?Mathematical formulae have been encoded as MathML and are displayed in this HTML version using MathJax in order to improve their display. Uncheck the box to turn MathJax off. This feature requires Javascript. Click on a formula to zoom.
?Mathematical formulae have been encoded as MathML and are displayed in this HTML version using MathJax in order to improve their display. Uncheck the box to turn MathJax off. This feature requires Javascript. Click on a formula to zoom.ABSTRACT
The concentrations of essential and non-essential metals were determined in four medicinal plants (Acacia abyssinica, Carissa spinarum, Dodonaea angustifolia and Euclea acemose) grown in Tara Gedam forest. The concentrations (in mg kg−1) of metals in D. angustifolia were 11.7 for Cu, 106 for Fe, 29.2 for Mn, 23.8 for Zn, 4.01 for Ni, 0.171 for Cd, and 3.11 for Pb; C. spinarum were 8.87 for Cu, 107 for Fe, 41.1 for Mn, 12.3 for Zn, 8.02 for Ni, 0.202 for Cd, and 8.53 for Pb; E. racemosa were 24.6 for Cu, 55.9 for Fe, 64.9 for Mn, 11.5 for Zn, 3.84 for Ni, 0.292 for Cd, and 0.771 for Pb; and A. abyssinica were 25.3 for Cu, 58.1 for Fe, 28.3 for Mn, 17.6 for Zn, 4.42 for Ni, 0.402 for Cd, and 4.04 for Pb. The percentage recoveries were ranged from 81.1% to 102.6%. The values of hazard index (HI) were greater than unity, thus there was non-carcinogenic adverse health risk for users. Since the carcinogenic risk (CR) due to Cd and Pb intake was lower than the recommended values (10−6), the use of these plants do not pose carcinogenic health risks.
KEYWORDS:
Introduction
For plants to grow properly healthy soils with critical minerals and nutrients are crucial.[Citation1] In recent years, the use of plants for medicinal purposes has increased in the developing countries.[Citation2] More than 80,000 plant species are utilized for medical purposes, and 80% of the world’s population use medicinal plants for treating diseases.[Citation3] The use of plants for herbal or natural health products with health advantages has expanded globally.[Citation4] In developing countries with wide biodiversity, the rural communities turn to traditional healers due to inadequate health facilities, high cost of medicines, lack of drugs, and other factors that increase the demand for medicinal plants.[Citation5] The effectiveness of medicinal plants for therapeutic purposes is related to the presence of chemical compounds such as alkaloids, flavonoids, minerals, terpenes, essential oils, glycosides, vitamins, and trace elements.[Citation6,Citation7]
Some heavy metals are essential (Cu, Mn, Fe, and Zn) to organisms and act as important components of proteins and enzymes in human body for enhancing enzyme activity; however, after accumulating in some parts of the human body, they can cause chronic toxicity.[Citation8] Medicinal plants may also contain toxic metals like As, Cd and Pb, which are extremely dangerous even at trace levels with no known vital biological roles.[Citation2,Citation9] Plants known for their applications in medicine can bioaccumulate heavy metals, up to many folds higher than the surrounding soil and this accumulation varies within the plant species.[Citation10]
Ethiopia is home to more than 6,500 higher plant species, of which about 887 have been used in traditional medicine.[Citation11,Citation12] In Ethiopia, people use medicinal plants to treat 90% of livestock and 80% of human ailments.[Citation13] People living around Adis Zemen have utilized plants which are widely grown in Tara Gedam forest, which is rich in medicinal plant species.[Citation14] Among these plants, Acacia abyssinica, Carissa spinarum, Dodonaea angustifolia and Euclear acemosa are the common ones used by the inhabitants to treat different ailments.
Acacia abyssinica (Fabaceae), is a tree with pinnate leaves, light yellow flowers found in heads and pods that are straight or slightly curved and indehiscent.[Citation15,Citation16] A. abyssinica is used to treat diarrhea, sore gum, diabetes, loose teeth, hemorrhage, and stomach sickness.[Citation17]
Carissa spinamm (Apocynaceae) is a creeping shrub or climber with ovate, elliptic, obovate or suborbicular leaf blade, and scented flowers.[Citation18] In Africa, it is distributed widely from Senegal to Somalia, from Sudan to the Transvaal and Namibia, and in Madagascar. It is also found in Yemen, India and Thailand, and on the islands of the Indian Ocean.[Citation18] C. spinarum has been used to treat snake bites, wounds, chest complaints, rheumatism, headaches, edema, gonorrhea, rabies, syphilis, and as a remedy for fever, cough, sickle cell anemia, toothache, ulcers, and worm infestation.[Citation19]
Dodonaea angustirolia (Sapindaceae), is a shrub with simple leaves and terminal inflorescence.[Citation16] It is distributed at the edges of upland vegetation, including secondary forest and scrub, invading recently cleared forest areas and overgrazing Acacia-Commiphora bushland.[Citation16] D. angustifolia is used to treat malaria, wound, fever, sore throat, rhinitis, sinusitis, and influenza.[Citation20]
Euclea racemosa (Ebenaceae) is an evergreen shrub or small tree with dark green leaves, and unbranched inflorescence.[Citation18] The plant is distributed in most floral regions of Ethiopia with open evergreen montane forest and bushland, and in Buxus-Acokanthera and Acacia–Commiphora bushland. The plant is widespread in eastern Africa, from Egypt, Sudan, through tropical East Africa and in Yemen and Oman.[Citation18] It is used for treating scabies, gonorrhea, abdominal pain, eczema, and constipation.[Citation21]
The objective of this study was to determine the elemental levels in A. abyssinica, C. spinarum, D. angustifolia and E. acemose to assess the risks involved in their use.
