ABSTRACT
This 12-week study evaluated the impact of krill meal (KM) in Nile tilapia, Oreochromis niloticus, reproductive performance. Two test feeds with 2% and 5% KM were compared to a commercial tilapia broodstock feed. Fish were stocked in 12 hapas with 50 females and 16 males each, allowing four replicate hapas per dietary treatment. Egg production exhibited a general decline. The control group yielded 16,066 ± 6,124 eggs per hapa, while the 2% KM diet showed an 18% increase (18,976 ± 6,417 eggs), and the 5% KM diet exhibited a 30% increase (20,947 ± 7,029 eggs). However, no statistically significant differences were observed in egg production. Spawning females declined over time, with 5% KM showing a 29% increase, but no statistical differences could be detected among dietary treatments. Egg hatching rates remained consistent, but the 5% KM diet hinted at a positive trend with 10% more larvae at day 10 despite the lack of statistical differences. Females fed KM spawned more frequently, and the 5% KM diet influenced higher fat content and elevated levels of omega-3 in Nile tilapia eggs. Overall, the study suggests positive effects on reproductive performance and larval survival in Nile tilapia with inclusion of krill meal in their diets.
Introduction
Farmed tilapia is currently the second most farmed finfish in aquaculture representing 5.27% of global aquaculture production (Miao and Wang Citation2020). Nile tilapia, Oreochromis niloticus, by far, is the most important tilapia species cultured accounting for 75% of the total farmed tilapia production (FAO Citation2022; Miao and Wang Citation2020). To support the increasing aquaculture potential for Nile tilapia, it is critical to provide high-quality offspring (Tsadik and Bart Citation2007). This necessitates the deployment of enhanced nutritional strategies, refined feeding practices, and effective broodstock management techniques (Bhujel Citation2013). The influence of lipids and essential fatty acids, pivotal determinants in broodstock nutrition, has been extensively recognized. Lipids and essential fatty acids, crucial determinants in broodstock nutrition, have been widely acknowledged for their influence on factors such as reproductive frequency, egg quality, and larval development (Izquierdo, Fernandez-Palacios, and Tacon Citation2001; Ng and Romano Citation2013).
Dietary manipulation has been shown to affect the immunity, health, and productivity of Nile tilapia broodstock and offspring (Abu-Elala et al. Citation2021). This has included the inclusion of dietary fermented extracts sourced from Saccharomyces cerevisiae (Abu-Elala et al. Citation2021), purified nucleotides (Nascimento et al. Citation2023), and taurine (Al-Feky, El-Sayed, and Ezzat Citation2016). Significantly, lipids and essential fatty acids, particularly n-3 highly polyunsaturated fatty acids (HUFAs) like EPA (eicosapentaenoic acid, 20:5n-3) and DHA (docosahexaenoic acid, 22:6n-3), have surfaced as vital contributors to reproductive performance, fecundity, and the quality of eggs and larvae across diverse fish species (Izquierdo, Fernandez-Palacios, and Tacon Citation2001; Suloma and Ogata Citation2012). A study on tropical freshwater fish of commercial importance, including Nile tilapia, Ayungin, and African catfish, underscore the prevalence of these n-HUFAs, highlighting their role in reproductive processes (Suloma and Ogata Citation2012). In addition, high protein levels (>40%) have been shown to play an important role to enhance the reproductive performance in broodfish Nile tilapia (El-Sayed, Mansour, and Ezzat Citation2003; Ribeiro, Evangelista, and Antônio Citation2017).
Fish meal and fish oil have been traditionally used as the main dietary source of lipids and proteins in many commercial fish feeds, respectively. However, the usage of these limited resources for direct human consumption and poultry, and their fluctuating prices have driven the aquaculture to look for more sustainable and efficient ingredients (Tacon Citation2020). Unfortunately, limited information is available on Nile Tilapia broodstock for alternative lipid and protein sources.
Krill meal (KM), obtained from Antarctic krill (Euphausia superba) has a rich nutrition profile including phospholipids, high levels of long-chain polyunsaturated fatty acids, such as EPA and DHA, astaxanthin, free amino acids, nucleotides, and trimethylamine N-oxide (TMAO). These nutrients have been vastly demonstrated to be beneficial in enhancing growth and health of different fish species including Nile tilapia (Gaber Citation2005; Kaur et al. Citation2022; Saleh et al. Citation2018; Tharaka et al. Citation2020; Torrecillas et al. Citation2021). Notably, there is an absence of research exploring the impact of KM on the performance of Nile tilapia broodstock. This research gap limits our understanding of its potential benefits, hindering the industry’s ability to make informed decisions regarding feed formulation and nutritional strategies which can improve fish health and reduce production costs.
