ABSTRACT
Objectives
This study tried to evaluate the impact of dexmedetomidine (DXM) infusion provided during sevoflurane (SEV) anesthesia on cognitive function (CF) of elderly patients undergoing elective arthroscopic shoulder surgeries.
Patients & Methods
A total of 140 patients were randomly allocated into Groups S and D. All patients received SEV (0.5–1 MAC) with placebo or DXM (0.6 µg/kg/h) infusions, respectively. CF was evaluated preoperatively, 48-h, 1-wk, and 2-wk postoperative (PO) using the Montreal Cognitive Assessment Test and Mini-Mental State Examination (MMSE). The study outcome is the frequency and severity of PO cognitive dysfunction (POCD).
Results
At 48-h PO, the frequency of normal CF and scorings were significantly decreased compared to preoperative findings, but were significantly higher in group D. At 1-wk PO, the frequency of normal CF and scores increased in both groups with significant difference in favor of group D, but differences were significantly lower than in preoperative measures. At 2-wk PO, 79.3% of patients regained their normal CF, with significantly higher frequency and score for group D, and the difference compared to preoperative data was insignificant in group D, but it was significant in group S. At 48-h, scorings were positively related to using DXM but were negatively related to age, obesity, and PO analgesia. Regression analysis defined old age as negative and the use of DXM as positive predictor for high scores.
Conclusion
SEV anesthesia induced reversible short-term POCD. Coupling of DXM infusion with SEV anesthesia decreased the frequency and scores of POCF and fastened resumption of normal CF.
1. Introduction
Postoperative cognitive dysfunction (POCD) is a common central nervous system complication that predominantly affects elderly patients with high incidence after cardiac surgery with cardiac bypass [Citation1]. Multiple perioperative independent factors; women, presence of mental illness, diabetes mellitus, dyslipidemia, aortic stenosis, type of surgery, especially cardiac surgery are risk factors for POCD [Citation2–4]. In addition to its health risks, POCD consumes a huge part of resources through extended hospital stays, higher costs, and workforce dropout [Citation5].
Sevoflurane (SEV) is one of the most widely used anesthetics for surgery for its advantages of easy and rapid induction and emergence from anesthesia, but unfortunately, SEV is one of the main causes of POCD [Citation6]. However, animal studies reported insignificant differences between SEV and isoflurane, another commonly used inhalational anesthetic (IA), on cognitive function (CF) [Citation7,Citation8]. Another animal study detected acute neuronal apoptosis in neonatal mice exposed to isoflurane or sevoflurane and found that the impact was more pronounced with isoflurane [Citation9].
Propofol total intravenous anesthesia (TIVA) was tried to replace IA to guard against POCD; however, the results of comparative trials are discrepant. A meta-analysis showed SEV was a risk factor for the emergence of delirium compared to propofol-based TIVA during various surgeries but not intracranial surgery [Citation10]. Another study reported an insignificant difference in the incidence of PO delirium between anesthesia maintenance with a volatile agent and propofol-based TIVA [Citation11].
Unfortunately, effective prevention methods remain elusive; experimental studies have shown that melatonin has an alleviating effect on SEV-induced cognitive deficits [Citation7], vitamin D supplementation improves anxiety and enhances learning ability and long-term memory after SEV anesthesia [Citation12], and metformin was found to reduce SEV-induced neurogenesis damage and neurocognitive defects [Citation13].
1.1. Hypothesis
The present study suggested the implementation of dexmedetomidine (DXM) infusion during induction of anesthesia may ameliorate the SEV impact on CF.
1.2. Objectives
The present study targets to evaluate the impact of DXM infusion on CF of elderly patients undergoing elective arthroscopic shoulder surgeries (ASS) under SEV maintenance anesthesia
1.3. Design
Prospective comparative multicenter study.
1.4. Setting
Anesthesia, Pain & ICU departments, Faculty of Medicine, Menoufia & Ain Shams Universities.
1.5. Ethical considerations
The study protocol was approved by the departmental committee before start of case collection at Jan 2021 and was approved by the Local Ethical Committee, Menufea University after the end of case collection and evaluation of CF at the last follow-up visit of the last case operated up on at 1–24/ANET3.
1.6. Patients
Patients over 60 years old who assigned for elective ASS were evaluated for demographic data: age, gender, height, weight, and calculation of body mass index (BMI) as weight divided by height in meter square. The presence of associated morbidities, indications for other elective or emergency surgery, ASA grade, and the presence of contraindication for general IA or allergy to the drugs to be used were reported. Patients underwent routine preoperative lab investigations and radiological workups.
1.7. Exclusion criteria
The presence of neurological or psychiatric disorders, previous cerebrovascular stroke, cardiac surgery, or manipulation of carotid vessels, the presence of uncontrolled diabetes mellitus or hypertension, chronic renal impairment, hepatic disorders, coagulopathies, and refusal to sign the written consent were the exclusion criteria. Patients who missed or had other surgical procedures during follow-up were also excluded from the study.
1.8. Inclusion criteria
Patients older than 60 years with ASA grade of I-III, assigned for elective ASS, available for follow-up, and were free of exclusion criteria were enrolled in the study.