Materials and Method
Study Area
The sampling site for medicinal plants was Tara Gedam, located very close to Addis Zemen town, northeast of Lake Tana. It is situated at 12° 351′- 12° 926′N and 37°266 ′- 37° 057′E, with altitudes ranging from 2217 to 2457 m.a.s.l. and covers around 875 ha.[Citation22] The mean annual minimum and maximum temperatures of the study area are 8°C and 32.8°C, respectively.
Sample Collection and Preparation
A 3 g of fresh leaves was collected from ten plants of each species separately and packed in polyethylene plastic bags, labeled, and brought to the laboratory for further processing. The samples were cleaned with tap water to get rid of the dirt and debris, rinsed with distilled water, dried naturally and ground using a blending instrument, and then dried at 105°C. After being cooled, powdered, and sieved, the plant samples were put in a polyethylene container until digestion.
For heavy metal (Cd, Pb, Cu, Mn, Zn, Fe and Ni) analysis, 0.5 g of C. spinarum was digested with 1 mL HClO4 and 4 mL HNO3 at 160°C for 2:30 h, D. angustifolia was digested with 6 mL HNO3 and 3 mL HClO4 at 180°C for 2:30 h; E. racemosa was digested with 1.5 mL HClO4 and 4.5 mL HNO3 at 160°C for 2:30 h and A. abyssinica was digested with 5 mL HNO3 and 2 mL HClO4 at 180°C for 2:30 h. The solutions were filtered into a 50 mL volumetric flask with Whatman filter paper (No. 41) after being diluted with 10 mL of de-ionized water. Similarly, the blank solution was digested under a similar procedure as that of the plant samples. Finally, both solutions (the samples and blank) were analyzed with Flame atomic absorption spectrophotometer (FAAS).
Analytical Methods and Quality Controls
All chemicals and reagents used were of analytical grades. The glassware were rinsed with HNO3 solution and then washed in deionized water. All analyses were conducted in triplicate. Standard solutions of metals were applied to prepare calibration curves. Linearity, precision, and accuracy were used to validate the analytical method in accordance the International Conference on Harmonization Guidelines.[Citation23]
The limit of detection (LoD) for each element was obtained by taking the ratio of three times the standard deviation of a series of blank signals to the calibration slope.[Citation24] The LoDs of Zn, Cu, Fe, Pb, Cd, Ni and Mn were 0.017, 0.137, 0.293, 0.134, 0.038, 0.048, and 0.052 mg kg−1, respectively (). The accuracy of the method and meta analyses were carried out by spiking the samples with known concentrations of metal standards. In this study, 5.8 μL of Cu, 50 μL of Fe, 14 μL of Mn, 11 μL of Zn, 2 μL of Ni, 0.8 μL of Cd and 1 μL of Pb from 1000 mg L−1 of stock solution were spiked with D. angustifolia (0.5 g), 4 μL of Cu, 53 μL of Fe, 20 μL of Mn, 6 μL of Zn, 4 μL of Ni, 1 μL of Cd and 4 μL of Pb from 1000 mg L−1 of stock solution spiked with C. spinarum (0.5 g), E. racemosa was spiked with 12 μL of Cu, 27 μL of Fe, 32 μL of Mn, 5 μL of Zn, 1.9 μL of Ni, 2 μL of Cd and 0.4 μL of Pb; and 12 μL of Cu, 29 μL of Fe, 14 μL of Mn, 8 μL of Zn, 2 μL of Ni, 2 μL of Cd and 2 μL of Pb were spiked with 0.5 g of A. abyssinica and digested under the same procedure as that of the unspiked samples. The percent recovery ranged from 81.1% to 103.6% ().
Table 1. Limits of detection (LOD) and recoveries of metals in acacia abyssinica, Carissa spinarum, Dodonaea angustifolia, and Euclea racemose.
