ABSTRACT
Background
Application of supraclavicular block generally comprises elbow, forearm, and hand surgery. Tramadol and dexamethasone were tested and compared in the current study as adjuvants to levobupivacaine in the supraclavicular block.
Patients and Methods
60 ASA Grade I and II patients of either sex, older than 18, participated in the current study. Two groups of patients were created: Group D (n = 30), who received 30 ml of 0.5% levobupivacaine and 2 ml of dexamethasone (8 mg). 30 ml of 0.5% levobupivacaine and 2 ml of 5% tramadol (100 mg) were given to Group T (n = 30). Statistics were used to compare the two groups.
Results
The tramadol group required substantially less time (13.4 ± 2.6 h) than the dexamethasone group (15.3 ± 2.8 h) for the first rescue analgesia request (P-value = 0.009). Within the first 24 hours following surgery, the mean total dose of rescue analgesia was considerably lower in group D (36 ± 12.2 mg) than in group T (44 ± 15.2 mg, P-value = 0.029). Group D had no side effects and considerably higher patient satisfaction (P = 0.042).
Conclusion
When levobupivacaine was used with dexamethasone in a supraclavicular brachial plexus block for forearm fractures, it worked better as an adjuvant than tramadol. Sensory and motor blockage, a quicker onset and longer duration of analgesia, and higher satisfaction levels were all present.
1. Introduction
Brachial plexus nerve blocks (BPBs) offer greater analgesia and lower the need for opioids during upper extremity surgery [Citation1].Footnote1 The effects of single-injection brachial plexus nerve blocks invariably wear off many hours after the surgical insult’s moderate to severe pain is revealed. Although recent research has shown that analgesic duration can be equivalently achieved with doses as low as 5 ml, efforts to extend the duration of brachial plexus nerve blocks by increasing local anesthetic dose are constrained by their small therapeutic window. They may even be ineffective [Citation2–4].
Commercial preparations of levobupivacaine have lower toxicity than bupivacaine and are a racemic mixture of its two enantiomers, levobupivacaine, S (-) isomer, and dextro-bupivacaine, R (+) isomer, are available. It has been demonstrated that the levorotatory isomers have a safer pharmacological profile with fewer deleterious cardiac and neurological effects [Citation5].
Placement of indwelling perineural catheters to allow prolonged infusion or the co-administration of adjuvants such as epinephrine, two agonists (i.e., clonidine and dexmedetomidine), midazolam, or dexamethasone are methods to extend brachial plexus nerve blocks analgesia past the pharmacological duration of the local anesthetic used [Citation6–8].
A peripheral nerve block is more effective and lasts longer when dexamethasone is used. This is supposed to be accomplished by lowering ectopic neuronal firing, attenuating the release of inflammatory mediators, and blocking the release of nociceptive C-fibers via potassium channels [Citation9–14].
Tramadol is a special type of opioid that inhibits pain in two ways, one mediated by the receptor and the other by the activity of the α 2-adrenergic and serotoninergic systems [Citation15,Citation16]. Tramadol suppresses the descending pain pathways by acting on monoamine receptors, which block nociceptive transmission at the spinal level [Citation17]. By inhibiting K+ channels, tramadol also has local anesthetic effects [Citation14]. The effects of tramadol as a local anesthetic adjuvant in brachial plexus block have been extensively studied [Citation18]. The current study assessed and compared Dexamethasone versus tramadol as adjuvants to levobupivacaine in the supraclavicular block.
2. Patients and methods
This randomized, prospective, double-blind comparative study received ethical approval from the Medical Research Ethics Council of the Faculty of Medicine (IRB 17,101,156). The research was done between the first of April 2020 and the last day of December 2020. It followed the principles of the Helsinki Declaration and was tracked on ClinicalTrials.gov (NCT0455188). All participants in this study don’t pay any charge for their medical services. The quality of care received by patients who declined to participate in the trial was unaffected. All patients provided informed written consent, and data confidentiality was maintained throughout the study. After describing the study’s purpose and introducing itself to each participant, the researcher asked them to participate. All patients were given thorough explanations of the study’s goals, anticipated advantages, complications, and disadvantages of the intervention. The entire project was conducted with the utmost ethical attention.