1.9. Sample size
A previous study compared POCD after SEV anesthesia versus propofol TIVA during cancer resection for 110 patients per group and detected insignificant differences in the frequency of patients who developed POCD: 29.1% vs. 27.3%, respectively [Citation14]. Thereafter, a similar comparative study included 83 women divided into two groups and detected affection of all items evaluating the neurocognitive function with varied extent between SEV inhalational anesthesia and propofol TIVA, but differences were insignificant [Citation15]. The study’s null hypothesis is the significant reduction of the extent of POCD after SEV anesthesia in conjunction with DXM infusion in comparison to the use of SEV alone. Considering that females are more vulnerable to POCD [Citation2], the current study evaluated the proposed regimen for anesthesia of patients undergoing ASS, especially females. To achieve a study power of 80%, using the G*Power (Version 3.1.9.2) computer system for sample size calculation [Citation16] with an effect size of 0.20, α-error factor of 5%, the required sample size to assure the reliability of the null hypothesis was calculated using the F-test model, to be 70 patients per group.
1.10. Randomization & grouping
Using a computer-generated random number sequence in a 1:1 ratio with the dropping of odd numbers, patients were categorized into two groups; Group S & Group D. Group titles were printed into cards that were enclosed in sealed envelopes and patients were asked to choose a closed envelope and present it to the anesthetist in charge. Categorization and grouping were performed by one of the authors: Salah MA.
1.11. Blindness
Assessment of cognitive function before and after surgery was the duty of an author, Helwa A, who was blinded by the type of infusion used. The preparation of intraoperative (IO) infusions and provision of anesthetic procedure was the duty of another author, El-Henawy T, who was blinded by the results of the preoperative assessment of CF. The interpretation of the results was conveyed by the 3rd author; Salah MA who was blinded by the Pre and PO assessments of CF.
1.12. Preparation of IO infusions
Using masked saline bottles, DXM infusion was prepared to provide patients of Group D with 0.6 µg/kg/h, while patients of Group S received an infusion of plain saline in masked bottles.
1.13. Anesthetic technique
Anesthesia was induced by propofol 1–2 mg/kg, fentanyl 1 µg/kg, and atracurium 0.5 mg/kg. DXM and placebo infusions were applied at a rate of 0.6 µg/kg/h infusion during anesthesia. Tracheal intubation was aided by gentle tracheal pressure and an endotracheal tube measuring 6.5 mm was inserted. After intubation of the trachea, the lungs were ventilated with 100% O2 in the air using a semi-closed circle system. Anesthesia was maintained with sevoflurane 0.5–1 MAC and top-up doses of atracurium if needed. Ventilation was controlled with a tidal volume of 6–8 ml/kg, and the ventilatory rate was adjusted to maintain an end-tidal carbon dioxide (paCO2) of 32–35 mmHg. The muscle relaxant was reversed using neostigmine 0.05 mg/kg with atropine 0.01 mg/kg.
1.14. Intraoperative monitoring
Continuous IO non-invasive monitoring for mean arterial pressure (MAP) and heart rate (HR) until the end of surgery. HR and MAP measures were recorded preoperatively, after intubation, every 30-min till the end of surgery and after extubation
Duration of surgery and development of IO complications were recorded.
1.15. Postoperative monitoring
HR and MAP measures were determined at PACU admission, 1-h, 2-h, 4-h thereafter and every 4-h till discharge.
Postoperative (PO) pain data
- All patients received PO analgesic protocol including paracetamol 1-g every 8 h and ketorolac 30 mg every 12 h.
- The numeric rating scale (NRS) was used to assess pain severity at PACU transfer and hourly for 4 h and at 8-h and 12-h PO. A higher score on NRS score indicated worse pain sensation and a score of ≥ 4 indicated the need for receiving rescue analgesia.(Citation17)
- Pain was evaluated during rest with the upper limb supported by the shoulder suspensor.
- Duration of PO analgesia was defined as the time elapsed between PACU admission and the 1st request for rescue analgesia.
- Rescue analgesia was provided as nalbufin 5–10 mg depending on the previous data documented by Seol et al.(Citation18), (2003) who used a combination of fentanyl (600 mg) and nalbufin (60 mg) for patient-controlled intravenous analgesia (PCIA) and reported an insignificant difference in analgesic efficacy and side effects between fentanyl alone or this combination. Aslo, Wang et al.(Citation19), (2022) found sufentanil combined with nalbuphine for PCIA provided superior analgesia compared with sufentanil alone after CS. Rescue analgesia was repeated whenever the NRS score was ≥4 up to a maximum dose of 100 mg. The frequency of need for rescue analgesia and the total doses of nalbuphine used to abolish pain were registered.
The frequency of PO adverse events: PO sedation was assessed at PACU admission, 30-min, 1-h, and 2-h thereafter using the 6-point modified Ramsey sedation scale (RSS) [Citation20]. PO nausea and vomiting (PONV) was evaluated using a 0–3 point scale for nausea; no, mild, moderate, and severe nausea, and 0–2 points for vomiting; no, one and >1 attack [Citation21]. Ondansetron (Zofran); 4 mg IV dose as previously documented [Citation22] was given and repeated according to PONV severity. Hypotension, bradycardia, respiratory depression, and the need for ICU transfer were recorded.
1.16. Evaluation of cognitive function
Cognitive function was evaluated preoperatively, 48-h, 1-wk, and 2-wk PO during attendance at the orthopedic clinic for follow-up using:
Montreal cognitive assessment (MoCA) test: MoCA is a 7-domain 30-point questionnaire evaluating executive/visuospatial function [5 points], naming [3 points], attention [6 points], language [3 points], abstraction [2 points], recall [5 points], and orientation [6 points] through answering 11 questions with a higher score indicates normal CF. MoCA test result was interpreted as follows: 25–30 points indicate normal CF; 18–25 indicate mild CD, 10–17 points indicate moderate, and a score <10 indicates severe CD(Citation23).