Non-Carcinogenic and Carcinogenic Risk Estimation
The non-carcinogenic risk for heavy metal can be expressed through target hazard quotient (THQ), which is calculated as the ratio of daily intake (ADI) to the chronic reference dose (RfD, mg kg−1 d−1). The ADI and THQ values were calculated using Eqn. 1 and Eqn. 2, respectively.[Citation25]
where CI is the concentration of metal in the plant (mg kg−1), IR is the ingestion rate of medicinal plants (20 g day−1),[Citation24] EF is the exposure frequency (365 days/year), ED is the exposure duration (70 y), BW is the body weight of exposed adults with a value of 65 kg,[Citation26] and AT is the time period over which the dose is averaged (365 days/year x number of exposure years, assuming 70 y).[Citation27]
The RfD values for Cd, Cu, Fe, Mn, Ni, Pb and Zn were 0.001, 0.04, 0.7, 0.14, 0.02, 0.0035 and 0.3 mg kg−1 d−1, respectively.
The non-carcinogenic risk can also be expressed in terms of the hazard index (HI), which is the sum of THQ values for all metals in the plants.[Citation24] If the HI values are >1, there will be a significant non-carcinogenic risk of metals for the exposed population.[Citation28]
The carcinogenic risk (CR) obtained from the intake of Cd and Pb was computed using the Eqn. 3
Where, EF is the exposure frequency (350 days/year), ED is the exposure duration (30 y)[Citation29] and AT is the averaging time for carcinogens (365 days/year × 70 years), and CSFo is the oral carcinogenic slope factor[Citation30] database, which were 0.38 and 0.0085 (mg kg−1 day−1) for Cd and Pb, respectively.
Generally, CR values ranging between 1 × 10−6 and 1 × 10−4 are acceptable, and CR less than 10−6 are considered to pose no significant health risks for humans, while CR values greater than 1 × 10−4 indicate potential harm to humans.[Citation31]
Statistical Analysis
Data were analyzed using one-way Analysis of Variance (ANOVA) using SPSS (ver. 20). The Pearson correlation was used to determine the correlation between metals in the plants. The Principal Component Analysis (PCA) was used to determine the difference between the plants and to investigate environmental pollution with respect to metals. Clusters analysis was used to relate on the nearness or similarity of the mineral composition.
Results
Concentration of Metals
In all the medicinal plants, all the seven metals were above the detection limit (). The mean concentration of Cd ranged from 0.171 to 0.402 mg kg−1 with the lowest and highest values being in D. angustifolia and A. abyssinica, respectively. The order of Cd concentrations in the plants was: D. angustifolia < C. spinarum <E. racemose < A. abyssinica, which attributed to the difference in the metal uptake in the soil and water by the plants.
Table 2. Concentration (mean ± SD, mg kg−1 dry weight) of essential and toxic metals in acacia abyssinica, Carissa spinarum, Dodonaea angustifolia, and Euclea racemose.
The mean concentration of Cu ranged from 8.87 mg kg−1 (C. spinarum) to 25.3 mg kg−1 (A. abyssinica). Generally, Cu concentration in the plants was: C. spinarum < D. angustifolia < E. racemose < A. abyssinica ()
Fe concentration ranged from 55.9 mg kg−1 to 107 mg kg−1, in the order of E. racemosa < A. abyssinica < D. angustifolia < C. spinarum.
The average Mn concentrations in the plants ranged between 28.3 mg kg−1 (in A. abyssinica) and 64.9 mg kg−1 (in E. racemosa). The highest concentration of Mn was found in E. racemose, followed by C. spinarum, D. angustifolia, and A. abyssinica. ()
The mean concentration of Ni followed the descending order as: C. spinarum (8.02 mg kg−1) > A. abyssinica (4.42 mg kg−1) > D. angustifolia (4.01 mg kg−1) > E. racemose (3.84 mg kg−1).
The concentrations of Pb were 0.771 mg kg−1 (E. racemosa), 3.10 mg kg−1 (D. angustifolia), 4.04 mg kg−1 (A. abyssinica), and 8.53 mg kg−1 (C. spinarum) ().
The Zn concentrations ranged from 11.5 mg kg−1 to 23.8 mg kg−1, with the lowest and highest being recorded in E. racemosa and D. angustifolia, respectively.
Pearson’s Correlation Analysis
Strong positive correlations were observed between Fe with Zn, Cu with Mn and Zn, and Pb with Cd and Ni in D. angustifolia (). However, there were high negative correlations of Fe with Mn, Cu and Ni, Mn with Zn. Similarly, in C. spinarum, strong positive correlations were observed between Fe with (Zn and Ni), Cu with Mn, Zn with Ni, Pb with (Cu, Mn and Ni). A high negative correlation was noted: Fe with Mn, Cu and Pb, Mn with Zn and Ni, Zn with Cu, Cd and Pb; and Cu with Ni in C. spinarum.
Table 3. Pearson correlations of metals in acacia abyssinica Carissa spinarum, Dodonaea angustifolia and euclea racemose.
