?Mathematical formulae have been encoded as MathML and are displayed in this HTML version using MathJax in order to improve their display. Uncheck the box to turn MathJax off. This feature requires Javascript. Click on a formula to zoom.
?Mathematical formulae have been encoded as MathML and are displayed in this HTML version using MathJax in order to improve their display. Uncheck the box to turn MathJax off. This feature requires Javascript. Click on a formula to zoom.ABSTRACT
This study presents an initial exploration of the phytochemical composition and biological properties of Salsola foetida, a wild plant native to southeastern Algeria. The investigation encompassed an analysis of the biochemical content, phytochemical profile, and bioactivity of the plant’s aerial parts. The biomass of S. foetida was found to comprise 51.2 ± 0.4% ash, with elemental analysis revealing the presence of carbon, oxygen, calcium, sodium, chlorine, magnesium, potassium, and sulfur. Carbohydrates were identified as the predominant constituents, surpassing the levels of proteins and lipids. Phytochemical screening, coupled with Fourier Transform Infrared (FT-IR) analysis, confirmed the presence of various secondary metabolites, including alkaloids and phenols. Reversed-phase high-performance liquid chromatography (RP-HPLC) analysis further delineated a spectrum of phenolic compounds, with rutin emerging as the predominant compound at 1.59 µg/ml. Quantification revealed a noteworthy phenolic content of 67.1 ± 0.2 µg GE/mg ED. The extract exhibited robust radical scavenging effects, with the radical 50% inhibitory concentration (IC50) being 61.8 ± 0.2 μg/ml, along with protective effects against H2O2-induced hemolysis and the ability to reduce ferric ions. Additionally, the extract demonstrated anti-inflammatory, antibacterial, and antidiabetic activities. Notably, it exhibited considerable sunscreen properties, boasting a sun protected factor (SPF) value of 21 ± 1. Overall, S. foetida presents promising antioxidant activity and a spectrum of potential biological activities, underscoring its significance as a novel antioxidant candidate for applications in animal nutrition, medicine, and natural product research.
Introduction
Studying the chemical profile of wild plants, particularly desert plants, holds significant scientific benefits. Firstly, it allows for the identification and characterization of the unique bioactive compounds present in these plants. These compounds may possess valuable properties such as antioxidant, antimicrobial, anti-inflammatory, and anticancer activities, among others [Citation1]. Understanding the chemical composition of wild desert plants can provide insights into their potential medicinal and nutritional applications [Citation2]. Additionally, analyzing the chemical profile of these plants contributes to our knowledge of their ecological adaptation and survival strategies in harsh desert environments. Desert plants have evolved specialized mechanisms to cope with extreme temperatures, water scarcity, and high levels of UV radiation. By studying their chemical constituents, scientists can gain a deeper understanding of the protective and adaptive mechanisms employed by these plants [Citation3].
This study highlights a particular species, Salsola foetida, which belongs to the botanical family Chenopodiaceae. This family is renowned for its widespread distribution in arid and semi-arid regions worldwide and is characterized by numerous halophytes species [Citation4]. This family is also very popular due to its various traditional uses across different cultures. Several species in the Chenopodiaceae family serve as fodder or herbal supplements for livestock, and some possess medicinal properties [Citation5, Citation6]. Furthermore, the family encompasses saline-alkaline species such as Ashnan, which is utilized for laundry purposes [Citation7]. Furthermore, certain members of this family, such as Suaeda, Salsola, and Salicornia, thrive in salt marshes, coastal regions, and deserts, and are utilized for extracting soda ash, which finds applications in soap, glass, and ceramics production [Citation8]. Finally, woody perennials like Salsola dendroides Pall. serve as fuelwood sources [Citation9]. In general, plants of the Chenopodiaceae family contain different groups of secondary metabolites, among which alkaloids, coumarins, lipids, essential oils, flavonoids, sterols, and steroidal estrogen-like substances are the most important [Citation10].
Salsola foetida Del. (S. barysoma; S. imbricata) is perennial shrubs. It is an edible halophyte, and it is considered one of the most important grazing plants for camels, as it has been verified as an anthelmintic for livestock [Citation11]. It has also been widely used to treat various diseases such as indigestion, diarrhea, dysentery, cold and asthma. It also relieves skin itching and sinus congestion and is considered as diuretic and female contraceptive [Citation12, Citation13]. Scientific research has also proven that the extract of the plant S. foetida has anti-inflammatory, hepatoprotective, antimicrobial, antispasmodic and bronchorelaxant activities [Citation12, Citation14–16]. It also possesses antidiabetic activity [Citation17], tyrosinase inhibitor [Citation18], and central nervous system stimulant. Scientific studies have shown that it contains antioxidants as it is known to have a role in the prevention of various diseases [Citation15, Citation17, Citation18].
Based on these established criteria, this study aims to explore the potential applications of the wild plant Salsola foetida as antioxidant nutritional supplements. This research is significant for the development of functional diets that can effectively address oxidative stress, prevent diseases, and reduce the need for therapeutic interventions in both humans and animals.
Materials and methods
Plant collection
The aerial part of S. foetida was gathered from the El Oued region (Debila) during the flowering stage in May, specifically at the coordinates 33° 31′N, 6° 56′E and an elevation of 54 m. The botanical identity of the species was authenticated by Prof. Noureddine Slimani from Department of Biology, Faculty of Natural Science and Life, El Oued University, Algeria. A voucher specimen (LOST.Sf05/010) was subsequently deposited at the herbarium of the Faculty of Life and Natural Sciences, El Oued University.
Biochemical analysis
Chlorophyll and Carotenoids Content
Chlorophyll and carotenoids content were determined following the method of Radwan and Soltan [Citation19] with some modification. Fresh leaves (0.1 g) were homogenized in 5 ml of 80% acetone, incubated for 24 hours at 4°C. The homogenate was centrifuged at 5,000×g for 10 min. The absorbance of supernatant was recorded at 663, 645 and 480 nm. The total chlorophyll and total carotenoid content was expressed as mg of plant per g of fresh weight.
Moisture and Ash Contents
Moisture and ash contents determined according to Afify, et al. [Citation20]. The moisture content was determined by drying the fresh plant material in an incubator at 105°C. The ash content was determined by burning the dry plant matter in a muffle furnace at 550°C.
Chemical elements and oxides content
Chemical elements and oxides content were determined using energy-dispersive X-ray spectroscopy by subjecting the powder of the aerial part of S. foetida to analysis [Citation21].
Macronutrient Composition
The determination of carbohydrate, lipid, and protein contents in the dry matter of the aerial part of S. foetida was conducted following the methodology reported by Chouikh, et al. [Citation22]. Each content was individually extracted using the following procedures: Firstly, 0.5 g of dried plant material was immersed in 5 mL of 20% trichloroacetic acid (TCA). The mixture was vigorously shaken on a vortex for five minutes, then centrifuged at 3000 rpm for 10 minutes, and the resulting supernatant was collected for carbohydrate quantification. Subsequently, 2 mL of a chloroform/methanol solution (1:1, v/v) was added to the remaining sediment, followed by another centrifugation at 3000 rpm for 15 minutes. The supernatant obtained was used for the determination of lipid content. Finally, to estimate the total protein content, 5 mL of 0.1N NaOH was added to the sediment, and the resulting supernatant was utilized for protein determination.
To determine the carbohydrate content, 2 mL of TCA extract was combined with 1 mL of 5% phenol and 5 mL of concentrated sulfuric acid. The resulting mixture was thoroughly shaken and incubated for 10 minutes, after which the absorbance was measured at 490 nm. The carbohydrate content was expressed as micrograms of glucose per milligram of dry matter. To determine the lipid content, 0.15 mL of sulfophosphovanillinic reagent was added to 0.1 mL of the fatty extract along with 1 mL of concentrated sulfuric acid. The resulting mixture was subjected to a water bath at 100°C for 10 minutes. Afterward, the mixture was vigorously shaken and incubated for 30 minutes, followed by measuring the absorbance at 530 nm. The fat content was expressed as micrograms of soybean oil/µg of dry matter.