In this context, the current study assessed the effects of varying levels of KM incorporation in diets for Nile tilapia broodstock. The study sought to elucidate effects on spawning, egg quantity and quality, and the survival of broodstock Nile tilapia larvae produced.
Materials and methods
Study site
The study was conducted in a commercial tilapia breeding facility located in Miami, USA, at the coordinates 25°34’07.6”N and 80°28’50.4”W. The experiment was performed according to the guidelines and protocols approved by the Aquaculture Best Management Practices Manual, incorporated into rule 5 L–3.004, F.A.C. (Aquaculture number AQ5223612) approved by the Florida Department of Agriculture and Consumer Services, Division of Aquaculture.
Experimental design
Twelve 40-m3 hapas were placed in a 1,500-m3 above ground pond to allow four replicate hapas per dietary treatment. Individual hapas were stocked with 50 females and 16 males, totaling 792 fish resulting in a 3:1 sex ratio. The weight of five randomly chosen females, and two males was recorded during fish stocking from each hapa. Total stocked fish biomass per hapa reached 98.6 ± 11.6 kg (coefficient of variation = 11.8%). Fish body weight was carried out at the end of the study to determine growth disparities between individual hapas and dietary treatments. Four hapas were assigned for each dietary treatment. Fish were fed twice daily for 12 weeks, at .2 g of feed/kg of stocked fish biomass over the course of the rearing period.
Feed composition
Three floating extruded feeds were commercially manufactured as 6.5-mm pellets. Two feeds contained krill meal (QRILL™ Aqua, Aker BioMarine Antarctic AS, Lysaker, Norway) included at 2.00 (2% KM) 5.00% (5% KM, % of the diet, as-is). One commercial tilapia broodstock feed produced in the USA with proprietary formulation was used as a control (CTL). Feeds were formulated using the linear formulation software Optimal Formula 2000 (Optimal Informatica Ltda., Campinas, Brazil) to be isonitrogenous and isocaloric ().
Table 1. Ingredient and proximate composition of feeds used in the present study.
One sample from each diet was chemically analyzed (AOAC Citation2023). Dry matter (DM) was determined by drying samples in a convection oven for 24 h at 105°C. The Dumas combustion method was applied to analyze CP (AOAC 968.06), while total lipids was determined through acid hydrolysis (AOAC 954.02). Ash content was determined by burning samples in a muffle furnace at 600°C for 2 h (AOAC 942.05) and crude fiber by enzymatic-gravimetric determination (AOAC 992.16). Fatty acid (FA) composition was determined using high-resolution gas chromatography (GC) with a flame ionization detection fitted with a capillary GC column.
Reproductive performance variables
The presence of eggs in the mouth of female fish was checked on a weekly basis in each hapa over the course of a 12-week period. When present, eggs were manually collected by opening the fish’s mouth, removing the eggs by hand, and weighing them. The number of mouth-brooding females at time of egg collection was also recorded. The number of spawning females indicated the number of females per hapa found with eggs in their mouth at time of egg collection. Collected eggs from each hapa were transferred to individual McDonald-type hatching jars. Egg hatching rate (%) was computed by assessing fish larval survival at day 8 of incubation. This was carried out by manually counting post-hatched fish larvae divided by the number of eggs transferred to each jar multiplied by 100. Fish larval survival was determined by dividing the initial number of eggs transferred to each jar by the number of live fish larvae 10 days after hatching. The FA composition of frozen tilapia eggs was determined using the same methodology as described for fish diets.
Statistical analysis
Homogeneity of variance was examined for all data by using Bartlett-Box F and Cochran’s C tests. Kurtosis and skewness and their standard error (i.e., s.e. kurtosis and s.e. skewness) were applied to the data as measures of asymmetry and tests of normality. Based on these results, data were transformed to a log(x) scale to normalize and homogenize the variances and to meet statistical assumptions. The effect of dietary treatment on fish broodstock reproductive parameters (number of spawning females, and number of eggs and larvae produced) were analyzed using One-Way Analysis of Variance (ANOVA). When significant differences were detected, they were compared two-by-two with Tukey’s HSD. The significance level of 5% was applied in all statistical analyzes. Statistical package SPSS 15.0 for Windows was used (SPSS Inc., Chicago, Illinois, United States). Results are reported as means ± s.e.