Strong positive correlations were observed in A. abyssinica between Fe with Cu, Mn, and Zn, Mn with Zn and Cu, Cu with Zn, Cd and Pb, Ni with Cd and Pb, Cd with Pb.
Strong positive correlations were observed between Fe with Cu, and Pb, Mn with Zn, Cu with Cd, Pb and Zn, Zn with Pb, in E. racemosa, indicating that they arose from common anthropogenic or natural sources of similar chemical properties (). High negative correlation of Fe with Zn and Mn, Mn with Cu and Pb confirmed that large absorption of one element may affect the absorption of the other elements in E. racemosa plant. Some elements show moderate positive and negative relationships (r values between 0.5 and 0.7, −0.5 and −0.7 respectively), suggesting that they may be influenced by similar factors.
Non-Carcinogenic and Carcinogenic Risks
The mean values of ADI decreased in the order of Fe > Mn > Cu > Zn > Ni > Cd > Pb for A. abyssinica, Fe > Mn > Zn > Cu > Pb > Ni > Cd for C. spinarum, Fe > Mn > Zn > Cu > Ni > Pb > Cd for D. angustifolia, and Mn > Fe > Cu > Zn > Cd > Ni > Pb for E. racemosa ().
Table 4. Non-cancer risk index of metals in acacia abyssinica, Carissa spinarum, Dodonaea angustifolia, and Euclea racemose.
The carcinogenic risk (CR) values for Cd ranged from 2.0310−7 (D. angustifolia) and 4.6 × 10−7 (E. racemosa), while the CR values for Pb ranged from 1.87 × 10−9 (E. racemosa) to 2.06 × 10−8 (C. spinarum) ().
Table 5. Carcinogenic risks (CR) of Cd and Pb due to medicinal use of acacia abyssinica, Carissa spinarum, Dodonaea angustifolia, and Euclea racemose.
Cluster Analysis and Principal Component Analysis
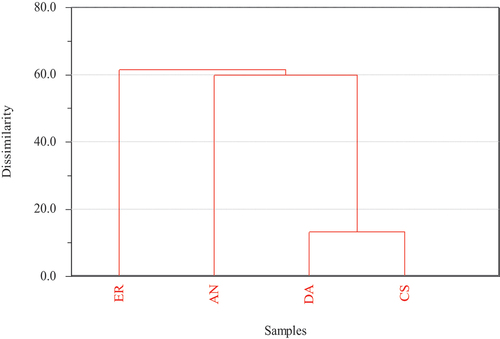
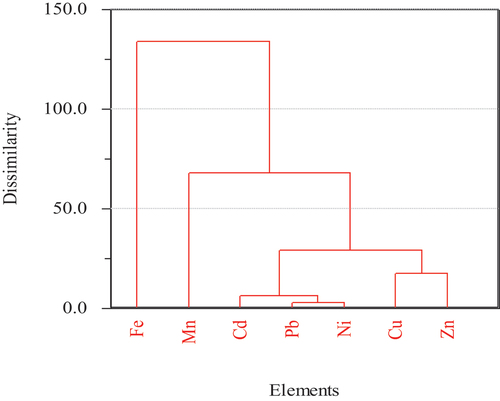
The cluster analysis confirmed the presence of three distinct clusters: cluster 1 consisted of Cd, Pb, Ni, Cu and Zn, which revealed that these metals had higher contamination levels; cluster 2 and cluster 3 contained Fe and Mn, respectively, which revealed the lowest contamination levels of Fe and Mn. Cd, Ni, and Pb had high similarities in their contents (). Cu and Zn had close proximities.
Figure 1. Hierarchical cluster analysis of metals in acacia abyssinica Carissa spinarum, Dodonaea angustifolia and euclea racemose.
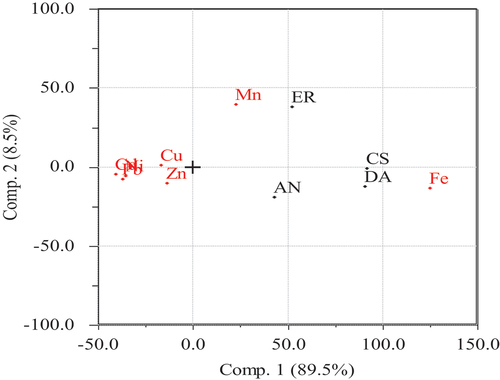
The hierarchical dendrograms of the plants showed three main clusters (). The 1st cluster represented ER (E. racemosa), the 2nd cluster AA (A. abyssinica) and the 3rd cluster included DA (D. angustifolia) and CS (C. spinarum). The DA and CS showed distributions generally in close regions with identical environmental conditions. The findings of the dendrograms supported the findings of PCA () which showed that the 1st component explained 89.5% of the total variance; the 2nd component accounted for 8.5%, and overall, the PCA accounted for 98% of the variance of the total variance. The first component showed a strong negative loading for Cd, Ni, and Pb and high positive loadings for Fe. The second component showed a strong positive loading for Mn.