The protein content of the sample was determined by mixing 0.2 mL of the NaOH extract with 1.8 mL of the Coomassie brilliant blue reagent, followed by incubation for 5 minutes before measuring the absorbance at a wavelength of 546 nm. The protein content was expressed as micrograms of bovine serum albumin (BSA) per milligram of dry matter.
Phytochemical profile
Preparation of the plant extract
The dry aerial parts powder of the plants (10 g) was macerated with 60 mL of methanol: water (70: 30%) at room temperature for 24 hours. The macerate process was repeated three times. The extracts were concentrated using a rotovap at 45 °C. The dry extracts were stored at 4 °C.
Phytochemical screening
Phytochemical examination was conducted following standard methods to assess the presence of alkaloids, terpenoids, saponins, coumarins, phenols, and tannins in the extract [Citation23].
FT-IR analysis
Fourier Transform Infrared (FT-IR) analysis was performed using Agilent Cary 630 Fourier transform infrared spectroscopy (Agilent, Santa Clara, CA, USA). The sample was directly placed on the platform, applying consistent pressure, to record spectra with an average of 8 scans at a resolution of 16 cm−1 within the range of 400–4000 cm−1.
Quantification of Phenolic Compounds by RP-HPLC
Phenolic compounds were assessed using reversed-phase high-performance liquid chromatography (RP-HPLC). Detection and quantification were performed utilizing a Shimadzu LC-20 HPLC system equipped with UV detection at a wavelength of 268 nm. The analysis was carried out on a C18 column (5 μm, 4.6 × 250 mm) with an injection volume of 20 μL. The mobile phase consisted of acetonitrile (A) and 0,1% acetic acid (B). A flow rate of 1 mL/min was maintained. The gradient method employed for separation started with 90% B and gradually reduced to 86% B within 6 minutes, 83% B within 16 minutes, 81% B within 23 minutes, 77% B within 28 minutes, and held at 77% B from 28 to 35 minutes. Subsequently, the gradient continued with a decrease to 60% B at 38 minutes, followed by a return to 90% B at 50 minutes. Gallic acid, chlorogenic acid, vanillic acid, caffeic acid, vanillin, coumaric acid, rutin, naringin, and quercetin were employed as reference standards. Identification and quantitative analysis were conducted by comparing with these standards. The quantity of each compound was expressed as micrograms per milligram of the extract.
Total bioactive components
For the determination of total saponin content, diosgenin was utilized as the reference standard. The studied sample were mixed with vanillin (8%) and subsequently treated with sulfuric acid (72%) in an equal volume. The resulting mixture was incubated at 60°C for 10 minutes and then cooled in an ice water bath for 15 minutes. Subsequently, the absorbance of the mixture was measured at 583 nm using a spectrophotometer [Citation23]. The total saponin content was expressed in diosgenin equivalents (µg DE/mg DE).
The total phenolic content of the crude extract was determined using the Folin-Ciocalteu method [Citation24]. To initiate the reaction, 200 µL of the extract or gallic acid concentrates were combined with 1000 µL of 10% Folin-Ciocalteu reagent and incubated for three minutes. Subsequently, 0.8 mL of 7.5% Na2CO3 was added, and the mixture was incubated for 30 minutes. The absorbance was measured at 765 nm. The content was expressed as µg of gallic acid per mg of dry extract.
The determination of hydrolyzed tannin content was performed following the Follin-Dennis method [Citation24]. 500 μL of the extract or gallic acid concentrates were mixed with 1000 μL of Folin-Denis reagent and 500 μL of 35% Na2CO3. The mixture was then diluted with distilled water to a total volume of 10 mL. After a 30-minute incubation in the dark, the absorbance was measured at 700 nm. The results were expressed as µg of gallic acid equivalents (QE) per mg of the sample.
The condensed tannin content was determined by the vanillin HCl method [7]. To 500 μL of extract or different concentrations of catechin were added 3000 μL of vanillin reagent (4%) and 1500 μL of 1N HCl and the mixture was mixed well. After incubation in the dark at 20°C for 15 minutes, the absorbance was read at 760 nm. Results were expressed as µg of catechin equivalents (CE) per mg of extract weight.
Biological activity
Antioxidant activity: DPPH• assays. The antioxidant capacity was evaluated by determining IC50 values, following the procedure outlined by Chekroun-Bechlaghem, et al. [Citation25].
β-carotene bleaching assay: Antioxidant activity to inhibit lipid peroxidation was evaluated using the β-carotene bleaching method as described by Chekroun-Bechlaghem [Citation25].
Anti-hemolytic assays: The efficiency of extract to inhibit human erythrocyte hemolysis was determined according to Chouikh [Citation22].
Reducing power: This assay was performed using the method of Muthukrishnan, et al. [Citation26]. Results are expressed as EC50. The concentration that provides absorption is 0.5.
Phosphomolybdenum reducing power assay: The total antioxidant capacity of extract was expressed as gallic acid equivalent (µg GAE/mg ED), based on the method of phosphomolybdate [Citation26].
Sun protected factor (SPF)
The photoprotective activity of the extract was tested by calculating SPF by applying the following equation, after measuring the absorbance of a sample dissolved in ethanol (1 mg/mL) at seven different wavelengths (290–320 nm) [Citation27]:
where: CF; correction factor (10). EE; erythemogenic effect of radiation with wavelength (λ) nm. I; solar intensity spectrum (λ) nm. DO (λ); spectrophotometric absorbance values at wavelength. The values of EE(λ)×I(λ) are constants. In this test, quercetin was used as a positive control.
Anti-inflammatory activity
The effectiveness of the extract in safeguarding protein (albumin) from heat-induced denaturation was determined through the following procedure: 1 mL of 5% albumin was mixed with 1 mL of various concentrations of the studied sample and 20 μg/mL of 1N HCl. The mixture was incubated at 37°C for 20 minutes, followed by placement in a water bath at 57°C for 3 minutes. After cooling, 2.5 mL of a phosphate buffer solution (0.1 M, pH = 6.4) was added. The absorbance was measured at 660 nm [Citation24]. Aspirin® was employed as a reference drug. The percentage protection from denaturation was computed using the formula:
Where the control refers to the solution containing all reagents except the test sample.
Antibacterial activity
Utilizing the Disk Diffusion method [Citation26] as a guide, the extract’s effectiveness was assessed against bacterial strains, including Bacillus subtilis ATCC-6633, Listeria innocua CIP-74915, Staphylococcus aureus ATCC-6633, Escherichia coli ATCC-8737, Pseudomonas aeruginosa ATCC-9027, and Salmonella typhimurium ATCC-14028. The active bacterial strains were spread onto Mueller-Hinton agar using a sterile cotton swab. Subsequently, discs containing 10 μL volume of the extract at varying doses (4–1 mg) were placed on an agar plate. After 24 hours of incubation at 37°C in an incubator, the zone of inhibition (mm) was observed.
The determination of Minimum Inhibitory Concentrations involved subjecting a series of broth dilutions to testing with Mueller-Hinton broth [Citation28]. The MIC represents the lowest extract concentration with no visible growth. Concentrations were prepared by sequentially adding 2 mL of Mueller-Hinton broth to 2 mL of the solution in each tube, starting with the highest concentration in the first tube. Then, 0.2 mL of bacterial suspension was added to each tube and gently mixed. Tubes were incubated at 37°C for 24 hours, and results were assessed for turbid or clear solutions. The MIC was calculated using the formula:
Where CClear is the extract concentration in the first tube with a clear solution, and CTurbid is the extract concentration in the last tube with a turbid solution.
Effect of the extract on reducing glucose levels
In vitro evaluation of extract antidiabetic activity involves three straightforward assays, providing insights into the potential antidiabetic properties of the sample.
Glycosylation assay
This assessment examines the impact of plant extract on glucose complex in vitro, following the procedure by Kebieche [Citation29] with modifications. Extract and the reference compound (Metformin) were dissolved in physiological water, along with a glucose solution. The mixture, including negative control with water, was incubated at 37°C for 15 minutes. Glucose not associated with the samples was quantified using a glucometer (On Call® Extra), and results were expressed in mg/dL.