Results
Egg production
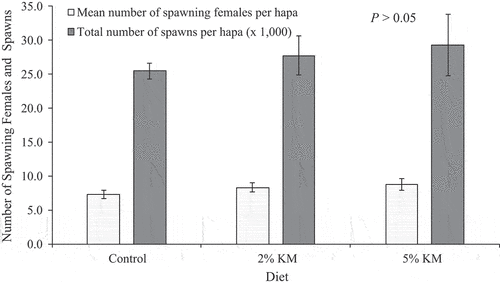
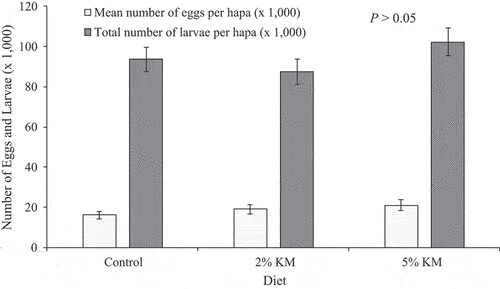
Over the course of 12 weeks, egg production showed a general decline regardless of dietary treatment. The control group exhibited an egg production of 16,066 ± 6,124 eggs per hapa per group, while fish fed the diet 2% KM achieved an egg production of 18,976 ± 6,417 eggs, which was 18% higher than control group, followed by 20,947 ± 7,029 eggs in fish fed 5% KM, which was 30% higher than the control group (). However, no statistically significant differences could be detected in egg production among broodstock under the different dietary treatments (p > 0.05). A total of 2,166 eggs were produced per female fed the control diet, followed by 2,313 (7% higher than the control group) for those fed 2% KM, and 2,493 (15% higher than control group) eggs per female for 5% KM diet, respectively. Female egg production was observed to remain stable throughout the experiment, and no significant differences in eggs produced per female were noted between dietary treatments (p > 0.05). Similarly, mean egg weight was similar among dietary treatments, remained relatively constant, with a means of 16.4 ± 4.3, 16.4 ± 4.2, and 16.0 ± 3.9 mg for fish fed the control, 2% KM, and 5% KM, respectively.
Figure 1. Mean number of eggs (x 1,000 ± standard error) per hapa and total number of tilapia larvae (x 1,000, ± se) per hapa (at day 10 post collection) produced over a 12-week period. Fish were fed a commercial diet (control), and diets containing 2 and 5% krill meal (KM). Each hapa was stocked with 50 female tilapia.
Spawning females
The number of females with eggs in their mouth declined as the study progressed, independent of diet. A total of 7 ± 4 spawning females per hapa was observed in the group fed the control diet, followed by 8 ± 5 (14% higher than control group) for those fed 2% KM, and 9 ± 6 (29% higher than control group) females under the 5% KM treatment (p > 0.05, ).
Larval survival 10 days post hatching
Egg hatching rate was similar among the different diet groups, with no clear trends throughout the feeding period (data not shown). There was a positive effect of 5% KM on the total number of larvae counted at day 10 post collection, with 10% higher larvae than the control group (409,057 and 374,355 larvae in 5% KM and control group, respectively). However, the number of larvae obtained in 2% KM (349,923) were numerically lower than the control group (). Despite these observed trends, no statistical differences could be observed among dietary treatments.
Most females spawned once (n = 17) when fed control diet, with few females that spawned twice (n = 8). However, most of the females spawned twice when fed 2% (n = 14) and 5% (n = 10) KM. Besides, the total number of spawning females was higher in both 2% (n = 66; 18% higher than control) and 5% (n = 62; 11% higher than control) KM when compared to the control (n = 56) group ().
Chemical composition of eggs
Proximate composition of tilapia eggs indicated a higher fat content in eggs when fish were fed 2% KM (4.05%) followed by 5% KM (3.67%), and the control diet (2.74%). In addition, the EPA+DHA (8.8% in 5% KM versus 8.3% in control) and total n-3 fatty acid level (13% in 5% KM versus 12% in control) was higher in the 5% KM in comparison to control group. No differences were observed for the crude protein content, ranging from 21.50% to 22.20%, total fiber between 1.01% and 1.90%, and ash between 2.38% and 2.45% ().