Discussion
Plants absorb minerals that are important for their growth through roots from the soil and leaf absorption from the air.[Citation32] The concentration of metals in medicinal plants is affected by factors such as the chemical properties of soil, industrialization, air pollution, and other climatic conditions.[Citation33]
The Cd concentrations were below recommended values set by WHO (0.3 mg kg−1) except in Acacia abyssinica, while Cu, Fe, Mn, Ni and Zn concentrations were within permissible limits set by WHO across all plant samples (WHO, 2007),[Citation34] indicating no severe effect from the use of these plants.[Citation2]
However, Pb concentrations in C. spinarum exceeded WHO values,[Citation35] which may be attributed to the use of chemicals such as fertilizers and pesticides as agricultural inputs to enhance agricultural productivity.[Citation36] Thus, the use of C. spinarum for medicinal purposes poses health risks to humans in terms of Pb content.[Citation37]
The amounts of Cd detected in this study were comparable with the medicinal plants reported from Ethiopia[Citation38] and India[Citation39]). However, these values were slightly higher than those found in medicinal plants from Iran.[Citation40] The amounts of Cu obtained in this study were higher than those found in medicinal plants reported from Ethiopia,[Citation38] Iran,[Citation40] and Tanzania[Citation41] and close to those reported from Ethiopia.[Citation26] The amounts of Fe found in this study were consistent with those reported in Tanzania,[Citation41] but higher than that found in plants from Iran,[Citation40] Ethiopia[Citation38] and India.[Citation39] The findings in this study regarding Mn and Ni concentrations agree with the results obtained in Tanzania,[Citation41] but are higher than the data reported elsewhere.[Citation38,Citation40] The concentrations of Pb were in agreement with the results reported by some researchers[Citation26] but higher than the data presented by others.[Citation38–40] Zn content noted in this study was comparable to that reported from Tanzania,[Citation41] but slightly higher than those in medicinal plants reported from Ethiopia,[Citation38] India[Citation39] and Iran.[Citation40] The variations in heavy metal contents in the plant samples in this study compared to studies in Ethiopia and other countries maybe due to differences in varieties, environments from where samples were collected, growth, anthropogenic activities, and analytical methods.[Citation42]
The pattern of the overall concentration of metals in plant samples was in the order for D. angustifolia: Fe > Mn > Zn > Cu > Ni > Pb> Cd, C. spinarum: Fe > Mn > Zn > Cu > Pb > Ni > Cd; E. racemosa: Mn > Fe > Cu > Zn > Cd > Ni > Pb and A. abyssinica: Fe > Mn > Cu > Zn > Ni > Pb > Cd. The results showed that the metal contents varied between the plants for some metals which may be due to variations in the morphology and physiology of these plants for metal uptake, accumulation, exclusion, and retention.[Citation36]
Calculating the Pearson correlation coefficients between the concentrations of different metals in plants gave an insight into the potential relationships between them. Some elements showed strong and moderate positive relationships, indicating that both metals may have common sources or may be influenced by similar factors. Elements which showed weak negative or positive correlations demonstrated that the presence or absence of one element can affect the other to a lesser extent. This poor relationship might be due to the different capacities of the plant to accumulate specific elements depending on the type of soil, environmental conditions, and the plant’s capacity to accumulate specific elements.
Non-Carcinogenic and Carcinogenic Risks
The mean values of ADI decreased in the order of Fe > Mn > Cu > Zn > Ni > Cd > Pb, Fe > Mn > Zn > Cu > Pb > Ni > Cd, Fe > Mn > Zn > Cu > Ni > Pb > Cd and Mn > Fe > Cu > Zn > Cd > Ni > Pb for A. abyssinica, C. spinarum, D. angustifolia and E. Racemosa, respectively ().
The THQ values for all metals except Cd in A. abysinica and D. angustifolia were less than unity, which are considered safe for human consumption. The HI values ranged from 1.0928 to 1.9153, and consequently, the users are at high risk due to the presence of Fe, Mn, Zn, Cu, Ni, Pb and Cd in these plants, and their regular use for treating various diseases is not recommended.
The CR values for Cd were between 2.03 × 10−7 (A. abyssinica) and 4.6 × 10−7 (E. racemosa), while the CR values for Pb ranged from 1.87 × 10−9 (E. racemosa) to 2.06 × 10−8 (C. spinarum) (). Since the CR values of Cd and Pb for medicinal plants were less than the USEPA standard (1×10−4), the consumption of medicinal plants due to Cd and Pb may not pose a cancer risk to adults.