Sixty patients (older than 18 years) of both sexes, ASA I-II with forearm fractures scheduled for internal fixation. Patients with coagulopathy or who were taking anticoagulants, infections near the site of the needle insertion, BMI >40 kg/m2, Significant organ dysfunction, drug, or alcohol abuse, neurological or neuromuscular diseases, patients with a history of epilepsy, any psychiatric conditions that might affect perception and assessment of pain, patient refusal, and patients with known allergies to a drug used in the study were excluded.
Before surgery, patients were instructed to assess their pain level using the Visual Analog Scale (VAS), which ranges from 0 to 10 and measures acute postoperative pain [Citation19].
3. Randomization and blindness
Patients were randomly allocated using a computer-generated randomizer program into one of 2 groups.
3.1. Group (D)
30 patients (dexamethasone group):
The patient received (30 ml) of 0.5% levobupivacaine plus (2 ml) 8 mg of dexamethasone.
3.2. Group (T)
30 patients (tramadol group):
The patient received (30 ml) of 0.5% levobupivacaine plus (2 ml) of 5% tramadol.
To avoid bias, infiltrate a total volume of 32 ml in each group.
A researcher not included in the intervention and patient observation prepared the research medications in a similar syringe. The syringe codes were kept in opaque envelopes with numbers from 1 to 60. The codes on the envelopes were only accessible to one anesthesiologist who packaged the envelopes, and he was not a study participant. All trial participants must know their group status, including the patients and the researchers who provided postoperative care.
The patients were positioned supinely in the operating room, and an 18-gauge catheter was put intravenously in the dorsum of the contralateral hand. 10 ml/kg of lactated ringer solution was administered intravenously over 10 minutes before the start of the supraclavicular block. The standard monitoring techniques included electrocardiography, non-invasive blood pressure, oxygen saturation, respiration rate, and temperature. By injecting 1–2 mg of midazolam, all groups were administered pre-emptive conscious sedation.
4. Study protocol
The supraclavicular block was carried out under ultrasound guidance using a linear multi-frequency 6–13 MHz transducer scanning probe and the Mindray Enterprise 8000. Sono Plex Stim canula (PAJUNK) with an echogenic needle of 22 gauge and 60 mm length was employed.
The ultrasound machine was on one side, while the anesthesiologist was behind the patient’s head. Without a pillow, the patient’s head was directly on the operation table, facing the opposite direction. The patient’s jaw was being pressed by the hand carrying the probe. The coronal oblique plane of the supraclavicular fossa received the probe. The lateral side was used to insert the needle. It was possible to identify the pulsing hypoechoic supraclavicular artery. The bed’s head was slightly lifted to allow for a small amount of shoulder flexion and opening of the supraclavicular joint. The pleura and first rib could be seen because of the probe’s position. The subclavian artery is generally surrounded by a collection of hypoechoic circular formations that look like a bunch of grapes and are superior and posterolateral to the subclavian artery, typically representing the nerve structures (trunks or divisions).
After sterilization and local anesthetic infiltration, the needle was advanced longitudinally to the ultrasound probe (in-plane approach); the shaft and the tip could be seen up until the commencement of sensory and motor blockade; sensory blockade of the circumflex, musculocutaneous, and radial cutaneous nerves of the arm was assessed every 5 minutes.
It was recorded when the medicine was injected. Patients were assessed every two minutes until the sensory and motor blocks were formed. Patients who did not have complete sensory or motor blockade after 45 minutes were removed from the research, and the attending anesthesiologist decided to provide additional anesthetic care. The sensory block was demonstrated by the loss of cold sensation in all dermatomes supplied by the brachial plexus when cotton was drenched in ether.