Non-Enzymatic hemoglobin glycosylation assay
This assay gauges the extract efficiency in inhibiting hemoglobin glycosylation. Following [Citation30], samples were prepared in phosphate buffer (0.01 M, pH = 7.4). A solution of samples (250, 500, and 1000 µg/mL), hemoglobin, Gentamicin (0.02%), and glucose was incubated in the dark at 37°C for 72 hours. Absorbance was measured at 520 nm.
Glucose uptake assay by yeast cells
This test measures glucose transport across yeast cell membranes. Saccharomyces cerevisiae was employed as a model. Yeast suspension was mixed with samples and glucose solution (10 mM) and incubated at 37°C for 10 minutes. Yeast suspension (10%) was added, and after an hour of incubation, absorbance was measured at 540 nm. Metformin served as an antidiabetic drug [Citation31].
Statistical analysis
The results were expressed as the mean ± standard deviation of three replicates. The statistical study was conducted using SPSS Statistic for Windows version 15.0. The differences between the two variables were determined using independent-sample Student’s t-test.
Results
Photosynthetic pigments, moisture, and ash content
The presents the measurements of photosynthetic pigments, moisture content, and ash content of the aerial part of S. foetida. The values reported are as follows: moisture content of 61.55 ± 0.96%, ash content of 51.2 ± 0.4%, chlorophyll a content of 5.11 ± 4.96 mg/mL, chlorophyll b content of 8.23 ± 1.97 mg/mL, and carotene content of 0.07 ± 0.09 mg/mL.
Table 1. Photosynthetic pigments, Moisture, and ash content of the aerial part of S. foetida.
Chemical elements and oxides content
EDX analysis () of dry milled plant material reveals its elemental composition. Carbon (C) and oxygen (O) dominate, comprising 40.13% and 43.27% of the total weight. Calcium (Ca), sodium (Na), chlorine (Cl), and magnesium (Mg) are present at 2.47%, 2.22%, 2.5%, and 1.41%, respectively. Potassium (K) and sulfur (S) account for 7.26% and 0.75%, respectively. Oxide forms of elements (CaO, Na2O, MgO, and SO3) are also detected, with percentages of 5.71%, 4.93%, 3.83%, and 3.08%. These insights provide a basis for further investigation into the chemical properties of the plant material.
Table 2. Results of EDX Analysis of the dried aerial part of S. foetida.
Macronutrient composition
shows the carbohydrate, protein, and lipid content of the aerial part of S. foetida. Carbohydrates were found to be the highest, followed by protein and lipids.
Table 3. Carbohydrate, Lipids, and Protein content of the aerial part of S. foetida.
Phytochemical profile
represents the phytochemical screening results of the methanolic extract obtained from the aerial part of S. foetida. The screening was conducted to determine the presence or absence of various phytochemical compounds in the extract. It is evident that all the bioactive compounds tested were present in the methanolic extract of S. foetida.
Table 4. Preliminary phytochemical screening of the aerial part of S. foetida.
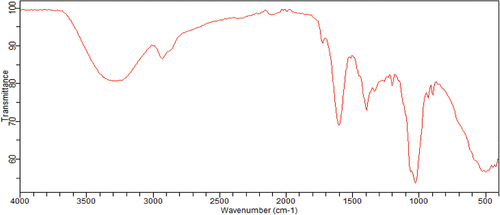
The spectrogram () and corresponding insights into the chemical composition of the methanol extract from the aerial part of S. foetida. Peaks in the mid-infrared region indicate the presence of phenolic compounds, alkane groups, aromatic compounds, aromatic amines, and diverse bioactive compounds.
presents the results of RP-HPLC analysis of individual phenolic compounds in the methanolic extract of S. foetida. The content of various phenolic compounds in the extract was determined, and their presence or absence is indicated. Among the analyzed compounds, rutin exhibited the highest content with a value of 1.59 µg/mg, making it the dominant phenolic compound in the extract. Chlorogenic acid and vanillin were also present in the extract with contents of 0.96 and 0.47 µg/mg, respectively. Vanillic acid showed a lower content of 0.13 µg/mg. On the other hand, gallic acid, caffeic acid, p-coumaric acid, naringin, and quercetin were not detected.Figure 2. Content of individual phenolic compounds in the methanolic extract of S. foetida, obtained from RP-HPLC analysis.
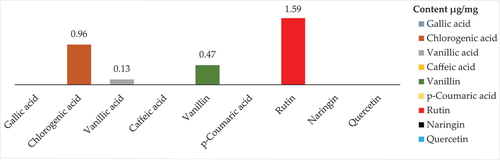
Table 5. FTIR peak values and of methanolic extract of S. foetida.
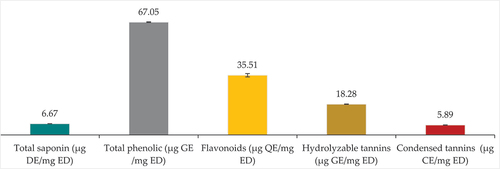
presents the quantification of bioactive compounds in S. foetida extracts, including saponins, total phenols, flavonoids, hydrolyzable tannins, and condensed tannins. Total phenolic content (67.1 ± 0.2 µg GE/mg ED) exceeds saponin content (6.67 ± 0.09 µg DE/mg ED). Flavonoids dominate the phenolic content, representing nearly half of it (35.51 ± 0.94 µg QE/mg ED), followed by soluble tannins (18.28 ± 1.12 µg GE/mg ED), and then condensed tannins with the lowest content (6.21 ± 0.09 µg CE/mg ED).
Figure 3. Saponins, total phenols, flavonoids, hydrolysable tannins and condensed tannins content of S. foetida extracts.
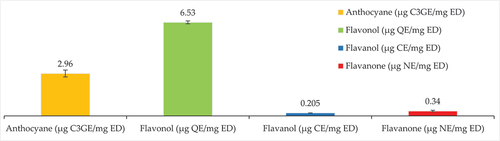
Flavonoid subclasses were quantified in the S. foetida extract, with flavonols being the dominant compound (6.53 ± 0.33 µg QAE/mg ED). Anthocyanes were moderately present (2.96 ± 1.48 µg C3GE/mg ED), while flavanol content was relatively lower (0.205 ± 0.06 µg CE/mg ED). Flavanones had the lowest abundance (0.34 ± 0 µg NE/mg ED).
Biological effects
Antioxidant activity: The results of the antioxidant activity of S. foetida extract are summarized in . The extract exhibited increasing inhibition of DPPH‧ free radicals with higher concentrations, indicating antioxidant activity. Statistical analysis demonstrated highly significant differences (p < .0001) between the extract and the positive control (Ascorbic acid). In the β-carotene bleaching assay, the extract demonstrated antioxidant activity, supported by statistical analysis (p < .0001) showing significant differences compared to α-tocopherol. Additionally, the methanolic extract of S. foetida displayed concentration-dependent anti-hemolysis effects, with decreasing hemolysis observed at higher concentrations. Ascorbic acid also showed reduced hemolysis, and statistical analysis confirmed the significant difference (p < .0001) between S. foetida and ascorbic acid. also presents the results of the reducing ability test, which showed that S. foetida extract had weak ability compared to ascorbic acid. The extract exhibited a higher total antioxidant capacity (176.17 ± 0.33 µg GAE/mg) compared to BHT (57.94 ± 1.54 µg GAE/mg).
Table 6. Values of radical scavenging activity, β-carotene bleaching activity, anti-hemolysis activity, ferrous reducing capacity, and total antioxidant capacity, that has been obtained for the methanolic extract of the aerial parts of S. foetida.
Photoprotective activity: shows the sun protection factor (SPF) values of the methanolic extract and quercetin were determined. The SPF values were found to be 21 ± 1.12 for S. foetida extract and 26.99 ± 0.08 for quercetin.
Table 7. SPF values for two methanolic extracts of S. foetida and quercetin. (n = 2, Student’s t-test, p < .0001.).