Mini-Mental State Examination (MMSE) is a 5-domain 30-point questionnaire for evaluation of orientation, registration, attention/calculation, recall, and language. MMSE score of 25–30 indicates normal CF, 21–24 indicates mild CD, 10–21 indicates moderate CF, and score <9 indicates severe CD.(Citation24)
1.17. Statistical analysis
Inter-group differences were evaluated using a paired t-test, and intra-group differences were evaluated using the one-way ANOVA test. The chi-square test was used to assess the differences in the percentage of data. Correlation between MMSE score at 48-h PO and perioperative data was examined using Pearson’s correlation analysis. Regression analysis was used to determine the predictor for obtaining normal CF as assessed by MMSE. The Receiver Operating Characteristic (ROC) curve was conducted to assure the predictability of correlated variates for regaining normal CF as judged by the area under the curve (AUC) and its significance concerning the area under the reference line (AUC = 0). The optimum cutoff point for significance was p < 0.05. Statistical analyses were conveyed using IBM® SPSS® Statistics software (Version 22, 2015; Armonk, USA).
1.18. Study outcomes
The primary outcome is the frequency and severity of POCD in patients of both groups.
Secondary outcomes included:
- PO analgesic efficacy of IO infusion was judged by the PO duration of analgesia and the frequency and dose of nalbuphine used.
- The frequency and severity of PO adverse effects need for ICU admission, and duration of PO hospital stay.
- The ability of the perioperative findings to be used as predictors for the oncoming CF.
2. Results
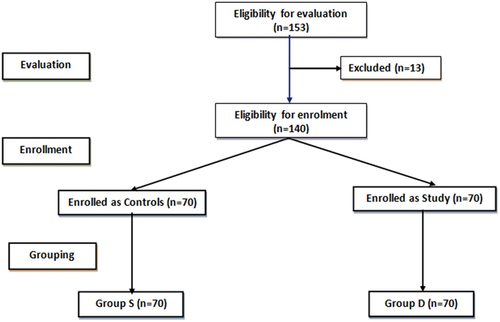
The preliminary evaluation included 153 patients; 5 patients had other indications for surgical interference, 4 patients had neuropsychiatric disorders and 2 patients refused to participate, while 140 patients were randomly divided into two study groups ().
There were insignificant differences between patients’ enrolment data as shown in .
Table 1. Baseline data of patients from both groups.
Duration of surgery ranged between 50 and 90 min with insignificant difference between both groups. Preoperative hemodynamic measures showed insignificant differences between both groups, while HR and MAP measures estimated during surgery, were significantly lower in patients of group D in comparison to that of patients of group S. No IO complications were encountered in patients of both groups ()
Table 2. The finding detected during surgery for patients from both groups.
At the time of PACU admission and till 8-h PO, HR measures were significantly lower in patients of group D than corresponding measures in patients of group S, while at the 12th PO hour, the difference was insignificant despite being lower in patients of group D. Similarly, MAP measures since PACU admission till 4-h PO were significantly lower in patients of group D, but at the 8th and 12th hours after surgery, MAP measures were non-significantly lower in patients of group D., NRS pain scores at time of PACU admission were non-significantly lower in patients of group D than group S. Then, pain scores started to increase progressively in all patients but were significantly lower in patients of group D than group S till 3-h PO, while at the 8th PO hour, pain scores were significantly (p = 0.034) higher in group D. At the 4th and 12th hour PO, the difference in pain scores was insignificant. The average 12-h PO pain score was significantly (p < 0.001) lower in patients of group D than in group S. Thirty patients did not request rescue PO analgesia; 8 in group S and 22 in group D with significant (p = 0.015) intergroup difference. The duration till the 1st nalbufin request was significantly (p = 0.0003) longer, and the total nalbufin consumed dose was significantly (p = 0.037) lower as shown in .
Table 3. Hemodynamic and pain data reported during postoperative course for patients from both groups.
The frequency of patients who had RSS of two increased progressively in group D, but decreased progressively in patients of group S with insignificant differences between both groups at 30-min and 120-min PO, while the frequency of patients who had RSS of two was significantly (p = 0.039) higher in group D at 60-min PO. No patient had respiratory depression or vomiting, and admission to ICU was not required. Regarding other adverse events, the differences between both groups were insignificant in favor of group D ().
Table 4. The reported postoperative adverse events affected patients from both groups.
