ABSTRACT
Objective
Spinal cord ischemia (SCI) is a potentially devastating complication of thoracic and thoraco-abdominal endovascular aortic repair (TEVAR and TAEVAR) that can result in varying degrees of short-term and permanent disability. This study was undertaken to evaluate the incidence, investigate the risk factors of SCI, and describe the clinical outcomes, reversibility, long-term functional impact, and influence on survival of SCI
Methods
This study employed a retrospective design to investigate patients who underwent TEVAR and TAEVAR successfully within the past 11 years between January 2012 and June 2022 in a single center. The analysis focused on factors such as incidence, personal history, and detailed assessment of medical and surgical risk factors. Data was retrieved from medical records. SCI was defined by any new lower neurologic deficit not attributable to another cause and diagnosed through clinical examination immediately after emergence from anesthesia and frequently postoperatively, as well as during follow-up outpatient clinic visits.
Results
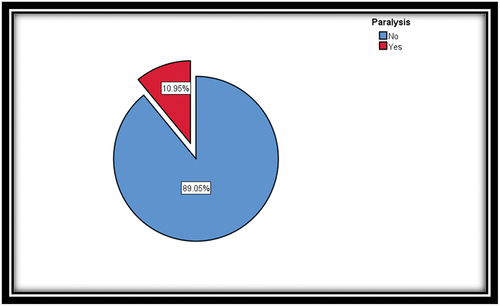
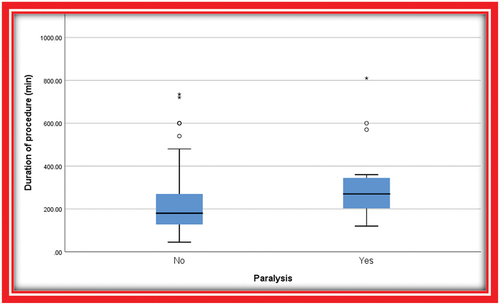
A total of 137 patients were enrolled in the study. Among them, 15 patients developed lower limb paralysis with an incidence of 10.95%, three patients (20.1%) developed paralysis immediately postoperatively, and five patients (33.3%) developed paralysis on day 1 postoperatively (33.3%). Furthermore, the remaining patients experienced delayed onset paralysis, with the most recent case occurring 26 days after the operation. In terms of reversibility, nine patients had complete reversal of their motor and sensory symptoms to the baseline preoperative status. Two patients experienced a partial reversal of their symptoms, and four patients had persistent symptoms without any improvement. Data showed that 12 out of 81 patients (14.8%) who had functioning spinal drains developed paralysis. The duration of the procedure was the most significant risk factor for paralysis. Patients with a mean duration of 270 min had a higher risk of paralysis.
Conclusion
Prediction of paralysis post-TEVAR and TAEVAR remains challenging, with data analysis indicating that the duration of the procedure is the sole statistically significant variable to consider.
1. Introduction
Spinal cord ischemia (SCI) and reversible or permanent paraplegia had always been severe complications of Thoracic and Thoracoabdominal aortic endovascular repair (TEVAR and TAEVAR). A number of patient and procedure-related factors have been shown in previous studies to be associated with the development of SCI after TEVAR and TAEVAR, including aortic treatment length, left subclavian artery coverage, obesity, blood loss, procedural urgency, adjunct procedures (e.g., conduit, embolization), renal insufficiency, hypotension and indication [Citation1].
Further, multimodality strategies have been reported for the prevention and treatment of SCI after TEVAR and include cerebrospinal fluid (CSF) drainage, left subclavian and/or hypogastric artery revascularization, augmentation of oxygen delivery and pharmacologically induced hypertension [Citation1]. Despite increased awareness of this problem and judicious application of these interventions, some patients continue to suffer this devastating complication. SCI leads to varying degrees of short- and long-term disability, ranging from mild transient paraparesis to permanent flaccid paralysis, and the occurrence of this complication has a known negative impact on long-term survival [Citation2].
2. Aim of the study
The purpose of this study was to evaluate the prevalence of and investigate risk for SCI after TEVAR and TAEVAR, provide an overview of the current evidence on the effectiveness of perioperative strategies to prevent SCI, and to define the outcomes of patients experiencing SCI after TEVAR and TAEVAR and determine differences in the evolution of long-term functional recovery, as well as the impact on survival.
3. Patients and methods
3.1. Type of Study
A single-center retrospective cross-sectional study.
3.2. Study Setting
The study was conducted on consecutive patients who underwent TEVAR and TAEVAR.
3.3. Study Population
3.3.1. Eligibility
All consecutive patients who underwent TEVAR and TAEVAR successfully in the past 11 years (1st of January 2011 until May 2022).
3.3.2. Exclusion Criteria
Patients were excluded if:
presence of neurologic deficits confirmed to be secondary to stroke or peripheral neuropathy;
failure to be evaluated for neurological outcomes after TEVAR;
presence of lower limb dysfunction before TEVAR;
stroke immediately after TEVAR;
femoral vascular access complications after TEVAR, resulting in lower limb dysfunction; and
patients with open surgical repair.
4. Ethical Considerations
The Research Ethical Committee of the Faculty of Medicine, Ain Shams University, approved the study.
4.1. Study Tools
Retrospective review of data and patients’ electronic medical records.