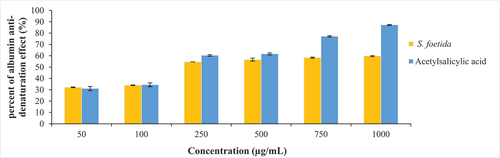
Anti-inflammatory activity: According to , the methanolic extract of S. foetida shows some anti-inflammatory activity by protecting albumin from temperature denaturation. However, its anti-denaturation effects are generally lower compared to the acetylsalicylic acid. Statistical analysis (p < .0001) confirms a significant difference in anti-inflammatory activity between S. foetida and acetylsalicylic acid.
Antibacterial activity: presents the mean inhibition zone diameter of S. foetida extract at various concentrations against pathogenic strains. The extract displayed weak effects against B. subtilis and S. typhimurium. However, it demonstrated significant antibacterial activity against L. innocua, with the largest inhibition zone diameter observed at 4 mg/disc. The extract also exhibited moderate activity against S. aureus, E. coli, and P. aeruginosa.
Table 8. Mean inhibition zone diameter (mm) of S. foetida extract on some pathogenic strain causing infection.
shows the minimum inhibitory concentrations (MICs) of S. foetida extract have been determined against several strains of microorganisms known to cause infectious diseases. The MIC values were recorded for B. subtilis, L. innocua, S. aureus, E. coli, P. aeruginosa, and S. typhimurium. These values indicate the concentration of the extract at which no visible growth of the respective microorganisms was observed.
Table 9. MIC values of S. foetida extract on some infective pathogenic strain.
Antihyperglycemic activity: The results of the in vitro screening revealed significant differences between S. foetida extracts and Metformin in terms of glucose levels and glucose uptake (). At a dose of 250 µg/mL, S. foetida extracts showed no inhibition of glucose levels, while metformin exhibited a substantial inhibitory effect (21 ± 1.00%). Similar trends were observed at higher doses of 500 µg/mL and 1 mg/mL. In terms of glucose uptake, S. foetida extracts at 250 µg/mL demonstrated a significant increase (59.18 ± 1.30%) compared to the minimal effect of Metformin (7.19 ± 1.95%). However, at 500 µg/mL, both S. foetida extracts (59.5 ± 0.0%) and Metformin (57.3 ± 0.00%) exhibited comparable glucose uptake. At the highest concentration of 1 mg/mL, no significant difference was observed between S. foetida extracts (69.7 ± 0.0%) and Metformin (70.04 ± 1.3) in terms of glucose uptake.
Table 10. In vitro screening of S. foetida extract for potential antidiabetic properties compared to metformin, with statistical significance determined using the Student’s t-test.
Discussion
Chemical profile of salsola foetida
Photosynthetic pigments play a crucial role in the process of photosynthesis, which is essential for the plant’s energy production and growth. Chlorophylls, including Chlorophyll a and Chlorophyll b, are the primary pigments responsible for capturing light energy during photosynthesis. They absorb light at specific wavelengths and transfer the energy to other molecules involved in the conversion of light energy into chemical energy [Citation32]. The content of Chlorophyll a and Chlorophyll b in S. foetida indicates the presence of a significant amount of chlorophylls, reflecting the plant’s capacity to efficiently capture light energy for photosynthesis. The relatively higher content of Chlorophyll b compared to Chlorophyll a suggests the potential adaptation of S. foetida to specific environmental conditions or growth strategies. The amount of chlorophyll in leaf tissues is affected by nutrient availability and environmental stresses such as drought, salinity, cold, heat, etc [Citation33]. Carotene, another important photosynthetic pigment, is responsible for absorbing light in different regions of the spectrum and transferring the energy to chlorophylls [Citation34]. The presence of Carotene in S. foetida indicates its contribution to light absorption and energy transfer processes during photosynthesis. The moisture content represents the amount of water present in the plant material. In this case, the aerial part of S. foetida was found to have a relatively high moisture content, indicating that it contains a significant amount of water. The moisture content of a plant material is an important parameter to consider as it can influence its stability, preservation, and processing. Additionally, the moisture content can impact the extraction efficiency of bioactive compounds present in the plant material [Citation35]. The ash content represents the inorganic residue left after the complete combustion of organic matter. It provides an estimate of the total mineral content present in the plant material. In the case of S. foetida, the ash content was determined to be 51.9%. The ash content is influenced by various factors, including the soil composition, plant species, and harvesting methods [Citation36]. The presence of a relatively high ash content suggests the presence of minerals and other inorganic components in the aerial part of S. foetida.
The dry matter EDX analysis of S. foetida showed differences in the weight of elements and oxides present in their aerial parts. This plant showed relatively low levels of sulfur, indicating their suitability for consumption as a food source [Citation37]. Additionally, it’s had higher levels of magnesium oxide and sodium, which could be beneficial for dietary intake.
presents the protein, carbohydrate, and lipid content of the aerial part of S. foetida. The results show that the highest content was observed for carbohydrates with a value of 8.99 ± 0.3 µg Glucose/mg DM. This indicates that S. foetida is rich in carbohydrates, which play a crucial role as a source of energy and structural components in plants [Citation38]. The protein content was found to be 6.69 ± 0.03 µg BSA/mg DM, suggesting a moderate protein concentration in the plant. Proteins are essential for various physiological processes and are involved in enzymatic reactions, cellular signaling, and structural support [Citation39]. On the other hand, lipids exhibited the lowest content at 3.18 ± 0.38 µg Soybean oil/mg DM. Lipids are important for energy storage and membrane structure in cells [Citation40]. These results contribute to a general understanding of the nutritional composition of S. foetida and provide valuable insights for potential applications in food and pharmaceutical industries. Further investigations are warranted to explore the specific types of carbohydrates, proteins, and lipids present in S. foetida and their potential bioactive properties.
Phytochemical profile of methanolic extract of Salsola foetida
The results of phytochemical screening contribute to the understanding of the chemical composition of the methanolic extract of the aerial part of S. foetida. The presence of alkaloids, terpenoids, saponins, coumarins, phenols, and tannins indicates the potential pharmacological and medicinal properties of the extract [Citation41]. This result is consistent with the findings of Ajaib [Citation11], who conducted phytochemical screening on the seeds and bark of the same plant. The screening revealed the presence of anthraquinones, reducing sugar, tannins, saponins, flavonoids, alkaloids, and cardiac glycosides.
The FT-IR analysis results confirmed the findings of the phytochemical screening, indicating that the methanol extract of S. foetida is a mixture of active compounds. The analysis revealed the presence of various functional groups, including alkanes, aliphatic amines, organic sulfur, aromatic functions, and phenol hydroxylase. The abundance of compounds in these extracts can be attributed to the crude extract’s nature, containing a wide range of phytochemical compounds, as well as the efficacy of methanol as the solvent for extracting a substantial content of phytochemical components [Citation42]. Additionally, the plant being in the germination stage may contribute to the presence of numerous compounds, as secondary metabolite production typically increases during flowering, particularly in leaves and flowers. These metabolites play a role in promoting flower and fruit formation and maturation. For instance, phenols and alkaloids can influence the behavior and foraging patterns of pollinators by either attracting or repelling them [Citation43, Citation44].
The results of RP-HPLC analysis () revealed the presence of several phenolic compounds, namely chlorogenic acid, rutin, quercetin, and vanillin, in the studied extract of S. foetida. Among these compounds, rutin was identified as the predominant one. However, other phenolic compounds such as gallic acid, caffeic acid, coumaric acid, and naringin were not detected. In comparison to a another study, the HPLC analysis of the aqueous ethanol extract obtained from the aerial part of the same plant grown in Pakistan demonstrated the presence of quercetin, gallic acid, syringic acid, benzoic acid, and chlorogenic acid [Citation45]. On the other hand, HPLC analysis of the same plant grown in Dubai identified 20 phenolic compounds, including coumaric acid, naringenin, quercetin, caffeic acid, gallic acid, as well as chlorogenic acid, vanillic acid, and rutin [Citation46]. In the case of S. foetida leaf extract cultivated in Saudi Arabia, HPLC analysis revealed the presence of the following compounds: catechin, vanillic acid, catechol, benzoic acid, β-hydroxy acid, caffeic acid, syringic acid, ferulic acid, rutin, ellagic acid, coumaric acid, resveratrol, quercetin, myristin, and kaempferol and health benefits [Citation47]. It is possible that the obvious difference in the presence and absence of some phenolic compounds between the results of the HPLC analyzes for one plant species is due to a difference between the analysis method, especially the difference in the mobile phase used and the wavelength. And the geographical location of plant growth also affects the phenolic compounds.