Table 2. Proximate composition of eggs from Nile tilapia broodstock fed on a commercial diet and on diets containing 2% and 5% krill meal (KM).
The composition of fatty acids in both the experimental diets and tilapia eggs exhibited higher concentrations of omega-3 (n-3) and highly unsaturated (HUFA) fatty acids in the 5% KM diet, followed by the CTL diet, and the 2% KM diet (). Conversely, diets containing higher proportions of KM showed higher concentrations of n-6, saturated (SFA), monounsaturated (MUFA), and polyunsaturated (PUFA) fatty acids when compared to the CTL diet. A similar trend was observed in the fatty acid composition of Nile tilapia eggs.
Table 3. Fatty acid profile (%, as-is) of diets and frozen tilapia eggs.
Discussion
This is the first pilot study conducted to determine the effect of two doses of KM (2% and 5%) on the reproductive performance, spawning, egg quantity and quality, and survival of larvae obtained after 12 weeks of feeding on test diets on 10-month-old broodstock Nile tilapia. The results obtained indicated positive effects of KM inclusion, which included higher egg production, higher number of spawning females, higher spawning frequency and higher larvae survival post 10-day hatching, in comparison to a control commercial diet (). The results are in line with the earlier studies conducted on the lipid part of krill meal (krill oil-KO), in broodstock of marine fish species and in shrimp (Liang et al. Citation2022; Watanabe et al. Citation1991). Watanabe et al. (Citation1991) conducted a study on red seabream broodstock, testing the effects of krill oil (KO) fractions on egg quality. After a 30-day feeding period, groups supplemented with krill showed improved egg quality compared to a control diet. The study concluded that both phosphatidylcholine from the polar fraction and astaxanthin from the nonpolar fraction of KO contributed to enhanced egg quality. Similarly, Liang et al. (Citation2022) compared three dietary phospholipid (PL) sources (4% KO, 4% egg yolk, and 4% soy lecithin) in female Pacific whiteleg shrimp broodstock diets. The results indicated that 4% KO supplementation led to higher growth, better immunity, increased gut microbiota diversity, and enhanced antioxidant capacity compared to other dietary PL sources.
In our study, the 5% KM treatment showed a trend toward a positive effect on the number of larvae counted at day 10 post-collection, with 10% more larvae than the control group. This was most likely driven by higher dietary levels of n-3 PUFA, particularly DHA (22:6n-3, docosahexaenoic acid) and EPA (20:5n-3, eicosapentaenoic acid). However, only higher levels of DHA were found in tilapia eggs when fish were fed the 5% KM diet. In a similar study, Ng and Wang (Citation2011) assessed the impact of different dietary lipid sources on the reproductive performance of Nile tilapia broodstock. Four isonitrogenous and isolipidic diets were formulated with fish oil (FO), FO plus crude palm oil (CPO), CPO alone, or linseed oil (LSO). The results highlighted that broodstock tilapia fed diets with CPO exhibited larger gonad sizes and lower intraperitoneal fat. Additionally, CPO-based diets led to earlier first spawning, higher spawning frequency, and a shorter inter-spawning interval. However, contrary to our findings, authors reported that high levels of EPA and DHA in the diet and gonads of tilapia fed the FO diet did not lead to improved broodfish reproductive performance. As opposed to the present study, the work of Ng and Wang (Citation2011) used diets with high lipid levels, between 10.1% and 10.6% (% of the diet, dry matter basis) compared to a range of 4.3% and 6.3% in the present study. This might have resulted in excessive levels of n-3 PUFAs, potentially contributing to the reported adverse effects.
Although we did not observe discernible differences in the number of eggs produced per female or the mean egg weight among the various dietary treatments, there were positive trends in the spawning frequency of females fed the 2% and 5% KM diets. Both these diets demonstrated a higher incidence of females spawning twice compared to the control group. This trend might be attributed to the elevated levels of digestible dietary protein and energy present in KM-containing diets. This notion finds support in the slightly higher crude protein levels detected in eggs from fish fed the 2% and 5% KM diets. Previous studies have indicated that the optimal spawning performance of Nile tilapia broodstock is associated with diets containing 40% dietary protein and 16.7 MJ/kg gross energy (El-Sayed and Kawanna Citation2008). In contrast, authors found that lower dietary protein levels, such as 30%, had detrimental effects on tilapia reproductive performance.
