study of a Thymol/γ-Terpinene/p-Cymene Type Oil of Ocimum gratissimum L. And Its Antioxidant and Antifungal Effects, Journal of the Brazilian Chemical Society. Journal of the Brazilian Chemical Society, 30, 930–938 (2019). doi: 10.21577/0103-5053.20180237.
- K. Kunihiro, Y. Kikuchi, S. Nojima and T. Myoda, Characteristic of aroma components and antioxidant activity of essential oil from Ocimum tenuiflorum leaves. Flavour and Fragrance Journal, 37(4), 210–218 (2022). doi: 10.1002/ffj.3701.
- A. Rattanamaneerusmee, K. Thirapanmethee, Y. Nakamura and M. Chomnawang, Differentiation-inducing effect in human colon cancer cells of essential oils. Pharmaceutical Sciences Asia, 45(3), 154–160 (2018). doi: 10.29090/psa.2018.03.154.
- V. Soundararajan, V. Kandasamy and P. Subramani: Antibiofilm, antioxidant and larvicidal activity of formulated nanoemulsion from Ocimum tenuiflorum, Materials Today: Proceedings, 45, 3438–3443, (2021).
- S.D. Thokchom, S. Gupta and R. Kapoor, Arbuscular mycorrhiza augments essential oil composition and antioxidant properties of Ocimum tenuiflorum L. – a popular green tea additive. Industrial Crops and Products, 153, 112418 (2020). doi: 10.1016/j.indcrop.2020.112418.
- I.U. Okagu, R.N. Aguchem, C.A. Ezema, T.P.C. Ezeorba, O.E. Eje and J.C. Ndefo, Molecular mechanisms of hematological and biochemical alterations in malaria: A review. Molecular and Biochemical Parasitology, 247, 111446 (2022). doi: 10.1016/j.molbiopara.2021.111446.
- I.U. Okagu, E.C. Aham, T.P.C. Ezeorba, J.C. Ndefo, R.N. Aguchem and C.C. Udenigwe, Osteo-modulatory dietary proteins and peptides: A concise review. Journal of Food Biochemistry, 46(10), e14365 (2022). doi: 10.1111/jfbc.14365.
- I.U. Okagu, T.P.C.C. Ezeorba, R.N. Aguchem, I.C. Ohanenye, E.C. Aham, S.N. Okafor, C. Bollati and C. Lammi, A Review on the Molecular Mechanisms of Action of Natural Products in Preventing Bone Diseases. A Review on the Molecular Mechanisms of Action of Natural Products in Preventing Bone Diseases, 23(15), 8468 (2022). doi: 10.3390/ijms23158468.
- P.E. Joshua, C.C. Ilo, U.G. Ukachukwu, D.C. Odimegwu, R.O. Asomadu and T.P.C. Ezeorba, Could eggshell membrane be an adjuvant for recombinant Hepatitis B vaccine?: A preliminary investigation. Future Journal of Pharmaceutical Sciences, 9(1), 28 (2023). doi: 10.1186/s43094-023-00481-5.
- P.E. Joshua, C.O. Nwauzor, D.C. Odimegwu, U.G. Ukachukwu, R.O. Asomadu, T.P.C. Ezeorba, T.P. Chidike Ezeorba and I. Chemin, Experimental and molecular predictions of the adjuvanticity of snail mucin on hepatitis B vaccine in albino mice. PLoS One, 16(7), e0246915 (2021). doi: 10.1371/journal.pone.0246915.
- G. Pizzino, N. Irrera, M. Cucinotta, G. Pallio, F. Mannino, V. Arcoraci, F. Squadrito, D. Altavilla and A. Bitto, Oxidative Stress: Harms and Benefits for Human Health, Oxidative Medicine and Cellular Longevity. doi:10.1155/2017/8416763.