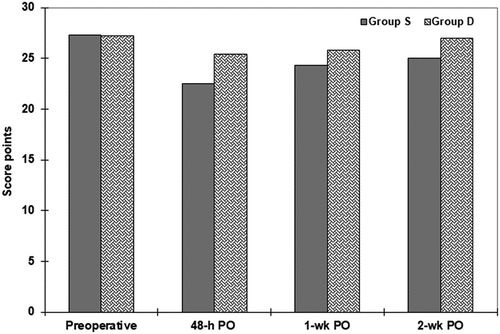
Preoperative MoCA score showed an insignificant difference between the enrolled patients and all patients had a normal cognitive function, but about 56.4% of patients (n = 79) had a preoperative score of <28 with an insignificant difference between both groups. At 48-h PO, 37 patients (26.4%) showed POCD with significantly (p = 0.013) lower incidence among patients of group D. However, the frequency of patients who had POCD was significantly higher in group S (p < 0.001) and group D (p = 0.0014) in comparison to preoperative frequency. Similarly, the mean value of MoCA score at 48-h PO was significantly (p < 0.001) lower in both groups compared to the preoperative score with significantly (p = 0.0001) lower score of patients of group S than group D. At the end of the 1st week PO, CF started to improve and 32 patients (22.9%) had POCD with significantly (p = 0.016) lower incidence, but significantly (p = 0.032) higher mean score with than without DXM. At the 2-wk PO visit, six patients (8.6%) of group D still had mild POCD, while 19 patients (27.1%) of group S had PCOD with significantly (p = 0.0066) lower incidence of POCD among patients of group D. The frequency of patients who had normal CF was significantly lower in group S (p = 0.00002) and group D (p = 0.012) in comparison to preoperative frequencies. Also, the mean value of the MoCA score was significantly (p = 0.0004) lower in group S, but insignificantly (p = 0.495) lower in comparison to their respective mean preoperative score (, ).
Table 5. Postoperative findings on Montreal Cognitive Assessment for patients from both groups.
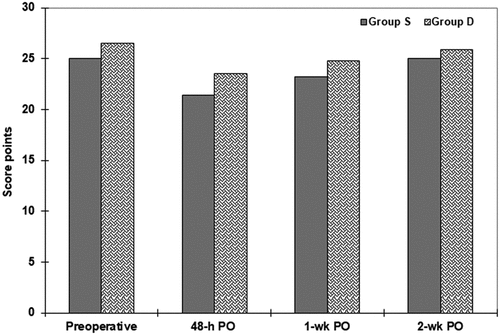
Evaluation of preoperative CF using MMSE defined 11 patients (7.9%) had mild CD with insignificant difference between both groups. The frequency of patients who had normal CF was significantly decreased at 48-h PO in comparison to preoperative frequency in both group S (p < 0.001) and group D (p = 0.0005) with significantly (p = 0.022) higher frequency among patients of group D. Similarly, MMSE score was decreased significantly (p < 0.001) compared to preoperative score of patients of both groups, but was significantly (p = 0.0011) higher for patients of group D than group S. At 1-wk PO, the frequency of patients who had normal CF increased in both groups with significantly (p = 0.014) higher difference in favor of group D, but in comparison to preoperative frequencies, normal CF was still significantly lower in patients of group S (p < 0.001) and group D (p = 0.001) in comparison to preoperative frequency. For the MMSE score, it was still significantly lower in both groups S & D (p < 0.001 & 0.00003, respectively) but was significantly (p = 0.0043) lower in group S than group D.
At 2-wk PO, 111 patients (79.3%) regained their normal CF with significantly (p = 0.018) higher frequency among group D than group S. In comparison to preoperative frequencies, the difference in group S was still significant (p = 0.0022) but was insignificant (p = 0.507) in group D. Similarly, the MMSE score was significantly (p = 0.048) higher in group D with insignificant (p = 0.100) difference versus preoperative score, while in group S the score was significantly (p = 0.038) lower than preoperative score (, ).
Table 6. Mini-mental state examination findings of patients from both groups.
Correlation analysis of the calculated CF scorings at 48-h PO showed a positive significant correlation with the use of DXM infusion but showed negative significant correlations with age, BMI, and the dose used of PO nalbufin. The correlation between the CF scorings with female gender was negative and significant in the case of MoCA, but insignificant in the case of MMSE. MMSE scores showed a negative significant correlation with the frequency of PO adverse events, but the relation with MoCA was negatively insignificant. CF scorings were negatively correlated with operative time and PO pain score, but the relation was insignificant ().
Table 7. Correlation analysis of the relation between cognitive function scorings and perioperative data.
The perioperative data significantly correlated with CF scorings were evaluated using the Regression analysis that defined old age as negative and the use of DXM as positive predictors for high scores on patients’ CF evaluation using MoCA or MMSE ().
Table 8. Regression analysis for the correlated perioperative data with CF scorings.
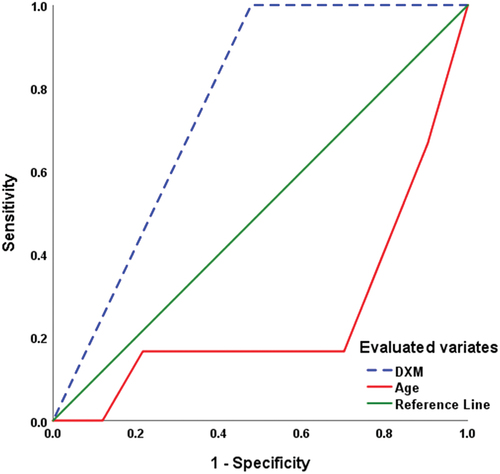
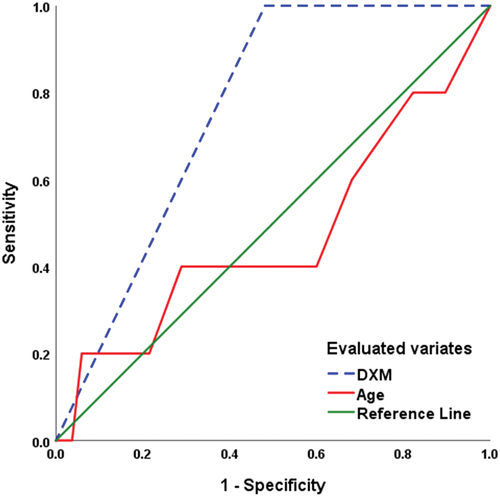
ROC curve analysis defined old age as a significant sensitive predictor for POCD with MMSE < 25 (AUC = 0.254 ± 0.113; p = 0.042; 95% CI: 0.032–0.475), while defining the use of DXM infusion as a significant specific predictor (AUC = 0.761 ± 0.063; p = 0.031; 95% CI: 0.637–0.885) for achieving normal CF with MMSE of >25 (). Also, ROC curve analysis predicted MMSE = 28 as the uppermost value of MMSE score at 48-h PO with the use of DXM infusion (AUC = 0.759 ± 0.069; p = 0.049; 95% CI: 0.625–0.894), while excluded age (AUC = 0.472 ± 0.150; p = 0.831; 95% CI: 0.178–0.766) as predictor for achieving MMSE = 28 ().