Fe was the highest in all samples and the lowest was Cd, except in E. racemosa, which was Pb. Except for Pb, the concentrations of all other metals were found below the permissible limit set by the WHO. Cd, Fe, Ni and Pb were the major contributors to principal component 1, while the concentration of Mn was the major contributor to principal component 2. The HI values calculated for adult exposure to heavy metals were between 1.0928 and 1.9153, which implied that using these medicinal plants is likely to cause adverse non-carcinogenic effects. However, the carcinogenic risk (CR) values of Cd and Pb were less than the recommended limit of 1.0 × 10−4, indicating that there were no carcinogenic risks for the users.
Author’s Contributions
Nigus Dagnew: Conceptualization, Formal analysis, Investigation, Interpretation of the data, Writing – Original Draft; Adisie Kassa: Formal analysis, Investigation, Interpretation of the data; Seada Ahmed: Formal analysis, Investigation, Data Curation; Atnafu Guadie: Analysis and Interpretation of the data; Molla Tefera: Formal analysis, Investigation, Interpretation of the data, Supervision; Walelign Wubet and Andualeme Ejigu: Writing – Review & Editing; Getinet Masresha: Conceptualization, Critical revising.
Disclosure statement
The authors declared no potential conflicts of interest with respect to the research, authorship, and publication of this article.
Data availability statement
Data will be made available on request.
References
- Kebede, T.; Gadisa, E.; Tufa, A. Antimicrobial Activities Evaluation and Phytochemical Screening of Some Selected Medicinal Plants: A Possible Alternative in the Treatment of Multi Drug Resistant Microbes. Plos. One. 2021, 16(3), e0249253. DOI: 10.1371/journal.pone.0249253.
- Karahan, F. Evaluation of Trace Element and Heavy Metal Levels of Some Ethnobotanically Important Medicinal Plants Used as Remedies in Southern Turkey in Terms of Human Health Risk. Biol. Trace Elem. Res. 2023, 201(1), 493–513. DOI: 10.1007/s12011-022-03299-z.
- Vaou, N.; Stavropoulou, E.; Voidarou, C.; Tsigalou, C.; Bezirtzoglou, E. Towards Advances in Medicinal Plant Antimicrobial Activity: A Review Study on Challenges and Future Perspectives. Microorganisms. 2021, 9(10), 2041. DOI: 10.3390/microorganisms9102041.
- Eshete, M. A.; Molla, E. L. Cultural Significance of Medicinal Plants in Healing Human Ailments Among Guji Semi-Pastoralist People, Suro Barguda District, Ethiopia. J. Ethnobiol. Ethnomed. 2021, 17(1), 1–18. DOI: 10.1186/s13002-021-00487-4.
- Ayalew, H.; Tewelde, E.; Abebe, B.; Alebachew, Y.; Tadesse, S. Endemic Medicinal Plants of Ethiopia: Ethnomedicinal Uses, Biological Activities and Chemical Constituents. J. Ethnopharmacol. 2022, 293, 115307. DOI: 10.1016/j.jep.2022.115307.
- Karahan, F.; Ozyigit, I. I.; Saracoglu, I. A.; Yalcin, I. E.; Ozyigit, A. H.; Ilcim, A. Heavy Metal Levels and Mineral Nutrient Status in Different Parts of Various Medicinal Plants Collected from Eastern Mediterranean Region of Turkey. Biol. Trace Elem. Res. 2020, 197(1), 316–329. DOI: 10.1007/s12011-019-01974-2.
- Zárate-Quiñones, R. H.; Custodio, M.; Orellana-Mendoza, E.; Cuadrado-Campó, W. J.; Grijalva-Aroni, P. L.; Peñaloza, R. Determination of Toxic Metals in Commonly Consumed Medicinal Plants Largely Used in Peru by ICP-MS and Their Impact on Human Health. Chem. Data Collect. 2021, 33, 100711. DOI: 10.1016/j.cdc.2021.100711.
- Woreta, G.; Guadie, A.; Mulu, M.; Beshaw, T.; Lijalem, T.; Ezez, D.; Kokeb, A.; Leggesse, M.; Tefera, M. Occurrence and Accumulation of Metals in Lupine Seeds in Ethiopia. J. Food Compost. Anal. 2023, 105218, 105218. DOI: 10.1016/j.jfca.2023.105218.
- Massadeh, A. M.; El-Rjoob, A. O.; Omari, M. N. Investigation of Metal Levels in Artemisia Herba-Alba Medicinal Plant and Soil Samples Collected from Different Areas in Jordan Country. Soil Sediment Contamin. Int. J. 2021, 30(2), 216–230. DOI: 10.1080/15320383.2020.1832041.
- Tripathi, S.; Sharma, P.; Singh, K.; Purchase, D.; Chandra, R. Translocation of Heavy Metals in Medicinally Important Herbal Plants Growing on Complex Organometallic Sludge of Sugarcane Molasses-Based Distillery Waste. Environ. Technol. Innov. 2021, 22, 101434. DOI: 10.1016/j.eti.2021.101434.