Patients were asked to elevate their arms (circumflex nerve), abduct, or adduct their thumbs (radial/ulnar nerve), oppose their thumbs (median nerve), and flex their forearms on their arms (musculocutaneous nerve) to test for motor block (musculocutaneous nerve). Patients were regarded to have a complete motor block when they could not actively raise their hands or move them. This moment was documented and recorded as the beginning of the motor blockade. If required, fentanyl I.V. incremental boluses were added to the intraoperative analgesia 50 ug up to a 100 ug maximum dose. The supraclavicular block was considered unsuccessful if a patient required more than 100 ug of fentanyl to complete the surgery. If fentanyl augmentation wasn’t enough to finish the procedure, propofol intravenously was used to administer general anesthesia (GA): Laryngeal mask airway and infusion. Those patients with unsuccessful blocks were excluded from the study.
After finishing the surgery, the patient was transferred to the post-anesthesia care unit (PACU), where visual analog scale (VAS) scores were used to quantify block regression and postoperative pain (ranging from 0 = no pain to 10 = the worst pain imaginable). Upon arrival at the PACU (time 0), the VAS score was assessed every hour for the following two hours. And then every two hours for the following 12 hours, and then every six hours up to 24 hours postoperatively in the ward. Rescue analgesia of 30 mg of ketorolac IV was administered at the patient’s request or when the VAS was above 4. The time it lasted before the first rescue analgesia was recorded.
For 24 hours after surgery, a nurse in the ward was also blinded to the drug administered; the patients were monitored for any adverse symptoms like pruritus, nausea, vomiting, hypotension, bradycardia, drowsiness, and neurologic abnormalities.
A 5-point Likert scale was used to evaluate patient satisfaction with the effectiveness of supraclavicular block, with 1 representing total dissatisfaction and 5 representing perfect satisfaction [Citation20].
The patients were returned home when pain-free and had no residual motor or sensory block. A second anesthesiologist in the PACU performed a postoperative assessment that was blind to the medicine administered, as was a nurse in the ward.
Length of analgesia was defined as the interval from the commencement of the sensory blocking, the patient’s pain score (VAS) reaching higher than four, and the delivery of rescue analgesia. Motor recovery was measured by the patient’s ability to squeeze the examiner’s hand.
5. Outcomes
The primary outcome was the first postoperative request for analgesia following surgery, or more specifically, the time from the supraclavicular injection to the first rescue analgesic. The secondary outcomes were the secondary outcomes of the length of effective analgesia, the time needed to establish sufficient motor and sensory block, the total amount of analgesia needed, the frequency of intraoperative hemodynamic changes, and side effects of itching, hypotension, nausea, and vomiting.
5.1. Power of the study
The time to the first analgesic request was the study’s primary outcome. Based on a preliminary analysis with T-tests and the G-Power calculator 3.1.9.7, a total sample size of 52 patients (26 in each group) would be sufficient for statistical testing. - Means: Difference between two independent means (two groups), with an effect size of 0.7, power of 0.8, and one-tailed type I error of 0.05. Four additional participants were added to each group to prevent patient dropout.
6. Statistical analysis
SPSS (statistical software for social science; SPSS Inc., Chicago, IL, USA) version 22 was used for all statistical calculations. Data were statistically represented as median and range when they were not normally distributed, mean and standard deviation (SD), frequencies (number of cases), and relative frequencies (percentages), as appropriate. The Mann-Whitney U test for non-normally distributed data and a student t-test for normally distributed data were used to compare quantitative variables. The Fisher’s exact or chi-square tests were used to compare categorical variables. Statistics were considered significant for P values < 0.05.
7. Results
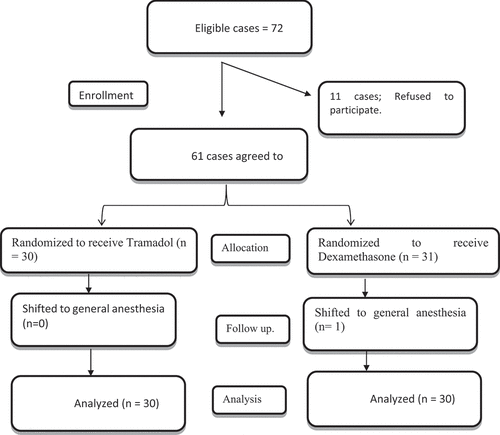
Sixty-one cases were randomly assigned to receive either Dexamethasone (n = 31) or Tramadol (n = 30), and finally, each arm had 30 cases (). Seventy-two patients were eligible for inclusion in the current study; 11 instances were refused participation in the current investigation ().