3. Discussion
Evaluated CF at 2-wk after surgery showed an insignificant difference in comparison to preoperative evaluation both as frequency and score in patients who received DXM infusion as adjunctive to SEV inhalational anesthesia (IA) with a significant difference in comparison to patients who received SEV alone. This outcome assured the null hypothesis of the study that perioperative use of DXM might be a decisive statement for the preservation of CF of geriatric patients going to receive IA.
In line with the reported ameliorative effect of DXM on PO cognitive dysfunction (POCD), multiple animal and clinical studies assured the obtained outcomes; animal models’ studies found perioperative DXM did only significantly reduce the incidence of POCD, but also improved PO neurocognitive function mostly through activation of the PI3K/Akt signaling pathway via acting on the α2 adrenergic receptor to inhibit the massive release of high mobility group protein B1 (HMGB1) and reduces its binding with Toll-like receptor-4 leading to inhibition of release of inflammatory mediators especially TNF-α, IL-1β [Citation25]. Moreover, extracellular HMGB1 was found to impair the stability of T regulatory cells, decrease its expression of cell surface marker molecules, and inhibit the secretion of the anti-inflammatory cytokine leading to an intensified autoimmune process; however, DXM was found to alleviate these effects [Citation26]. In addition to the downregulating effect of DXM on HMGB1 release and action, DXM was also found to decrease the rate of apoptosis in the hippocampus [Citation27].
Clinically, a review of recent literature assured the positive effects of DXM on CF, especially for PO neurocognitive (NC) disorders and found DXM may help improve POCD, particularly for PO acute events and delayed NC recovery [Citation28]. Regarding geriatric patients, a review of the literature documented the enhanced early recovery of CF in old aged patients undergoing abdominal surgery with the intraoperative use of DXM [Citation29]. Moreover, another review of trials evaluating CF of elderly patients who underwent non-cardiac surgery under various anesthetics and adjuvants found sufentanil and DXM was ranked as the top in reducing the incidence of POCD by 87.4% and 81.5%, respectively [Citation30].
Furthermore, recent randomized comparative clinical studies supported the superiority of DXM as a neuroprotective drug, where Chawdhary et al. [Citation31] reported that DXM is anesthetic sparing and in combination with bispectral index-guided monitoring for titrating the depth of anesthesia is an invaluable tool for reducing POCD than propofol and Yoo et al. [Citation32] in a placebo comparative study detected significant difference in the Mini-Cog© score over time in favor of DXM and found the probability of perioperative CD decreased by 0.48 times on day-3 after SEV/DXM anesthesia for geriatric patients.
Also, Racman et al. [Citation33] reported significantly lower incidence of delayed NC recovery and better cognitive outcomes on procedural sedation with DXM compared to propofol. Additionally, El-Ghazaly et al. [Citation34] found intravenous injection of a DXM bolus before induction of anesthesia is associated with the lowest incidence of delirium and CD after isoflurane anesthesia in comparison to pre-induction injection of ketamine or placebo and Regression analysis revealed reduction of PO delirium and CD by 32% and 62%, respectively, with DXM than placebo, while ketamine increased the risk by 3-fold and 4.5 times, respectively, than placebo.
Concerning the impact of SEV on CF and the benefit of DXM as an adjuvant, in a similar comparative study, Zhang et al. [Citation35] found SEV anesthesia for old-aged patients resulted in a higher incidence of POCD compared to those received combination of SEV and DXM and found that DXM alleviates POCD through decreasing plasma levels of TNF-α and IL-6. Neimark et al. [Citation36] documented that sevoflurane anesthesia for old-aged patients who underwent laparoscopic cholecystectomy developed moderate POCD during early PO observation. Zeng et al. [Citation30] through a meta-analysis documented that DXM significantly reduced the incidence of POCD in comparison to SEV
As previously documented that understanding the mechanism of SEV-induced POCD may allow its prevention and treatment [Citation37]; SEV was found to activate microglia and induce apoptosis in hippocampus through overexpression of the peroxisome proliferator-activated receptor gamma [Citation38] and induce imbalanced cytoplasmic calcium homeostasis through activation of NMDA receptors leading to necroptosis of hippocampus neurons which affects cognitive performance in aged mice [Citation39]. On the contrary, DXM was found to increase the number of microglia and alleviate SEV-induced upregulation of proinflammatory cytokines’ levels, overexpressed purinergic ionotropic-4 receptor and NOD-like receptor protein-3 in the hippocampus, leading to amelioration of CD [Citation40]. Also, DXM improved POCD by targeting the CCAAT/enhancer-binding protein-β to activate the c-Jun N-terminal kinase/p-38 signaling pathway and inhibiting microglial M1 polarization-mediated inflammation in the central nervous system [Citation41]. Further, DXM was found to exert its neuroprotective effect through the upregulation of expression of microRNA miR-204-3p resulting in suppressed expression of F-box/LRR-repeat protein 7 in tissues with subsequent alleviation of neuroinflammation [Citation42].