- Gonfa, N.; Tulu, D.; Hundera, K.; Raga, D.; Yildiz, F. Ethnobotanical Study of Medicinal Plants, Its Utilization, and Conservation by Indigenous People of Gera District, Ethiopia. Cogent. Food.Agric. 2020, 6(1), 1852716. DOI: 10.1080/23311932.2020.1852716.
- Tesfaye, S.; Belete, A.; Engidawork, E.; Gedif, T.; Asres, K. Ethnobotanical Study of Medicinal Plants Used by Traditional Healers to Treat Cancer-Like Symptoms in Eleven Districts, Ethiopia. Evid. Based Complement. Altern. Med. 2020, 2020, 1–23. DOI: 10.1155/2020/7683450.
- Abebe, B. A.; Chane Teferi, S.; Ghayur, M. N. Ethnobotanical Study of Medicinal Plants Used to Treat Human and Livestock Ailments in Hulet Eju Enese Woreda, East Gojjam Zone of Amhara Region, Ethiopia. Evid. Based Complement. Altern. Med. 2021, 2021, 1–11. DOI: 10.1155/2021/6668541.
- Chekole, G.; Asfaw, Z.; Kelbessa, E. Ethnobotanical Study of Medicinal Plants in the Environs of Tara-Gedam and Amba Remnant Forests of Libo Kemkem District, Northwest Ethiopia. J. Ethnobiol. Ethnomed. 2015, 11(1), 1–38. DOI: 10.1186/1746-4269-11-4.
- Hassan, R. A.; Hamdy, R. S. Synoptic overview of exotic Acacia, Senegalia and Vachellia (Caesalpinioideae, mimosoid clade, Fabaceae) in Egypt. Plants. 2021, 10(7), 1344. DOI: 10.3390/plants10071344.
- Hedberg, I. Flora of Ethiopia; Edwards, S. eds.; Addis Ababa: Ethiopia, Uppsala, Sweden, 1989. Vol.3.
- Moges, A.; Moges, Y. Ethiopian Common Medicinal Plants: Their Parts and Uses in Traditional Medicine-Ecology and Quality Control. Plant. Sci-Struct. Anatomy. Physiol Plant. Cultured Vivo. Vitro,IntechOpen. 2019, 21.
- Hedberg, I.; Edwards, S., and Nemomissa, S. eds., Flora of Ethiopia; The Department of Systematic, Botany: Uppsala, Sweden, 2003 Vol. 4 part 1
- Sisay, M. A.; Yaya, E. E.; Mammo, W. Essential oil and smoke components of Carissa spinarum. Bull. Chem. Soc. Ethiop. 2022, 36(3), 641–649. DOI: 10.4314/bcse.v36i3.13.
- Amelo, W.; Nagpal, P.; Makonnen, E. Antiplasmodial Activity of Solvent Fractions of Methanolic Root Extract of Dodonaea Angustifolia in Plasmodium Berghei Infected Mice. BMC Complementary Altern. Med. 2014, 14(1), 1–7. DOI: 10.1186/1472-6882-14-462.
- Asres, K.; Gibbons, S.; Bucar, F. Radical Scavenging Compounds from Ethiopian Medicinal Plants. Ethiopian Pharm. J. 2006, 24(1), 23–30. DOI: 10.4314/epj.v24i1.35095.
- Gedefaw, M.; Soromessa, T. Status and Woody Plant Species Diversity in Tara Gedam Forest, Northern Ethiopia. Sc. Technol. Arts Res. J. 2014, 3(2), 113–118. DOI: 10.4314/star.v3i2.15.
- ICH (2005) Validation of Analytical Procedures: Text and Methodology Q2 (R1). International Conference On Harmonisation. https://www.gmp-compliance.org/guidemgr/files/Q2(R1).
- Guadie, A.; Mohammed, I.; Beshaw, T.; Tefera, M. Analysis and Health Risk Assessments of Some Trace Metals in Ethiopian Rice (White and Red) and Imported Rice. Heliyon. 2022, 8(5), e09374. DOI: 10.1016/j.heliyon.2022.e09374.
- Ahmed, M. S.; Yesmin, M.; Jeba, F.; Hoque, M. S.; Jamee, A. R.; Salam, A. Risk Assessment and Evaluation of Heavy Metals Concentrations in Blood Samples of Plastic Industry Workers in Dhaka, Bangladesh. Toxicol. Rep. 2020, 7, 1373–1380. DOI: 10.1016/j.toxrep.2020.10.003.
- Meseret, M.; Ketema, G.; Kassahun, H.; Cardeal, Z. Health Risk Assessment and Determination of Some Heavy Metals in Commonly Consumed Traditional Herbal Preparations in Northeast Ethiopia. J. Chem. 2020, 2020, 1–7. DOI: 10.1155/2020/8883837.