Age, gender, height, weight, ASA class, type, and length of surgery did not significantly differ between the two groups; neither did their height or weight ().
Table 1. Demographic data, type, and duration of surgery.
Hemodynamics: The mean arterial pressure (MAP), mean heart rate (HR), and mean oxygen saturation (SpO2) were not significantly different across the groups at any point during the study (data not presented).
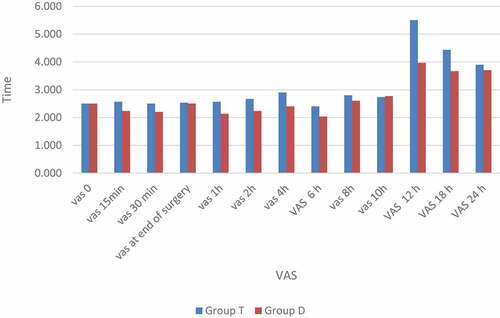
The time to reach the motor block was significantly faster in the dexamethasone group (p = 0.000), and the time to the onset of the sensory block was significantly slower in the dexamethasone group (p = 0.006). The mean duration time of analgesia in Group D was 16.3 ± 0.75 hr., and in Group T was 13.8 ± 0.76 hr. This difference was statistically significant (P < 0.001) ().
Table 2. Comparison of sensory and motor blockade (minutes), time of first rescue of analgesia (hours), total consumption, and numbers of patients’ requests for rescue analgesia between both studied groups.
Postoperative pain profile: The dexamethasone group consumed significantly less rescue analgesia overall in the first 24 hours postoperatively than the tramadol group, and the mean time to first request for IV ketorolac rescue analgesia was significantly longer in the dexamethasone group (15.3 ± 2.8) than in the tramadol group (13.4 ± 2.6; p = 0.009) (p < 0.05) ().
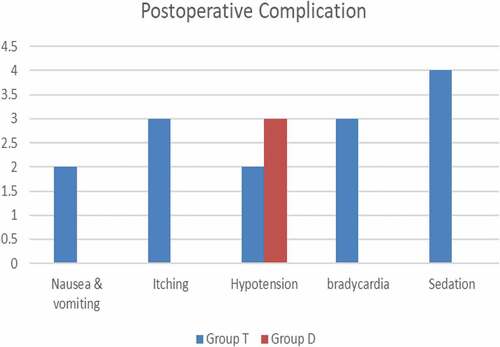
In Group D, 27 patients did not experience any side effects, compared with 16 patients in Group T. Sedation was much more frequent in Group T than in Group D. Still, no other side effects showed a significant difference between the two groups. None of the patients complained of neurological problems. ().
Regarding patient satisfaction, the “Likert scale” showed that 96.6% of the dexamethasone group and 86.6% of the tramadol group were pleased (extremely satisfied, satisfied, or neutral), respectively ().
Table 3. Comparison of the LIKERT scale between both studied groups.
8. Discussion
In this study, we conducted ultrasound-guided supraclavicular block on patients undergoing upper limb surgery. We have compared tramadol and dexamethasone as adjuvants to levobupivacaine to perform the ultrasound-guided supraclavicular block. We studied the onset of sensory and motor blockade, analgesia duration, and motor blockade duration. The supraclavicular block provides anesthesia for entire upper limb procedures consistently. When it is performed at the division level of the brachial plexus and with high volume, the trunk level of the plexus may also be blocked in this approach. According to one study, ultrasound-guided supraclavicular block prolongs analgesia more than landmark-guided block because it enables more precise drug deposition nearer to the nerve fibers [Citation21].
According to our research, adding dexamethasone to LA for a supraclavicular block significantly improved postoperative analgesia duration, pain scores, and analgesic use.
It is thought that glucocorticoids cause modest local vasoconstriction, which could limit the absorption of local anesthetics, lengthen the time the local anesthetic spends in touch with the nerve, and lengthen the period the sensory block lasts. Another hypothesis proposes that a rise in the activity of potassium channel inhibitors on nociceptive C fibers mediates dexamethasone’s analgesic effects. However, other authors suggest that its analgesic impact is primarily or solely systemic [Citation22].