However, the obtained results showed an interesting finding that despite the reported SEV-induced CD, 32.9% of patients of group S had normal CF at 48-h PO, and this may suggest individual vulnerability for CD on SEV exposure. Furthermore, by 2-wk post-exposure to SEV, about 40% of affected patients regained their normal CF, thus indicating the transitory nature of SEV-exposure CD. Similarly, a meta-analysis detected a comparable effect regarding the occurrence of short-term POCD after inhalation versus intravenous anesthesia for old-aged patients undergoing non-cardiac surgery [Citation43] and Karakurt et al. [Citation44] found MMSE at 1-h after SEV was significantly lower than preoperative and 24-h PO scores but was comparable to TIVA.
Such observation may be explained according to the result of the recent experimental work that found SEV exposure of aged rats induced overexpression of sestrin2, which is stress-inducible gene and exerts neuroprotective effects against brain injury, overexpressed sestrin2 in hippocampus alleviated SEV-induced CD through inhibition of SEV-induced inflammasome activation, production of pro-inflammatory factors, oxidative stress, and neuronal apoptosis [Citation45]. Additionally, sestrin2 counteracts SEV-induced CD through enhanced mitochondrial function and mitophagy through sestrin2-mediated activation of SIRT1, which is a nuclear NAD-dependent deacetylase [Citation46].
4. Conclusion
SEV anesthesia induced reversible short-term POCD. Using IO infusion of DXM during SEV anesthesia decreased the frequency and scores of POCD and allowed faster resumption of normal CF.
4.1. Limitation
The study limitation is the short-term follow-up for these old-aged patients who found repeated attendance at the orthopedic clinic is tedious
4.2. Recommendation
Similar comparative studies of DXM-based versus propofol-based TIVA to establish the preservative effect of DXM for CF after major surgeries, especially when high-risk patients are recommended.
List of Abbreviation
| ASS | = | Arthroscopic shoulder surgeries |
| AUC | = | Area under the curve |
| BMI | = | Body mass index |
| CF | = | Cognitive function |
| DXM | = | Dexmedetomidine |
| HR | = | Heart rate |
| IA | = | Inhalational anesthetic |
| IO | = | Intraoperative |
| MAP | = | Mean arterial pressure |
| MMSA | = | Mini-Mental State Examination |
| MoCA | = | Montreal cognitive assessment |
| NRS | = | Numeric rating scale |
| PCIA | = | Patients-controlled intravenous analgesia |
| PO | = | Postoperative |
| POCD | = | Postoperative cognitive dysfunction |
| PONV | = | Postoperative nausea and vomiting |
| ROC | = | Receiver Operating Characteristic |
| RSS | = | Ramsey sedation scale |
| SEV | = | Sevoflurane |
| TIVA | = | Total intravenous anesthesia |
Availability of data and material
Data are available when requited.
Disclosure statement
No potential conflict of interest was reported by the author(s).
References
- Kasputytė G, Bukauskienė R, Širvinskas E, et al. The effect of relative cerebral hyperperfusion during cardiac surgery with cardiopulmonary bypass to delayed neurocognitive recovery. Perfusion. 2023 Nov;38(8):1688–1696. doi: 10.1177/02676591221129737
- Lam G, Damant R, Ferrara G, et al. Characterizing long-COVID brain fog: a retrospective cohort study. J Neurol. 2023 Oct;270(10):4640–4646. doi: 10.1007/s00415-023-11913-w
- Chen F, Duan Z, Wu Z, et al. Plasma lipidomics reveals potential lipid markers for the prediction of delayed neurocognitive recovery after cardiac surgery with cardiopulmonary bypass. Clin Chim Acta. 2023, Aug 1;548:117504. doi: 10.1016/j.cca.2023.117504
- Díaz-Leiva J, Arrondo-Gómez P, Alonso-Sendín E, et al. Delayed cognitive impairment in patients with aortic stenosis treated with surgical valve replacement and transcatheter aortic valve implantation: a comparative study. Rev Neurol. 2023 Nov 1;77(9):205–214. doi: 10.33588/rn.7709.2022346
- Wang Y, Du W, Wang J. Impact of Transcutaneous electrical acupoint stimulation on delayed neurocognitive recovery in elderly patients following bronchoscopy. Altern Ther Health Med. 2023 Sep 1. AT8743.