S. foetida had the largest content of total phenols, flavonoids, anthocyanins, condensed tannins, and soluble tannins; 35.5 ± 0.92 µg QE/mg, 2.96 ± 1.5 µg C3GE/mg, 5.89 ± 0.09 µg CE/mg, and 18.3 ± 1.1 µg GAE/mg respectively. It also had a significate content of saponins, flavonol, flavanone, and a very poor content of flavanol; 6.68 ± 0.09 µg DE/mg, 6.53 ± 0.09 µg QE/mg, 0.34 ± 0.00 µg NE/mg, and 0.2 ± 0.06 µg CE/mg respectively. In comparison with the results of the study by Al-Omar, et al. [Citation48] on the ethanol extract of the same plant species grown in the highly saline Al Qassim region in the Kingdom of Saudi Arabia, the total phenolic and flavonoids content were estimated at 360 mg/g and 70.5 ± 0.9 mg/g of the extract, they are very high contents compared to the current study.
The results of the quantitative estimates showed that the flavonoid content of S. foetida was diverse and characterized by a significant content of flavonols (). Flavonols can have different ecological functions that are closely related to environmental conditions. Laoué, et al. [Citation49] mentioned that rapid climate change represented by drought, heat, ultraviolet radiation and salinity can enhance the production of flavonols in plants. It may also be due to the high content of flavanols due to exposure of the plant to light or ultraviolet radiation for a long time, as the flavanols are considered as protectors of ultraviolet radiation and light, because they absorb strongly in both UV-A and UV-B wavelengths [Citation50].
Biological activity of methanolic extract of Salsola foetida
Antioxidant activity: The methanolic extract demonstrated effective radical scavenging activity with an IC50 value of 61.8 ± 0.2 μg/ml, while the positive control exhibited an IC50 value of 1.4 ± 0.2 μg/ml. This finding is comparable to the results obtained by Al-Omar [Citation48], who investigated the aqueous ethanol extract of the same plant and the same part (aerial part), reporting an IC50 value of 66.3 ± 2.3 μg/ml.
Generally, the effectiveness of plant extracts in β-carotene bleaching activity is due to their antioxidant properties. β-carotene is a precursor of vitamin A and is easily oxidized by reactive oxygen species (ROS) [Citation25, Citation51], whereas plant extracts are rich in antioxidants, including phenolic compounds, which are capable of scavenging ROS and preventing the oxidation of β-carotene [Citation41].
Based on the percentage of hemolysis induced by H2O2, it is evident that the extract exhibits a protective effect on biological aggregates against oxidative reactions caused by free radicals. Specifically, at a concentration of 1 mg/mL, the extract was able to safeguard over 60 percent of red blood cells. However, this protective activity is comparatively weaker when contrasted with the positive control, which exhibited such effects at a concentration of 0.25 mg/mL. This diminished activity could be attributed to the substantial saponin content of the plant (6.67 ± 0.09 µg DE/mg ED), as saponins are known to induce hemolysis, likely by increasing the permeability of the plasma membrane [Citation52]. According to what was obtained during this study, the extract had moderate reducing power (EC50 = 883 ± 1.7 µg/mL). In contrast, it had good activity in the reduction of Mo (VI) to Mo (V) by the phosphomolybdenum assays results (176.2 ± 0.3 µg GAE/mg).
Photoprotective activity: Determine the sun protection factor is an simple laboratory measure to determine the effectiveness of products in protection the skin from UV-A and UV-B rays, it means more protection and prevention of sunburn and other skin damage [Citation53, Citation54]. The methanol extract of the aerial part of S. foetida had the best SPF values (21.3 ± 0.03), due to its high content of phenols and flavonoids, specifically the anthocyanin content. Anthocyanins, a class of water-soluble flavonoids widely found in fruits and vegetables [Citation55], have been reported to exhibit photoprotective activity. Recent studies have demonstrated that anthocyanins can absorb UV radiation and have a sun protection factor (SPF) effect [Citation56]. The mechanism of action of anthocyanins in UV protection is mainly attributed to their strong antioxidant and free radical scavenging activities. In addition, some studies have suggested that anthocyanins can inhibit the synthesis of melanin and reduce the expression of matrix metalloproteinases (MMPs), which play important roles in skin photoaging [Citation57–59].
Anti-inflammatory activity: Through the data obtained from the assay of stabilization of protein against thermal denaturation, it is clear that the halophytes of S. foetida had anti-inflammatory activity, which was demonstrated by giving them a high percentage of protection from denaturation. Also, study for Osman, et al. [Citation60] this halophyte shown clear anti-inflammatory activities with no toxicity on RAW 264.7 macrophage cells. Several in vitro and in vivo studies have demonstrated the ability of halophyte extracts to inhibit the production of pro-inflammatory cytokines and enzymes, including TNF-α, IL-6, and COX-2 [Citation61, Citation62]. These findings suggest that halophyte extracts may be a promising source of natural anti-inflammatory agents. Additionally, the ability of halophytes to tolerate extreme environmental conditions may confer other unique properties, such as enhanced antioxidant activity, that could have further health benefits [Citation63].
Antibacterial activity: The following bacterial strains: L. innocua, S. aureus, E. coli, P. aeruginosa, and S. typhimurium had moderate sensitivity to S. foetida extract, and B. subtilis had weak sensitivity. These findings also suggested that S. foetida extract has the potential to be developed as an alternative treatment for infections caused by these pathogenic strains. In order to confirm the antibacterial activity of the studied plant extracts, MICs values were determined. Where the MICs values proved that the extracts had a clear growth inhibition on the six bacterial strains, where the values were mostly not more than 9 mg/mL. Kaur and Bains [Citation64] also studied the antimicrobial activity of the flavonoid extracts of the roots, fruits and shoots of this plant growing in India by agar disk-diffusion method against four bacterial strains (Staphylococcus aureus, Bacillus cereus, Salmonella typhimurium and Escherichia coli) and the pathogenic fungus Candida albicans. This study concluded that all the extracts did not show effectiveness against all the tested pathogens, except for the flavonoid extracts of the fruit, which showed effectiveness against Salmonella typhimurium only. Al-Omar [Citation48] also concluded that the aqueous ethanol extract of the aerial part of the same plant had no activity against methicillin-resistant Staphylococcus aureus, Enterococcus faecalis, Escherichia coli, Pseudomonas aeruginosa, Proteus mirabilis, Klebsiella pneumoniae, and Candida albicans. It had weak activity against Staphylococcus aureus. The difference between studies can be justified by the different types of extracts and their effect on the content of active compounds. In view of the activities provided by the methanol extract to the aerial part of S. foetida, it is possible to include it in foods or feed preparation as an antibiotic that helps stabilize the product.
In vitro antihyperglycemic activity: The results obtained from the three assays, glycosylation assay, glucose uptake by yeast cells, and non-enzymatic glycosylation of hemoglobin, showed that the S. foetida has antihyperglycemic activity and may be further developed as natural alternatives to standard antidiabetic drugs. Al-Omar [Citation48] and Khacheba [Citation17] confirmed that the extracts of Salsola foetida, had a strong inhibitory effect on both α-amylase and α-glucosidase enzymes. Several studies have also investigated the hypoglycemic effects of halophytes, including the genus Salsola. For example, a study conducted on the ethyl acetate extract of Salsola collina leaves found that the extract was able to reduce blood glucose levels in diabetic rats [Citation65]. Another study on Salsola arabica and Salsola villosa extract showed that it had significant anti-diabetic [Citation66]. The antihyperglycemic activity of S. foetida is likely attributed to its antioxidant content, particularly phenolic compounds. This is because it exhibits both direct and indirect antioxidant capacities by stimulating endogenous protective enzymes and exerting positive regulatory effects on signaling pathways. Its antioxidant power depends on the presence of substitution or glycosylation [Citation67]. It should also be noted that the content of the good plant S. foetida of good anthocyanins proved that the quantitative estimate for this study exceeded 2 µg C3GE/mg ED (), as anthocyanins are among the proposals as antidiabetic agents because they have valuable health effects documented in many from in vivo and in vitro studies. It can affect several signaling pathways for glucose metabolism [Citation67].