- Adebiyi, F. M.; Ore, O. T.; Ogunjimi, I. O. Evaluation of Human Health Risk Assessment of Potential Toxic Metals in Commonly Consumed Crayfish (Palaemon hastatus) in Nigeria. Heliyon. 2020, 6(1), e03092. DOI: 10.1016/j.heliyon.2019.e03092.
- Getaneh, A.; Guadie, A.; Tefera, M. Levels of Heavy Metals in Ginger (Zingiber Officinale Roscoe) from Selected Districts of Central Gondar Zone, Ethiopia and Associated Health Risk. Heliyon. 2021, 7(4), e06924. DOI: 10.1016/j.heliyon.2021.e06924.
- USEPA 2006 National Primary Drinking Water Regulations: Ground Water Rule; Final Rule. 71 FR 65573. November 8, 2006.
- USEPA. Integrated Risk Information System (IRIS); United States Environmental Protection. https://cfpub.epa.gov/ncea//risk/human/index.htm, 2010.
- Kharazi, A.; Leili, M.; Khazaei, M.; Alikhani, M. Y.; Shokoohi, R. Human Health Risk Assessment of Heavy Metals in Agricultural Soil and Food Crops in Hamadan, Iran. J. Food Compost. Anal. 2021, 100, 103890. DOI: 10.1016/j.jfca.2021.103890.
- Obiora, S. C.; Chukwu, A.; Chibuike, G.; Nwegbu, A. N. Potentially Harmful Elements and Their Health Implications in Cultivable Soils and Food Crops Around Lead-Zinc Mines in Ishiagu, Southeastern Nigeria. J. Geochem. Explor. 2019, 204, 289–296. DOI: 10.1016/j.gexplo.2019.06.011.
- Briffa, J.; Sinagra, E.; Blundell, R. Heavy Metal Pollution in the Environment and Their Toxicological Effects on Humans. Heliyon. 2020, 6(9), e04691. DOI: 10.1016/j.heliyon.2020.e04691.
- Hussain, A.; Priyadarshi, M.; Dubey, S. Experimental Study on Accumulation of Heavy Metals in Vegetables Irrigated with Treated Wastewater. Appl. Water Sci. 2019, 9(5), 1–11. DOI: 10.1007/s13201-019-0999-4.
- WHO/FAO. Joint FAO/WHO Food Standard Programme Codex Alimentarius, 2007.
- Pandiyan, J.; Mahboob, S.; Govindarajan, M.; Al-Ghanim, K. A.; Ahmed, Z.; Al-Mulhm, N.; Jagadheesan, R.; Krishnappa, K. An Assessment of Level of Heavy Metals Pollution in the Water, Sediment and Aquatic Organisms: A Perspective of Tackling Environmental Threats for Food Security. Saudi J. Biol. Sci. 2021, 28(2), 1218–1225. DOI: 10.1016/j.sjbs.2020.11.072.
- Kormoker, T.; Proshad, R.; Islam, M. S.; Shamsuzzoha, M.; Akter, A.; Tusher, T. R. Concentrations, Source Apportionment and Potential Health Risk of Toxic Metals in Foodstuffs of Bangladesh. Toxin. Rev. 2021, 40(4), 1447–1460. DOI: 10.1080/15569543.2020.1731551.
- Hailemariam, A. Determination of Levels of Some Metals in Selected Traditional Medicinal Plants in Wolaita Zone. Intl. J. Curr. Res. 2019, 11(3), 1839–1844.
- Kulhari, A.; Sheorayan, A.; Bajar, S.; Sarkar, S.; Chaudhury, A.; Kalia, R. K. Investigation of Heavy Metals in Frequently Utilized Medicinal Plants Collected from Environmentally Diverse Locations of North Western India. Springer. Plus. 2013, 2(1), 1–9. DOI: 10.1186/2193-1801-2-676.
- Kohzadi, S.; Shahmoradi, B.; Ghaderi, E.; Loqmani, H.; Maleki, A. Concentration, Source, and Potential Human Health Risk of Heavy Metals in the Commonly Consumed Medicinal Plants. Biol. Trace Elem. Res. 2019, 187(1), 41–50. DOI: 10.1007/s12011-018-1357-3.
- Nkuba, L.; Mohammed, N. Heavy Metals and Essential Elements in Selected Medicinal Plants Commonly Used for Medicine in Tanzania. Chem. Sci. Int. J. 2017, 19(2), 1–11. DOI: 10.9734/CSJI/2017/31963.
- Adjei-Mensah, R.; Ofori, H.; Tortoe, C.; Johnson, P. N. T.; Aryee, D.; Frimpong, S. K. Effect of Home Processing Methods on the Levels of Heavy Metal Contaminants in Four Food Crops Grown in and Around Two Mining Towns in Ghana. Toxicol. Rep. 2021, 8, 1830–1838. DOI: 10.1016/j.toxrep.2021.11.001.