- Huang Y, Yang Y, Ye C, et al. The m6A reader YTHDF1 improves sevoflurane-induced neuronal pyroptosis and cognitive dysfunction through augmenting CREB-BDNF signaling. Neurochem Res. 2023 Dec;48(12):3625–3638. doi: 10.1007/s11064-023-04007-6
- Zou X, Zhang X, Qiang T, et al. Melatonin attenuates sevoflurane-induced hippocampal damage and cognitive deficits in neonatal mice by suppressing CypD in parvalbumin neurons. Brain Res Bull. 2023 Nov;204:110809. doi: 10.1016/j.brainresbull.2023.110809
- Abdelkareem E, Tayee E, Taha A, et al. Effect of Sevoflurane and isoflurane on post-anaesthesia cognitive dysfunction in normal and type II diabetic rats. Arch Razi Inst. 2023 Feb 28;78(1):151–159. doi: 10.22092/ARI.2022.358340.2202
- Zhao S, Fan Z, Hu J, et al. The differential effects of isoflurane and sevoflurane on neonatal mice. Sci Rep. 2020 Nov 9;10(1):19345. doi: 10.1038/s41598-020-76147-6
- Yang Y, Feng L, Ji C, et al. Inhalational versus Propofol-based intravenous maintenance of anesthesia for emergence delirium in adults: a meta-analysis and trial sequential analysis. J Neurosurg Anesthesiol. 2023 Apr 1;35(2):177–186. doi: 10.1097/ANA.0000000000000830
- Jiang J, Zhang L, He L, et al. Volatile versus total intravenous anesthesia on postoperative delirium in adult patients undergoing cardiac valve surgery: a randomized clinical trial. Anesth Analg. 2023 Jan 1;136(1):60–69. doi: 10.1213/ANE.0000000000006257
- Zhang J, Zhang X, Zhao J, et al. The effects of vitamin D on movement and cognitive function in senile mice after sevoflurane anaesthesia. Exp Aging Res. 2023 Nov;22:1–15. doi: 10.1080/0361073X.2023.2282350
- Fan P, Lu Y, Wei H, et al. Metformin attenuates sevoflurane-induced neurogenesis damage and cognitive impairment: involvement of the Nrf2/G6PD pathway. Metab Brain Dis. 2023 Aug;38(6):2037–2053. doi: 10.1007/s11011-023-01218-2
- Guo L, Lin F, Dai H, et al. Impact of sevoflurane versus propofol anesthesia on post-operative cognitive dysfunction in elderly cancer patients: a double-blinded randomized controlled trial. Med Sci Monit. 2020, Feb 15;26:e919293. doi: 10.12659/MSM.919293
- Chen C, Wang Y, Rao J, et al. Propofol versus sevoflurane general anaesthesia for selective impairment of attention networks after gynaecological surgery in middle-aged women: a randomised controlled trial. Front Psychiatry. 2022 Jul 14;13:917766.
- Faul F, Erdfelder E, Lang AG, et al. G*power 3: a flexible statistical power analysis program for the social, behavioral, and biomedical sciences. Behav Res Methods. 2007;39(2):175–191. doi: 10.3758/BF03193146
- Williamson A, Hoggart B. Pain: a review of three commonly used pain rating scales. J Clin Nurs. 2005;14(7):798–804. doi: 10.1111/j.1365-2702.2005.01121.x
- Seol GY, Choi JS, Park CH, et al. A comparison of the effect of fentanyl and fentanyl-nalbuphine for postoperative analgesia using IV-PCA. Korean J Anesth. 2003;45(4):481–485. doi: 10.4097/kjae.2003.45.4.481
- Wang L, Wang Y, Ma Y, et al. Sufentanil combined with nalbuphine via patient-controlled intravenous analgesia after cesarean section: a retrospective EvaluationDrug design. Dev And Therapy. 2022;Volume 16:16 3711–3721. doi: 10.2147/DDDT.S380292
- Sessler CN, Grap MJ, Ramsay MAE. Evaluating and monitoring analgesia and sedation in the intensive care unit. Crit Care. 2008;12(Suppl 3):S2. doi: 10.1186/cc6148
- Watcha MF. White PF: postoperative nausea and vomiting. Its etiology, treatment, and prevention. Anesthiology. 1992;77(1):162–184. doi: 10.1097/00000542-199207000-00023
- Garbe C, Drechsler S, Fiedler H, et al. Dose comparison of tropisetron (navoban) 5 mg and 10 mg orally in the prophylaxis of dacarbazine-induced nausea and emesis. Semin Oncol. 1994 Oct;21(5 Suppl 9):12–6.
- Nasreddine ZS, Phillips NA, Bédirian V, et al. The Montreal cognitive assessment, MoCA: a brief screening tool for mild cognitive impairment. J Am Geriatr Soc. 2005;53:695–699. doi: 10.1111/j.1532-5415.2005.53221.x
- Folstein MF, Folstein SE, McHugh PR. Mini-mental state: a practical method for grading the cognitive state of patients for the clinician. J Psychiatr Res. 1975;12:189–198.