In summary, the observed effects of KM inclusion on egg production, spawning frequency, and some aspects of egg composition can be attributed to the rich nutritional profile of KM, which includes essential fatty acids. While some trends are apparent, such as effect of essential fatty acids, especially n-3 PUFA like EPA and DHA, the absence of clear patterns in certain parameters emphasizes the need for further research to unravel the complex interactions between nutrition, reproductive physiology, and the overall performance of broodstock and their offspring.
Conclusion
In this study focused on the impact of KM on the reproductive performance of Nile tilapia broodstock, positive effects were observed. Both the 2% and 5% KM treatments demonstrated higher egg production, increased spawning frequency, and improved larvae survival compared to a control commercial diet. Particularly noteworthy was the positive trend associated with the 5% KM treatment, showing a 10% increase in the number of larvae at day 10 post-collection. The results align with earlier studies on the lipid components of krill meal, reinforcing the potential of KM to enhance reproductive parameters in aquatic species. The observed positive trends, especially in larvae survival, suggest that incorporating KM into broodstock diets could be a promising strategy for improving breeding success in Nile tilapia. While the study sheds light on the beneficial effects of KM, it is crucial to recognize the need for further research to fully understand the intricate interactions between nutrition, reproductive physiology, and overall broodstock performance.
Acknowledgments
The first author acknowledges the support from a research productivity fellowship (CNPq/MCTI, PQ# 306144/2020-4).
Disclosure statement
GL and KK are employed by Aker BioMarine Antarctic AS, Norway that has provided the krill meal and has sponsored the study.
Data availability statement
The data that support the findings of this study are available from the corresponding author upon reasonable request.
Additional information
Funding
References
- Abu-Elala, N. M., T. El-Sayed Ali, N. M. Ragaa, S. E. Ali, R. M. Abd-Elsalam, N. A. Younis, D. A. Abdel-Moneam, A. H. Hamdien, M. Bonato, and M. A. O. Dawood 2021. Analysis of the productivity, immunity, and health performance of nile tilapia (oreochromis niloticus) broodstock-fed dietary fermented extracts sourced from Saccharomyces cerevisiae (hilyses): A field trial. Animals 11: 815. 3. doi:10.3390/ani11030815.
- Al-Feky, S. S. A., A.-F. El-Sayed, and A. A. Ezzat. 2016. Dietary taurine improves reproductive performance of Nile tilapia (Oreochromis niloticus) broodstock. Aquaculture Nutrition 22:392–399. doi:10.1111/anu.12256.
- AOAC. 2023. Official Methods of Analysis of AOAC International. Oxford University Press, New York.
- Bhujel, R. C. 2013. On-farm feed management practices for nile tilapia (oreochromis niloticus) in Thailand. P. 159–89. In: On-farm feeding and feed management in aquaculture. FAO Fisheries and Aquaculture Technical Paper No. 583. Rome.
- El-Sayed, A.-F. M., and M. Kawanna 2008. Effects of dietary protein and energy levels on spawning performance of nile tilapia (oreochromis niloticus) broodstock in a recycling system, Aquaculture 280:179–184. 1–4. doi:10.1016/j.aquaculture.2008.04.030.
- El-Sayed, A. -C. R. Mansour, A. A. Ezzat. 2003. Effects of dietary protein level on spawning performance of nile tilapia (oreochromis niloticus) broodstock reared at different water salinities. Aquaculture 220:619–632. 1–4. doi:10.1016/S0044-8486(02)00221-1.
- FAO. 2022. The state of world fisheries and aquaculture 2022. Towards Blue Transformation. Rome. https://www.fao.org/3/cc0461en/online/cc0461en.html.
- Gaber, M. M. 2005. The effect of different levels of krill meal supplementation of soybean-based diets on feed intake, digestibility, and chemical composition of juvenile nile tilapia oreochromis niloticus, L. Journal of the World Aquaculture Society 36:346–353. 3. doi:10.1111/j.1749-7345.2005.tb00338.x.
- Izquierdo, M., H. Fernandez-Palacios, and A. Tacon. 2001. Effect of broodstock nutrition on reproductive performance of fish. Aquaculture 197 (1–4):25–42. doi:10.1016/S0044-8486(01)00581-6.