Contrary to Choi et al. investigation, perineurally given dexamethasone was added to LA extended motor block recovery following brachial plexus block [Citation6]. Moreover, we discovered that dexamethasone administration extended the recovery of both sensory and motor blockages.
Neurological side effects from the brachial plexus block procedure, such as respiratory distress and Horner’s syndrome, have been reported [Citation23]. Many investigations supported the safety of perineural dexamethasone injection by demonstrating that it did not result in the onset of Horner’s syndrome, nausea, vomiting, numbness/tingling, respiratory distress, or any of these other side effects [Citation24,Citation25].
Moreover, we discovered that administering perineural dexamethasone for brachial plexus blocks did not prevent the emergence of these effects.
Dexamethasone is now the most effective adjuvant treatment for extending the time sensory blocks last, exceeding clonidine, epinephrine, and midazolam [Citation26,Citation27]. Moreover, its safety profile is promising, with a low risk of neurotoxicity. Perineural use in diabetic patients does not significantly change blood glucose levels. Hyperglycemia induced by steroids has only been confirmed in high-dose regimens of intravenous dexamethasone [Citation28].
In our investigation, neither dexamethasone nor tramadol-related neurotoxicity was evident in the patient’s signs or symptoms. However, the sample size required to be expanded to detect unusual findings, and patients were not followed up after the last surgeon follow-up assessment date, which was seven days.
Regarding PONV, studies have indicated that this complication is diminished in patients for whom dexamethasone was used as a perineural adjuvant after 24 postoperative hours [Citation9,Citation28]. Although Group D had a decreased incidence of PONV in the current study, there was no statistical significance because of the low occurrence. The impact can be attributed to Group D receiving a greater dose of dexamethasone, even though this was not the study’s intended objective.
Also, our study showed that the mean analgesic duration in the tramadol group was prolonged but less than that of the dexamethasone group. Its cerebral and peripheral analgesic actions may be the cause of this. It is a mild opioid receptor agonist that increases serotonin and noradrenaline release while preventing noradrenaline and serotonin reuptake.
According to the results of the current study, adding an adjuvant medication, such as dexamethasone, was less costly, increased the quality of the supraclavicular block, and extended the duration of analgesia without having any major negative effects.
8.1. Limitations
The study’s major limitation was the outpatient nature of the surgical procedure, restricting patient outcome follow-up to 24 hours. Secondly, the variation in intraoperative or postoperative pain depending on the type of surgery performed, such as open reduction and internal fixation with plates and screws or rods, could not be standardized.
8.2. Recommendations
Further studies are needed to determine the proper dosage of drugs, the most beneficial pharmacologic combinations, and the synergic effects of the coadministration of adjuvants. A large-size multicenter study in a standard type of surgery is required.
9. Conclusions
As an additive to levobupivacaine, dexamethasone was superior to tramadol, lowering postoperative pain scores and increasing the duration of postoperative pain relief. Moreover, it had a longer time for the first analgesic request, lower analgesic requirement, and fewer incidences of adverse effects. Consequently, dexamethasone can be used as an adjuvant to levobupivacaine in supraclavicular block patients undergoing upper limb surgery.
Author contributions
1. Marwa M. Abdelrady: concept, design, literature search, manuscript preparation, editing, review, and final drafting.
2. Wesam Nashat Ali: definition of intellectual content, literature search, and manuscript review.
3. Mohamed Hassan Bakri: clinical studies, editing, and review.
4. Norhan M Bakri: literature search and review.
5. Esraa Gamal Abdel Nasser Fathy: clinical studies, methodological design, and final drafting.
6. Ola Wahba: statistical analysis, editing.
Acknowledgments
Thank the orthopedic surgery consultants, nurses, residents, and other surgical theatre staff for their contributions.
Disclosure statement
The authors declare that they have no conflicts of interest.
Additional information
Funding
Notes
1 WHAT IS KNOWN? The co-administration of dexamethasone with local anesthetics into the supraclavicular block has decreased postoperative analgesic requirements and has a significant analgesic effect. These advantages of dexamethasone over other adjuvants indicate the need for further studies of the utility of dexamethasone in postoperative analgesia.