Conclusion
This work investigated the chemical and phytochemical profile, bioactive components, and biological activities of Salsola foetida. The plant contains significant amounts of chlorophylls and carotene, indicating efficient light absorption during photosynthesis. It also has a high carbohydrate content, moderate protein concentration, and low lipid content, suggesting potential nutritional value. The phytochemical analysis identified various compounds, including alkaloids, terpenoids, saponins, phenols, and tannins, indicating potential medicinal properties. The plant extract exhibited antioxidant activity, protection against oxidative reactions, photoprotective, antibacterial, anti-inflamatory, and antidiabetic properties. Salsola foetida shows promise as a source of bioactive compounds with potential applications in various industries, but further research is needed to explore specific compounds and their bioactive properties.
Ethical approval and consent to participate
The study does not involve human or animal trials.
Disclosure statement
No potential conflict of interest was reported by the author(s).
References
- Hulkko, L.S.S., R.M. Rocha, R. Trentin, M. Fredsgaard, T. Chaturvedi, L. Custódio, and M.H. Thomsen. Bioactive Extracts from Salicornia Ramosissima J. Woods Biorefinery As a Source of Ingredients for High-Value Industries. Plants. 2023, 12(6), 1251. DOI: 10.3390/plants12061251.
- Radha, M. Kumar, S. Puri, A. Pundir, S.P. Bangar, S. Changan, P. Choudhary, E. Parameswari, A. Alhariri, M.K. Samota, R.D. Damale, S. Singh, M.K. Berwal, S. Dhumal, A.G. Bhoite, M. Senapathy, A. Sharma, B. Bhushan, and M. Mekhemar. Evaluation of Nutritional, Phytochemical, and Mineral Composition of Selected Medicinal Plants for Therapeutic Uses from Cold Desert of Western Himalaya. Plants. 2021, 10(7), 1429. DOI: 10.3390/plants10071429.
- Shahid, M.; Singh, R. K.; Thushar, S. Proximate Composition and Nutritional Values of Selected Wild Plants of the United Arab Emirates. Molecules. 2023, 28(3), 1504. DOI: 10.3390/molecules28031504.
- Sukhorukov, A., P.-L. Liu, and M. Kushunina. Taxonomic Revision of Chenopodiaceae in Himalaya and Tibet. PhytoKeys. 2019, 116, 1–141. DOI: 10.3897/phytokeys.116.27301.
- Murshid, S.S.A., D. Atoum, D.R. Abou-Hussein, H.M. Abdallah, R.H. Hareeri, H. Almukadi, and R. Edrada-Ebel. Genus Salsola: Chemistry, Biological Activities and Future Prospective—A Review. Plants (Basel). 2022, 11(6), 714. DOI: 10.3390/plants11060714.
- Nyerges, C. Foraging Wild Edible Plants of North America: More Than 150 Delicious Recipes Using Nature’s Edibles; Rowman & Littlefield: US.
- Barkoudah, Y. and J. Henderson. Plant Ashes from Syria and the Manufacture of Ancient Glass: Ethnographic and Scientific Aspects. J. Glass Stud. 2006, 297–321.
- Tite, M.s., A. Shortland, Y. Maniatis, D. Kavoussanaki, and S. Harris. The Composition of the Soda-Rich and Mixed Alkali Plant Ashes Used in the Production of Glass. J. Archaeol. Sci. 2006, 33(9), 1284–1292. DOI: 10.1016/j.jas.2006.01.004.
- Öztürk, M., V. Altay, E. Altundağ, and S. Gücel. 18 - Halophytic Plant Diversity of Unique Habitats in Turkey: Salt Mine Caves of Çankırı and Iğdır, in Halophytes for Food Security in Dry Lands; M.A. Khan; Editors. Academic Press: US, 2016; 291–315.
- Singh, P., Y. Shivhare, A. Singhai, and A. Sharma. Pharmacological and Phytochemical Profile of Chenopodium album Linn. Res. J. Pharm. Technol. 2010, 3(4), 960–963.
- Ajaib, M., S. Farooq, K.M. Khan, S. Perveen, and S. Shah. Phytochemical Analysis and Anthelmintic Activity of Salsola Imbricata. J. Chem. Soc. Pak. 2019, 41(1), 198–198. DOI: 10.52568/000714/JCSP/41.01.2019.
- Aslam, N.; Janbaz, K. H. Antispasmodic and Bronchorelaxant Activities of Salsola Imbricata Are Mediated Through Dual Ca+ 2 Antagonistic and β-Adrenergic Agonistic Effects. Pharm. Biol. 2017, 55(1), 1131–1137. DOI: 10.1080/13880209.2017.1291691.
- Shehab, N. G.; Abu-Gharbieh, E. Phenolic Profiling and Evaluation of Contraceptive Effect of the Ethanolic Extract of Salsola Imbricata Forssk. in Male Albino Rats. Evid. Based Complement. Altern. Med. 2014, 2014, 1–8. DOI: 10.1155/2014/695291.
- Osman, S.M., W.A. El Kashak, M. Wink, and M.A. El Raey. New Isorhamnetin Derivatives from Salsola Imbricata Forssk. Leaves with Distinct Anti-Inflammatory Activity. Pharmacogn. Mag. 2016, 12(Suppl 1), S47. DOI: 10.4103/0973-1296.176110.
- Shehab, N. G.; Abu-Gharbieh, E.; Bayoumi, F. A. Impact of Phenolic Composition on Hepatoprotective and Antioxidant Effects of Four Desert Medicinal Plants. BMC Complementary Altern. Med. 2015, 15(1), 1–12. DOI: 10.1186/s12906-015-0919-6.
- Gannoun, S., A. Mahfoudhi, G. Flamini, A. Helal, and Z. Mighri. Chemical Composition and Antimicrobial Activities of Tunisian Salsola Vermiculata L. 2016.
- Khacheba, I., A. Djeridane, A. Kameli, and M. Yousfi. The Inhibitory Effect of Some Algerian Plants Phenolics Extracts on the α-Glucosidase and α-Amylase Activities and Their Antioxidant Activities. Curr. Enzyme Inhib. 2014, 10(1), 59–67. DOI: 10.2174/15734080113099990001.
- Khan, K.M., G.M. Maharvi, A. Abbaskhan, S. Hayat, M.T.H. Khan, T. Makhmoor, M.I. Choudhary, and F. Shaheen, Atta-Ur-Rahman. Three Tyrosinase Inhibitors and Antioxidant Compounds from Salsola Foetida. Helv. Chim. Acta. 2003, 86(2), 457–464. DOI: 10.1002/hlca.200390045.
- Radwan, D.E.M. and D.M. Soltan. The Negative Effects of Clethodim in Photosynthesis and Gas-Exchange Status of Maize Plants Are Ameliorated by Salicylic Acid Pretreatment. Photosynthetica. 2012, 50(2), 171–179. DOI: 10.1007/s11099-012-0016-8.
- Afify, A., A. Abdallah, A. Elsayed, B. Gamuhay, A. Sabry, M. Hassan, M. Ataalla, and A. Mohamed. Survey on the Moisture and Ash Contents in Agricultural Commodities in Al-Rass Governorate, Saudi Arabia in 2017. Assuit J. Agri. Sci. 2017, 48(6), 55–62. DOI: 10.21608/ajas.1999.5752.