- Kaur, K., T. M. Kortner, T. Benitez-Santana, and L. Burri 2022. Effects of Antarctic krill products on feed intake, growth performance, fillet quality, and health in salmonids. Aquaculture Nutrition. 1–14. doi:10.1155/2022/3170854. (in press).
- Liang, X., X. Luo, H. Lin, F. Han, J. G. Qin, L. Chen, C. Xu, and E. Li. 2022. Growth, health, and gut microbiota of female pacific white shrimp, litopenaeus vannamei broodstock fed different phospholipid sources. Antioxidants 11 (6):1143. doi:10.3390/antiox11061143.
- Miao, W., and W. Wang 2020. Trends of aquaculture production and trade: Carp, tilapia, and shrimp. Asian Fisheries Science 33S:1–10. doi:10.33997/j.afs.2020.33.S1.001.
- Nascimento, C. Z., F. Meurer, S. Romão, L. H. Cazarolli, S. Marcon, T. V. Chagas, and R. A. Bombardelli. 2023. Feed for nile tilapia broodstock and offspring supplemented with purified nucleotides boosts the juvenile’s health, growth, and the resistance face to transport and Aeromonas hydrophila challenges. Animal Feed Science and Technology 297:115568. doi:10.1016/j.anifeedsci.2023.115568.
- Ng, W. K., and N. Romano. 2013. A review of the nutrition and feeding management of farmed tilapia throughout the culture cycle. Reviews in Aquaculture 5 (4):220–254. doi:10.1111/raq.12014.
- Ng, W.-K., and Y. Wang. 2011. Inclusion of crude palm oil in the broodstock diets of female nile tilapia, oreochromis niloticus, resulted in enhanced reproductive performance compared to broodfish fed diets with added fish oil or linseed oil. Aquaculture 314 (1–4):122–31. doi:10.1016/j.aquaculture.2011.01.034.
- Ribeiro, M. J. P., M. M. Evangelista, and E. Antônio 2017. Crude protein in diets for Nile tilapia broodfish. Boletim do Instituto de Pesca 43:35–46. doi:10.20950/1678-2305.2017.35.46.
- Saleh, R., L. Burri, T. Benitez‐Santana, S. Turkmen, P. Castro, and M. Izquierdo. 2018. Dietary krill meal inclusion contributes to better growth performance of gilthead seabream juveniles. Aquaculture Research 49 (10):3289–3295. doi:10.1111/are.13792.
- Suloma, A., and H. Y. Ogata. 2012. Lipid and fatty acid composition of commercially important tropical freshwater fish gonads: Guidelines for specific broodstock diet. Turkish Journal of Fisheries and Aquatic Sciences 12 (4):743–749. doi:10.4194/1303-2712-v12_4_02.
- Tacon, A. G. 2020. Trends in global aquaculture and aquafeed production: 2000–2017. Reviews in Fisheries Science & Aquaculture 28:43–56. 1. doi:10.1080/23308249.2019.1649634.
- Tharaka, K., T. Benitez‐Santana, B. E. Gunathilaka, M. G. Kim, C. Lee, J. Shin, and K. J. Lee. 2020. Evaluation of Antarctic krill (euphausia superba) meal supplementation in diets for olive flounder (paralichthys olivaceus). Aquaculture Research 51 (6):2291–2302. doi:10.1111/are.14573.
- Torrecillas, S., D. Montero, M. Carvalho, T. Benitez-Santana, and M. Izquierdo. 2021. Replacement of fish meal by Antarctic krill meal in diets for European sea bass dicentrarchus labrax: Growth performance, feed utilization and liver lipid metabolism. Aquaculture 545:737166. doi:10.1016/j.aquaculture.2021.737166.
- Tsadik, G. G., and A. N. Bart 2007. Effects of feeding, stocking density and water-flow rate on fecundity, spawning frequency and egg quality of nile tilapia, Oreochromis niloticus (L.). Aquaculture 272:380–388. 1–4. doi:10.1016/j.aquaculture.2007.08.040.
- Watanabe, T., T. Fujimura, M.-J. Lee, K. Fukusho, S. Satoh, and T. Takeuchi. 1991. Nutritional studies in the seed production of fish-XXI. Effect of polar and nonpolar lipids from krill on quality of eggs of red seabream pagrus major. Nippon Suisan Gakkaishi 57 (4):695–698. doi:10.2331/suisan.57.695.