WHAT IS NEW? The ultrasound-guided supraclavicular block effectively provides postoperative analgesia in upper limb surgery patients. Using a mixture of local anesthetic and dexamethasone provides better analgesia than tramadol. dexamethasone as an adjuvant to levobupivacaine to perform the ultrasound-guided supraclavicular block was found to be superior in providing longer duration to the first analgesic request, lower analgesic requirement, and lower pain scores.
References
- Fredrickson MJ, Krishnan S, Chen CY. Postoperative analgesia for shoulder surgery: a critical appraisal and review of current techniques. Anaesthesia. 2010;65(6):608–624. doi: 10.1111/j.1365-2044.2009.06231.x
- Gautier P, Vandepitte C, Ramquet C, et al. The minimum effective anesthetic volume of 0.75% ropivacaine in ultrasound-guided interscalene brachial plexus block. Anesthesia & Analgesia. 2011;113(4):951–955. doi: 10.1213/ANE.0b013e31822b876f
- Renes SH, Rettig HC, Gielen MJ, et al. Ultrasound-guided low-dose interscalene brachial plexus block reduces the incidence of hemidiaphragmatic paresis. Reg Anesth Pain Med. 2009;34(5):498–502. doi: 10.1097/AAP.0b013e3181b49256
- Riazi S, Carmichael N, Awad I, et al. Effect of local anaesthetic volume (20 vs 5 ml) on the efficacy and respiratory consequences of ultrasound-guided interscalene brachial plexus block. Br J Anaesth. 2008;101(4):549–556. doi: 10.1093/bja/aen229
- Bardsley H, Gristwood R, Baker H, et al. A comparison of the cardiovascular effects of levobupivacaine and rac-bupivacaine following intravenous administration to healthy volunteers. Br J Clin Pharmacol. 1998;46(3):245–249. doi: 10.1046/j.1365-2125.1998.00775.x
- Choi S, Rodseth R, McCartney C. Effects of dexamethasone as a local anaesthetic adjuvant for brachial plexus block: a systematic review and meta-analysis of randomized trials. Br J Anaesth. 2014;112(3):427–439. doi: 10.1093/bja/aet417
- Knezevic NN, Anantamongkol U, Candido KD. Perineural dexamethasone added to local anesthesia for brachial plexus block improves pain but delays block onset and motor blockade recovery. 2015.
- Pöpping DM, Elia N, Marret E, et al. Clonidine as an adjuvant to local anesthetics for peripheral nerve and plexus blocks: a meta-analysis of randomized trials. J Am Soc Anesthesiologists. 2009;111(2):406–415. doi: 10.1097/ALN.0b013e3181aae897
- Albrecht E, Kern C, Kirkham K. A systematic review and meta‐analysis of perineural dexamethasone for peripheral nerve blocks. Anaesthesia. 2015;70(1):71–83. doi: 10.1111/anae.12823
- Baeriswyl M, Kirkham KR, Jacot-Guillarmod A, et al. Efficacy of perineural vs systemic dexamethasone to prolong analgesia after peripheral nerve block: a systematic review and meta-analysis. BJA. Br J Anaesth. 2017;119(2):183–191. doi: 10.1093/bja/aex191
- Heesen M, Klimek M, Imberger G, et al. Co-administration of dexamethasone with peripheral nerve block: intravenous vs perineural application: systematic review, meta-analysis, meta-regression and trial-sequential analysis. Br J Anaesth. 2018;120(2):212–227. doi: 10.1016/j.bja.2017.11.062
- Huynh TM, Marret E, Bonnet F. Combination of dexamethasone and local anaesthetic solution in peripheral nerve blocks: a meta-analysis of randomised controlled trials. Eur J Anaesthesiol. 2015;32(11):751–758. doi: 10.1097/EJA.0000000000000248
- Pehora C, Pearson AM, Kaushal A, et al. Dexamethasone as an adjuvant to peripheral nerve block. Cochrane Database Syst Rev. 2017;2017(11). doi: 10.1002/14651858.CD011770.pub2