- Gheraissa, N., A.E. Chemsa, N. Cherrada, E. Erol, E.R. Elsharkawy, D. Ghemam-Amara, S. Zeghoud, A. Rebiai, M. Messaoudi, B. Sawicka, M. Atanassova, and M.S. Abdel-Kader. Biochemical Profile and In Vitro Therapeutic Properties of Two Euhalophytes, Halocnemum Strobilaceum Pall. and Suaeda Fruticosa (L.) Forske. Grown in the Sabkha Ecosystem in the Algerian Sahara. Molecules. 2023, 28(8), 3580. DOI: 10.3390/molecules28083580.
- Chouikh, A., A.E. Chemsa, C. Aounallah, I. Aounallah, and F. Alia. Phytochemical Study, Nutritive Value, Antioxidant and Anti-Inflammatory Activities of Phenolic Extracts from Desert Plant Calligonum Comosum L’Hér. Algerian J. Biosci. 2020, 1(2), 68–75. DOI: 10.57056/ajb.v1i2.29.
- Zeghib, K. Evaluation of the Pharmacological Effects of Atriplex Halimus L. Aqueous Extract Against Pathophysiological Alterations Induced by Benzene and Sodium Benzoate (Food Additive): Experimental Study in Rats; University of Eloued, 2020.
- Gheraissa, N., A.E. Chemsa, E. Elsharkawy, and N. Cherrada. Phenolic Compound Profile, and Evaluation of Biological Properties of Bassia muricata (L.) Asch. Aerial Part. Int. J. Sec. Metabo. 2022, 9(3), 335–347. DOI: 10.21448/ijsm.1080537.
- Chekroun-Bechlaghem, N., N. Belyagoubi-Benhammou, L. Belyagoubi, A. Gismondi, V. Nanni, G. Di Marco, L. Canuti, A. Canini, I.A. El Haci, and F. Atik Bekkara. Phytochemical Analysis and Antioxidant Activity of Tamarix africana, Arthrocnemum Macrostachyum and Suaeda fruticosa, Three Halophyte Species from Algeria. Plant Biosystems - An Int. J. Deal. Asp. Plant Biol. 2019, 153(6), 843–852. DOI: 10.1080/11263504.2018.1555191.
- Muthukrishnan, S.; Kumar, T. S.; Gangaprasad, A.; Maggi, F.; Rao, M. V. Phytochemical Analysis, Antioxidant and Antimicrobial Activity of Wild and In Vitro Derived Plants of Ceropegia Thwaitesii Hook – an Endemic Species from Western Ghats, India. J. Genet. Eng. Biotechnol. 2018, 16(2), 621–630. DOI: 10.1016/j.jgeb.2018.06.003.
- Mansur, J.d.S., M.N.R. Breder, M.C.d.A. Mansur, and R.D. Azulay. Determinaçäo Do Fator de proteçäo Solar Por Espectrofotometria/Determination of Sun Protection Factor by Spectrophotometry. An. Bras. Dermatol. 1986, 121–124.
- Bbosa, G., D. Kyegombe, J. Ogwal-Okeng, R. Bukenya-Ziraba, O. Odyek, and P. Waako. Antibacterial Activity of Mangifera Indica (L.). African J. Ecol. 2007, 45, 13–16. DOI: 10.1111/j.1365-2028.2007.00731.x.
- Kebieche, M. Activité biochimique des extraits flavonoïdiques de la plante Ranunculus repens L.: effet sur le diabète expérimental et l’hépatotoxicité induite par l’Epirubicine; Biochimie, These de Doctorat: US, 2009.
- Nagarajan, Y., M. Ali, and V. Anuradha. In Vitro Evaluation of Antidiabetic Potential of Gymnema Sylvestre and Dregea Volubilis a Medicinally Important Asclepiadaceae Plant. Int. J. Comp. Res. Biol. Sci. 2014, 1(1), 9–12.
- Gheraissa, N., A.E. Chemsa, N. Cherrada, E. Erol, and E. Elsharkawy. Anabasis Oropediorum Maire. As< a Health-Promoting Source: Phytochemical Content, in vitro Antioxidant, Antidiabetic, Antibacterial, and Anti-Inflammatory Potential. J. Res. Pharm. 2023, 27(5)(27(5)), 1924–1935. DOI: 10.29228/jrp.2023.00.
- Kuczynska, P.; Jemiola-Rzeminska, M.; Strzalka, K. Photosynthetic Pigments in Diatoms. Mar. Drugs. 2015, 13(9), 5847–5881. DOI: 10.3390/md13095847.
- Palta, J.P. Leaf Chlorophyll Content. Rem. Sens. Rev. 1990, 5(1), 207–213. DOI: 10.1080/02757259009532129.
- Maoka, T. Carotenoids as Natural Functional Pigments. J. Nat. Med. 2020, 74(1), 1–16. DOI: 10.1007/s11418-019-01364-x.
- Jha, A. K.; Sit, N. Extraction of Bioactive Compounds from Plant Materials Using Combination of Various Novel Methods: A Review. Trends Food Sci. Technol. 2022, 119, 579–591. DOI: 10.1016/j.tifs.2021.11.019.
- Bridgeman, T.G., L.I. Darvell, J.M. Jones, P.T. Williams, R. Fahmi, A.V. Bridgwater, T. Barraclough, I. Shield, N. Yates, S.C. Thain, and I.S. Donnison. Influence of Particle Size on the Analytical and Chemical Properties of Two Energy Crops. Fuel. 2007, 86(1), 60–72. DOI: 10.1016/j.fuel.2006.06.022.
- Komarnisky, L.A., R.J. Christopherson, and T.K. Basu. Sulfur: Its Clinical and Toxicologic Aspects. Nutrition. 2003, 19(1), 54–61. DOI: 10.1016/S0899-9007(02)00833-X.
- Hartmann, H.; Adams, H. D.; Hammond, W. M.; Hoch, G.; Landhäusser, S. M.; Wiley, E.; Zaehle, S. Identifying Differences in Carbohydrate Dynamics of Seedlings and Mature Trees to Improve Carbon Allocation in Models for Trees and Forests. Environ. Exp. Bot. 2018, 152, 7–18. DOI: 10.1016/j.envexpbot.2018.03.011.
- Brown, M.V., J.S. Reader, and E. Tzima. Mammalian Aminoacyl-tRNA Synthetases: Cell Signaling Functions of the Protein Translation Machinery. Vasc. Pharmacol. 2010, 52(1), 21–26. DOI: 10.1016/j.vph.2009.11.009.
- Welte, M.A. and A.P. Gould. Lipid Droplet Functions Beyond Energy Storage. Biochimica Et Biophysica Acta (BBA) - Molecular And Cell Biology Of Lipids. 2017, 1862(10, Part B), 1260–1272. DOI: 10.1016/j.bbalip.2017.07.006.
- Mujeeb, F., P. Bajpai, and N. Pathak. Phytochemical Evaluation, Antimicrobial Activity, and Determination of Bioactive Components from Leaves of Aegle Marmelos. Biomed Res. Int. 2014, 2014, 497606. DOI: 10.1155/2014/497606.
- Truong, H.; Nguyen, D.; Ta, N.; Bui Anh, V.; Do, T.; Nguyen, H. C. Evaluation of the Use of Different Solvents for Phytochemical Constituents, Antioxidants, and in vitro Anti-Inflammatory Activities of Severinia Buxifolia. J. Food Qual. 2019, 2019, 1–9. DOI: 10.1155/2019/8178294.
- Teoh, E.S. Secondary Metabolites of Plants, in Medicinal Orchids of Asia; Springer International Publishing: US, 2016; pp 59–73.
- Sosenski, P. and V. Parra-Tabla. Secondary Metabolites: Attracting Pollinators, in eLS; 1–9.
- Javed, F.; Jabeen, Q. Salsola Imbricata Forssk. Ameliorates Acetic Acid-Induced Inflammatory Bowel Disease by Modulating Dysregulated Antioxidant Enzyme System and Cytokine Signaling Pathways in Mice. Asian Pac. J. Trop. Biomed. 2021, 11(12), 527. DOI: 10.4103/2221-1691.331268.