- O.C. Enechi, E.S. Okeke, O.N. Isiogugu, B.U. Umeh, C.G. Eze, S.C. Emencheta, T.P. Ezeorba, C. Izuchukwu, N.C. Agbo, L. Ugwu and C.V. Iloh, Evaluation of the anti-inflammatory and antioxidant properties of flavonoid-rich seed extract of buchholzia coriacea engler (Capparaceae). Tropical Journal of Natural Product Research, 2022, 6, 1727–1732.
- I.G. Munteanu and C. Apetrei, Analytical Methods Used in Determining Antioxidant Activity: A Review. International Journal of Molecular Sciences, 22, 3380 (2021). doi: 10.3390/ijms22073380.
- C.A. Ezema, T.P.C. Ezeorba, R.N. Aguchem and I.U. Okagu, Therapeutic benefits of Salvia species: A focus on cancer and viral infection. Heliyon, 8(1), e08763 (2022). doi: 10.1016/j.heliyon.2022.e08763.
- W. Boonyanugomol, K. Rukseree, P. Prapatpong, O. Reamtong, S.C. Baik, M. Jung, M.K. Shin, H.L. Kang and W.K. Lee: An in vitro Anti-Cancer Activity of Ocimum Tenuiflorum Essential Oil by Inducing Apoptosis in Human Gastric Cancer Cell Line, Medicina, doi:10.3390/MEDICINA57080784.
- F. Ocheng, F. Bwanga, M. Joloba, A. Softrata, M. Azeem, K. Pütsep, A.K. Borg-Karlson, C. Obua and A. Gustafsson, Essential Oils from Ugandan Aromatic Medicinal Plants: Chemical Composition and Growth Inhibitory Effects on Oral Pathogens, Evidence-based Complementary and Alternative Medicine. doi:10.1155/2015/230832.
- T. Manaharan, R. Thirugnanasampandan, R. Jayakumar, M.S. Kanthimathi, G. Ramya and M.G. Ramnath, Purified Essential Oil from Ocimum sanctum Linn. Triggers the Apoptotic Mechanism in Human Breast Cancer Cells. Pharmacognosy magazine, 12, S327 (2016). doi: 10.4103/0973-1296.185738.
- A. Khan, A. Ahmad, L.A. Khan and N. Manzoor, Ocimum sanctum (L.) essential oil and its lead molecules induce apoptosis in Candida albicans. Research in Microbiology, 165, 411–419 (2014). doi: 10.1016/j.resmic.2014.05.031.
- S. Baptista-Silva, S. Borges, O.L. Ramos, M. Pintado and B. Sarmento, The progress of essential oils as potential therapeutic agents: a review. Journal of Essential Oil Research, 32, 279–295 (2020). doi: 10.1080/10412905.2020.1746698.
- P. Chitprasert and P. Sutaphanit, Holy basil (Ocimum sanctum Linn.) essential oil delivery to swine gastrointestinal tract using gelatin microcapsules coated with aluminum carboxymethyl cellulose and Beeswax. Journal of Agricultural and Food Chemistry, 62(52), 12641–12648 (2014). doi: 10.1021/jf5019438.
- M.F. Sallam, H.M.S. Ahmed, A. El‑Nekeety and K.A. Diab, S. H. Abdel‑Aziem, H.A. Sharaf and M.A. Abdel-Wahhab, Assessment of the Oxidative Damage and Genotoxicity of Titanium Dioxide Nanoparticles and Exploring the Protective Role of Holy Basil Oil Nanoemulsions in Rats. Biological trace element research. doi:10.1007/s12011-022-03228-0
- V.K. Singh, S. Das, A.K. Dwivedy, R. Rathore and N.K. Dubey, Assessment of chemically characterized nanoencapuslated Ocimum sanctum essential oil against aflatoxigenic fungi contaminating herbal raw materials and its novel mode of action as methylglyoxal inhibitor. Postharvest Biology and Technology, 153, 87–95 (2019). doi: 10.1016/j.postharvbio.2019.03.022.