- Zhao W-L, Ou X, Liu J, et al. Perineural versus intravenous dexamethasone as an adjuvant in regional anesthesia: a systematic review and meta-analysis. J Pain Res. 2017;10:1529. DOI:10.2147/JPR.S138212
- Grond S, Sablotzki A. Clinical pharmacology of tramadol. Clin Pharmacokinet. 2004;43(13):879–923. doi: 10.2165/00003088-200443130-00004
- Kayser V, Besson J-M, Guilbaud G. Evidence for a noradrenergic component in the antinociceptive effect of the analgesic agent tramadol in an animal model of clinical pain, the arthritic rat. Eur J Pharmacol. 1992;224(1):83–88. doi: 10.1016/0014-2999(92)94822-D
- Arcioni R, della Rocca M, Romanò S, et al. Ondansetron inhibits the analgesic effects of tramadol: a possible 5-HT3 spinal receptor involvement in acute pain in humans. Anesthesia & Analgesia. 2002;94(6):1553–1557. doi: 10.1213/00000539-200206000-00033
- Nagpal V, Rana S, Singh J, et al. Comparative study of systemically and perineurally administered tramadol as an adjunct for supraclavicular brachial plexus block. J Anaesthesiol Clin Pharmacol. 2015;31(2):191. doi: 10.4103/0970-9185.155147
- Revill S, ROBINSON JO, ROSEN M, et al. The reliability of a linear analogue for evaluating pain. Anaesth Intensive Care. 1976;31(9):1191–1198. doi: 10.1111/j.1365-2044.1976.tb11971.x
- Likert R. A technique for the measurement of attitudes. Archives of psychology; 1932.
- Raghove P, Singh K, Taxak S, et al. Comparison of ultrasound guided technique with conventional landmark technique for supraclavicular brachial plexus nerve block in patients undergoing upper limb surgery. Int J Pharmacol Clin Sci. 2016;5(1):1–4. doi: 10.5530/ijpcs.5.1.1
- Waldron NH, Jones CA, Gan TJ, et al. Impact of perioperative dexamethasone on postoperative analgesia and side-effects: systematic review and meta-analysis. Br J Anaesth. 2013;110(2):191–200. doi: 10.1093/bja/aes431
- Conroy PH, Awad IT. Ultrasound-guided blocks for shoulder surgery. Curr Opin Anaesthesiol. 2011;24(6):638–643. doi: 10.1097/ACO.0b013e32834c155f
- Desmet M, Braems H, Reynvoet M, et al. I.V. and perineural dexamethasone are equivalent in increasing the analgesic duration of a single-shot interscalene block with ropivacaine for shoulder surgery: a prospective, randomized, placebo-controlled study. Br J Anaesth. 2013;111(3):445–452. doi: 10.1093/bja/aet109
- Kim YJ, Lee GY, Kim DY, et al. Dexamathasone added to levobupivacaine improves postoperative analgesia in ultrasound guided interscalene brachial plexus blockade for arthroscopic shoulder surgery. Korean J Anesthesiol. 2012;62(2):130–134. doi: 10.4097/kjae.2012.62.2.130
- El-Baradey GF, Elshmaa NS. The efficacy of adding dexamethasone, midazolam, or epinephrine to 0.5% bupivacaine in supraclavicular brachial plexus block. Saudi J Anaesth. 2014;8(Suppl 1):S78–83. doi: 10.4103/1658-354X.144083
- Shah DM, Arora M, Trikha A, et al. Comparison of dexamethasone and clonidine as an adjuvant to 1.5% lignocaine with adrenaline in infraclavicular brachial plexus block for upper limb surgeries. J Anaesthesiol Clin Pharmacol. 2015;31(3):354–359. doi: 10.4103/0970-9185.161672
- Sakae TM, Marchioro P, Schuelter-Trevisol F, et al. Dexamethasone as a ropivacaine adjuvant for ultrasound-guided interscalene brachial plexus block: A randomized, double-blinded clinical trial. J Clin Anesth. 2017;38:133–136. DOI:10.1016/j.jclinane.2017.02.004