- Shehab, N.G. and E. Abu-Gharbieh. Phenolic Profiling and Evaluation of Contraceptive Effect of the Ethanolic Extract of Salsola Imbricata Forssk. in Male Albino Rats. Evid. Based Complement. Alternat. Med. 2014, 2014, 695291. DOI: 10.1155/2014/695291.
- Soliman, M.M., S.S. Alotaibi, S. Sayed, M.M. Hassan, F. Althobaiti, A. Aldhahrani, G. Youssef, and A.M. El-Shehawi. The Protective Impact of Salsola Imbricata Leaf Extract from Taif Against Acrylamide-Induced Hepatic Inflammation and Oxidative Damage: The Role of Antioxidants, Cytokines, and Apoptosis-Associated Genes. Front. Vet. Sci. 2022, 8, 1661. DOI: 10.3389/fvets.2021.817183.
- Al-Omar, M.S., H.A. Mohammed, S.A.A. Mohammed, E. Abd-Elmoniem, Y.I. Kandil, H.M. Eldeeb, S. Chigurupati, G.M. Sulaiman, H.K. Al-Khurayyif, B.S. Almansour, P.M. Suryavamshi, and R.A. Khan. Anti-Microbial, Anti-Oxidant, and α-Amylase Inhibitory Activity of Traditionally-Used Medicinal Herbs: A Comparative Analyses of Pharmacology, and Phytoconstituents of Regional Halophytic Plants’ Diaspora. Molecules. 2020, 25(22), 5457. DOI: 10.3390/molecules25225457.
- Laoué, J., C. Fernandez, and E. Ormeño. Plant Flavonoids in Mediterranean Species: A Focus on Flavonols As Protective Metabolites Under Climate Stress. Plants. 2022, 11(2), 172. DOI: 10.3390/plants11020172.
- Flamini, R.; Mattivi, F.; De Rosso, M.; Arapitsas, P.; Bavaresco, L. Advanced Knowledge of Three Important Classes of Grape Phenolics: Anthocyanins, Stilbenes and Flavonols. Int. J. Mol. Sci. 2013, 14(10), 19651–19669. DOI: 10.3390/ijms141019651.
- Grune, T., G. Lietz, A. Palou, A.C. Ross, W. Stahl, G. Tang, D. Thurnham, S.A. Yin, and H.K. Biesalski. Beta-Carotene Is an Important Vitamin a Source for Humans. J. Nutr. 2010, 140(12), 2268s–2285s. DOI: 10.3945/jn.109.119024.
- Das, T.K., D. Banerjee, D. Chakraborty, M.C. Pakhira, B. Shrivastava, and R. Kuhad. Saponin: Role in Animal System. Vet. World. 2012, 5(4), 248–254. DOI: 10.5455/vetworld.2012.248-254.
- Khan, M. A. Sun Protection Factor Determination Studies of Some Sunscreen Formulations Used in Cosmetics for Their Selection. J. Drug Delivery Ther. 2018, 8(5–s), 149–151. DOI: 10.22270/jddt.v8i5-s.1924.
- Sharma, T.; Tyagi, V.; Bansal, M. Determination of Sun Protection Factor of Vegetable and Fruit Extracts Using UV–Visible Spectroscopy: A Green Approach. Sustainable Chem. Pharm. 2020, 18, 100347. DOI: 10.1016/j.scp.2020.100347.
- Mattioli, R.; Francioso, A.; Mosca, L.; Silva, P. Anthocyanins: A Comprehensive Review of Their Chemical Properties and Health Effects on Cardiovascular and Neurodegenerative Diseases. Molecules. 2020, 25(17). DOI: 10.3390/molecules25173809.
- Chan, C. F.; Lien, C. Y.; Lai, Y. C.; Huang, C. L.; Liao, W. C. Influence of Purple Sweet Potato Extracts on the UV Absorption Properties of a Cosmetic Cream. J. Cosmet. Sci. 2010, 61(5), 333–341.
- Hwang, J. M.; Kuo, H. C.; Lin, C. T.; Kao, E. S. Inhibitory Effect of Liposome-Encapsulated Anthocyanin on Melanogenesis in Human Melanocytes. Pharm. Biol. 2013, 51(8), 941–947. DOI: 10.3109/13880209.2013.771376.
- Karunarathne, W., I.M.N. Molagoda, S.R. Park, J.W. Kim, O.K. Lee, H.Y. Kwon, M. Oren, Y.H. Choi, H.W. Ryu, S.R. Oh, W.S. Jo, K.T. Lee, and G.Y. Kim. Anthocyanins from Hibiscus syriacus L. Inhibit Melanogenesis by Activating the ERK Signaling Pathway. Biomolecules. 2019, 9(11), DOI: 10.3390/biom9110645.
- Shin, D.Y., J.N. Lu, G.Y. Kim, J.M. Jung, H.S. Kang, W.S. Lee, and Y.H. Choi. Anti-Invasive Activities of Anthocyanins Through Modulation of Tight Junctions and Suppression of Matrix Metalloproteinase Activities in HCT-116 Human Colon Carcinoma Cells. Oncol. Rep. 2011, 25(2), 567–572. DOI: 10.3892/or.2010.1104.
- Osman, S.M., W.A. El Kashak, M. Wink, and M.A. El Raey. New Isorhamnetin Derivatives from Salsola Imbricata Forssk. Leaves with Distinct Anti-Inflammatory Activity. Pharmacogn. Mag. 2016, 12(Suppl 1), S47–51. DOI: 10.4103/0973-1296.176110.
- Hsouna, A.B., S. Dhibi, W. Dhifi, R.B. Saad, F. Brini, N. Hfaidh, and W. Mnif. Essential Oil from Halophyte Lobularia Maritima: Protective Effects Against CCl(4)-Induced Hepatic Oxidative Damage in Rats and Inhibition of the Production of Proinflammatory Gene Expression by Lipopolysaccharide-Stimulated RAW 264.7 Macrophages. R.S.C. Adv. 2019, 9(63), 36758–36770. DOI: 10.1039/c9ra05885k.
- Abdallah, H. M.; Esmat, A. Antioxidant and Anti-Inflammatory Activities of the Major Phenolics from Zygophyllum Simplex L. J. Ethnopharmacol. 2017, 205, 51–56. DOI: 10.1016/j.jep.2017.04.022.
- Chagas, M.d.S.S., M.D. Behrens, C.J. Moragas-Tellis, G.X.M. Penedo, A.R. Silva, and C.F. Gonçalves-de-Albuquerque. Flavonols and Flavones As Potential Anti-Inflammatory, Antioxidant, and Antibacterial Compounds. Oxid. Med. Cell. Longev. 2022, 2022, 9966750. DOI: 10.1155/2022/9966750.
- Kaur, P. and N.S. Bains. Antimicrobial Activity of Flavonoids from in vitro Tissue Culture and Plant Parts of Important Halophytes of Western Rajasthan. Current Biotica. 2012, 6(1), 1–7.
- Zhao, X.; Wang, H.; Zhang, Z.; Jin, H.; Gong, Y. Effects of Ethyl Acetate Extract of Salsola Collina on Brain-Gut Peptides and Interstitial Cells of Gastric Cajal in Rats with Diabetic Gastroparesis. Iran. J. Basic Med. Sci. 2020, 23(9), 1218–1224. DOI: 10.22038/ijbms.2020.43521.10223.
- Amin, E., M.S. Abdel-Bakky, M.A. Darwish, H.A. Mohammed, S. Chigurupati, K.A. Qureshi, and M.H.A. Hassan. The Glycemic Control Potential of Some Amaranthaceae Plants, with Particular Reference to in vivo Antidiabetic Potential of Agathophora Alopecuroides. Molecules. 2022, 27(3), 973. DOI: 10.3390/molecules27030973.
- Shehadeh, M.B., G. Suaifan, and A.M. Abu-Odeh. Plants Secondary Metabolites As Blood Glucose-Lowering Molecules. Molecules. 2021, 26(14), 4333. DOI: 10.3390/molecules26144333.