- Meng L, Li L, Lu S, et al. The protective effect of dexmedetomidine on LPS-induced acute lung injury through the HMGB1-mediated TLR4/NF-(B and PI3K/Akt/mTOR pathways. Mol Immunol. 2018;94:7–17. doi: 10.1016/j.molimm.2017.12.008
- Zhang J, Chen L, Wang F, et al. Extracellular HMGB1 exacerbates autoimmune progression and recurrence of type 1 diabetes by impairing regulatory T cell stability. Diabetologia. 2020;63(5):987–1001. doi: 10.1007/s00125-020-05105-8
- He H, Zhu M, Lyu Y, et al. Effects and possible mechanisms of dexmedetomidine on post-operative cognitive dysfunction Chin. Med J (Engl). 2023 Oct 5;136(19):2392–2394. doi: 10.1097/CM9.0000000000002372
- Yu S, Leng Y, Wang Y, et al. A review of the biological mechanisms of Dexmedetomidine for postoperative neurocognitive disorders med Sci Monit. Med Sci Monit. 2022 Oct 25;28:e937862. doi: 10.12659/MSM.937862
- Yang Z, Wu A, Zhang M. Effects of dexmedetomidine on early cognitive function in elderly patients after abdominal surgery: a meta-analysis. Minerva Anestesiol. 2023 Nov;89(11):1034–1041. doi: 10.23736/S0375-9393.23.17399-8
- Zeng K, Long J, Li Y, et al. Preventing postoperative cognitive dysfunction using anesthetic drugs in elderly patients undergoing noncardiac surgery: a systematic review and meta-analysis. Int J Surg. 2023 Jan 1;109(1):21–31. doi: 10.1097/JS9.0000000000000001
- Chawdhary AA, Kulkarni A, Nozari A. Substitution of propofol for dexmedetomidine in the anaesthetic regimen does not ameliorate the post-operative cognitive decline in elderly patients. Indian J Anaesth. 2020 Oct;64(10):880–886. doi: 10.4103/ija.IJA_365_20
- Yoo S, Jue M, Kim Y, et al. The effect of Dexmedetomidine on the mini-cog score and high-mobility group box 1 levels in elderly patients with postoperative neurocognitive disorders undergoing orthopedic surgery. J Clin Med. 2023 Oct 19;12(20):6610. doi: 10.3390/jcm12206610
- Racman P, Kšela J, Racman M, et al. Comparison of procedural sedation with propofol and dexmedetomidine during transcatheter aortic valve replacement using the transfemoral approach. J Cardiothorac Vasc Anesth. 2023 Oct;37(10):1894–1900. doi: 10.1053/j.jvca.2023.05.009
- El-Ghazaly H, Hemaida T, Zaher Z, et al. A pre-anesthetic bolus of ketamine versus dexmedetomidine for prevention of postoperative delirium in elderly patients undergoing emergency surgery: a randomized, double-blinded, placebo-controlled study. BMC Anesthesiol. 2023 Dec 11;23(1):407. doi: 10.1186/s12871-023-02367-8
- Zhang H, Wu Z, Zhao X, et al. Role of dexmedetomidine in reducing the incidence of postoperative cognitive dysfunction caused by sevoflurane inhalation anesthesia in elderly patients with esophageal carcinoma. J Cancer Res Ther. 2018;14(7):1497–1502. doi: 10.4103/jcrt.JCRT_164_18
- Neimark M, Shmelev V, Rakhmonov A, et al. Correction of cognitive dysfunction after laparoscopic cholecystectomy under inhalation anesthesia with sevoflurane. Khir Z im N I Pirogova. 2022;5(5):52–58. doi: 10.17116/hirurgia202205152
- Jiang S, Xiong Y, Wang X Engeletin ameliorates sevoflurane-induced cognitive impairment by activating PPAR -gamma in neonatal mice. Neuropathology. 2023 Dec;43(6):431–440. doi: 10.1111/neup.12905
- Wang C, Chen W, Zhang Y, et al. Update on the mechanism and treatment of sevoflurane-induced postoperative cognitive dysfunction. Front Aging Neurosci. 2021 Jul 8;13:702231. Vitamin cocktail supplementation might improve cognitive dysfunction after acute traumatic brain injury. doi:10.3389/fnagi.2021.702231
- Liu X, Yu J, Tan X, et al. Necroptosis involved in sevoflurane-induced cognitive dysfunction in aged mice by activating NMDA receptors increasing intracellular calcium. Neurotoxicology. 2023 Dec 7;100: 35–46. doi:10.1016/j.neuro.2023.12.006.
- Li N, Ma Y, Li C, et al. Dexmedetomidine alleviates sevoflurane-induced neuroinflammation and neurocognitive disorders by suppressing the P2X4R/NLRP3 pathway in aged mice. Int J Neurosci. 2022 Sep;13:1–11. doi: 10.1080/00207454.2022.2121921
- Fu S, Zhao X, Li Y, et al. Dexmedetomidine alleviates hippocampal neuronal loss and cognitive decline in rats undergoing open surgery under sevoflurane anaesthesia by suppressing CCAAT/enhancer-binding protein beta. Eur J of Neuroscience. 2023 Nov 20;59(1):36–53.
- Lian X, Zhang X, Chen W, et al. Dexmedetomidine mitigates neuroinflammation in an Alzheimer’s disease mouse model via the miR-204-3p/FBXL7 signaling axis. Brain Res. 2024 Jan 1;1822:148612.
- Huang L, Zhang Y. The effect of intravenous and inhalation anesthesia in general on the cognition of elderly patients undergoing non-cardiac surgery: a systematic review and meta-analysis. Front Med. 2023 Nov 15;10:1280013. doi: 10.3389/fmed.2023.1280013
- Karakurt T, Kuyrukluyıldız U, Onk D, et al. Evaluation of the effects of total intravenous anesthesia and inhalation anesthesia on postoperative cognitive recovery. Anaesthesiologie. 2023 Dec;72(Suppl 1):19–24. doi: 10.1007/s00101-021-01083-7
- Sun L, Li Y, Wang D, et al. SESN2 attenuates sevoflurane-induced cognitive impairment and neuroinflammation in rats. Exp Brain Res. 2023 Dec 21;242(2):375–384.
- Sun L, Hong X, Wang D, et al. Overexpression of SESN1 improves mitochondrial damage and mitophagy, a potential therapeutic strategy for cognitive dysfunction after anaesthesia. Eur J Of Neuroscience. 2023 Dec 17;59(2):208–219.