- C. Onyebuchi and D. Kavaz, N, N-Trimethyl Chitosan Nanoparticle Encapsulation of Ocimum Gratissimum Essential Oil: Optimised Synthesis, in vitro Release and Bioactivity. International Journal of Nanomedicine, 14, 7707–7727 (2019). doi: 10.2147/IJN.S220202.
- A.P.Z. De Melo, C.G. Da Rosa, W.G. Sganzerla, M.R. Nunes, C.M. Noronha, M.V. De Oliveira Brisola Maciel, M.A. Villetti, F.C. Bertoldi and P.L.M. Barreto, Synthesis and characterization of zein nanoparticles loaded with essential oil of Ocimum gratissimum and Pimenta racemosa. Materials Research Express, 6(9), 095084 (2019). doi: 10.1088/2053-1591/ab2fc1.
- R.M. Brandão, G. Cardoso, L.R. Batista, A.R.S. Caetano, A.C.C. Lemos, M.A. Martins, D.L. Nelson and J.E. De Oliveira, Antifungal and physicochemical properties of Ocimum essential oil loaded in poly(lactic acid) nanofibers, Letters in Applied Microbiology. Letters in Applied Microbiology, 74(5), 765–776 (2022). doi: 10.1111/lam.13661.
- P. Sutaphanit and P. Chitprasert, Optimisation of microencapsulation of holy basil essential oil in gelatin by response surface methodology. Food Chemistry, 150, 313–320 (2014). doi: 10.1016/j.foodchem.2013.10.159.
- N. Ngamakeue and P. Chitprasert, Encapsulation of holy basil essential oil in gelatin: effects of palmitic acid in Carboxymethyl cellulose emulsion coating on antioxidant and antimicrobial activities. Food and Bioprocess Technology, 9(10), 1735–1745 (2016). doi: 10.1007/s11947-016-1756-4.
- K.O. Omeje, B.O. Ezema, C.N. Onaebi, S.C. Onoyima, T.P.C. Ezeorba and S.O.O. Eze, HPLC fingerprint of flavonoids, enzyme inhibition and antioxidant activity of Newbouldia laevis stem-bark: an in vitro and in silico study. Future Journal of Pharmaceutical Sciences, 9(1), 1–22 (2023). doi: 10.1186/s43094-023-00486-0.
- T.P. Chidike Ezeorba, A.L. Ezugwu, I.F. Chukwuma, E.G. Anaduaka and C.C. Udenigwe, Health-promoting properties of bioactive proteins and peptides of garlic (allium sativum). Food Chemistry, 435, 137632 (2024). doi: 10.1016/j.foodchem.2023.137632.
- I.F. Chukwuma, T.P.C. Ezeorba, F.N. Nworah, V.O. Apeh, K. Mohammad and H.S. Sherouk, Bioassay-guided identification of Potential Alzheimer’s Disease Therapeutic Agents from Kaempferol-Enriched Fraction of Aframomum melegueta seeds using in vitro and Chemoinformatics Approaches. Arabian Journal of Chemistry, 105089(9), 105089 (2023). doi: 10.1016/j.arabjc.2023.105089.
- E.S. Okeke, E.J. Nweze, E.G. Anaduaka, C.O. Okoye, C.A. Anosike, P.E. Joshua and T.P.C. Ezeorba: Plant-derived nanomaterials (PDNM): a review on pharmacological potentials against pathogenic microbes, antimicrobial resistance (AMR) and some metabolic diseases, 3 Biotech, DOI: 10.1007/S13205-023-03713-W.
- T.P.C. Ezeorba, E.S. Okeke, I.U. Okagu, E.J. Nweze, R.O. Asomadu, W.F.C. Ezeorba, I.F. Chukwuma, C.P. Ononiwu, C.A. Ezema, E.M. Okorigwe, V.O. Nwanelo and P.E. Joshua, Transcriptomic Diversity of Solanum tuberosum Varieties: A Drive towards Future Analysis of Its Polyploidy Genome. Biology and Life Sciences Forum, 2022, 11, 46.