4.2. Primary Outcome
Occurrence of new neurological insult affecting lower limbs after TEVAR and TAEVAR. SCI was defined as any new onset of transient or permanent paraplegia or paraparesis after surgery, manifested as deficit in motor or sensory function of the lower extremities. Consultation with neurology and/or confirmatory imaging with spinal MRI or CT were obtained in equivocal cases. Patients were divided into SCI group and non-SCI group depending on new onset of SCI symptoms or signs after surgery.
5. Methodology
Retrospective review of data and patients’ electronic medical records was carried out, and the following data was collected:
unidentifiable personal data: age, sex, family history, BMI, pre-operative ambulatory status, and special habits: smoking, alcoholism, and drug abuse.
Medical comorbidities: diabetes mellitus, hypertension, perioperative hypotension (MAP < 70 mmHg), renal insufficiency (serum creatinine >1.5 mg/dl or GFR < 60), COPD, degenerative aneurysm pathology, congestive heart failure, coronary artery disease and the presence of any malignancy.
Anesthetic data: agents used in anesthesia, duration of the procedure, central venous pressure, hemoglobin level, systemic blood pressure, CSF pressure, spinal cord perfusion pressure, hypothermia (temperature below 36 °C), usage of vasopressors or inotropic agents and CSF drainage.
Surgical data: indication, urgency of the procedure, the extent of aortic coverage, left subclavian artery coverage, coverage of hypogastric arteries, device type, number of stents, duration of the procedure, amount of blood loss, leaking aortic aneurysm, previous repair, presence of endo leak post-endovascular repair and surgical protective strategies including temporary aneurysm sac perfusion, minimally invasive segmental artery coil embolization, surgical staging of procedure.
Patients were deemed as high risk for SCI if [Citation1,Citation2]:
more than one endograft was implanted in the descending aorta, or the distal thoracic aorta was covered (the end of endograft reaching within 3 cm to the origin of celiac artery);
the patient had previous aortic repair, either thoracic or abdominal, endovascular or surgical.
the patient had unrepaired infrarenal aneurysm.
All high-risk patients received prophylactic measures unless contraindicated. Prophylactic measures included left subclavian artery revascularization as indicated, blood pressure augmentation, and CSF pressure control after TEVAR and TAEVAR [Citation1]. CSF pressure control was implemented after TEVAR and TAEVAR by lumbar puncture and CSF withdrawal. When the reading of CSF pressure was higher than 15 mmHg, CSF was withdrawn to a target pressure reduction of 30%. If the reading of CSF pressure was greater than 10 mmHg and lower than 15 mmHg, CSF was withdrawn to a target pressure <10 mmHg. Peri-operative management of the spinal drain was based on a previously published standardized protocol [Citation2]. The current indications for spinal drainage are listed in the following table [Citation3].
5.1. Statistical Analysis
Statistical analyses were performed using SPSS software to calculate means, standard deviations and frequencies. Numerical variables were compared using either an unpaired t-test or the Mann–Whitney test as appropriate. Categorized data were compared using the Chi-squared test or Fisher’s exact test. The level of statistical significance was set at P < 0.05.
6. Results
6.1. A total of 137 patients who underwent TEVAR/TAEVAR between January 2012 and June 2022 were screened for the study.
demonstrates the incidence of SCI, the timing of presentation, either immediate or delayed, neuroimaging (MRI spine and CT spine) for patients who developed paralysis, and recovery and functional improvement of paralysis. The latest case occurred 26 days postoperatively when the patient developed paralysis in both lower limbs.
Table 1. Incidence of Spinal cord injury (SCI).
Postoperative paresis/paralysis was detected by clinical examination immediately after emergence from anesthesia and frequent examination in the post-anesthesia care unit, surgical intensive care unit and cardiovascular nursing ward. It presented as lower limb weakness or complete paralysis, parathesis, or tingling sensation in lower limbs affecting either one lower limb or both lower limbs.
As regard the outcomes and functional improvement of the SCI patient’s cohort, nine patients had complete neurologic recovery of their motor and sensory symptoms to the baseline preoperative status. In addition, two patients had some degree of neurologic recovery and were either independently ambulating or ambulating with minimal assistance, and four patients had achieved any neurologic recovery with persistent symptoms and without any improvement despite routine management which include elevating MAP, lowering CSF pressure and naloxone administration.
Analysis of the demographic data, clinical variables, anesthetic agents, operative anesthetic and surgical details of patients with and without SCI did not detect any significant differences when comparing both groups (). Notably, only the duration of surgery was more significantly associated with the development of SCI (p = 0.004).
Table 2. Patients’ characteristics.
Table 3. Medical history.
Table 4. The impact of anesthetics used on SCI.
Table 5. The impact of operative anesthetic details on SCI.
Table 6. The impact of surgical data on SCI.
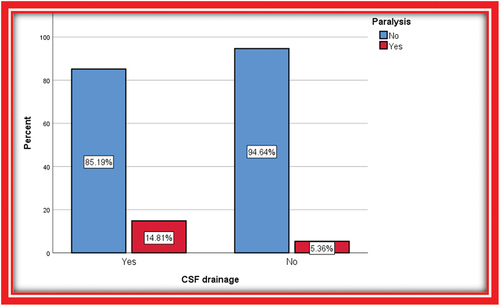
Overall, 81 (59.1%) of patients received a perioperative spinal drain, and no significant difference was noted when comparing patients with and without SCI regarding the rate of perioperative spinal drainage (p = 0.08) ().
Table 7. Number of patients who underwent CSF drainage.
Table 8. Relation between CSF drainage and SCI.
further details the survival rate in all patients, only one patient died in the SCI group at day 2 post-operatively due to complicated gut ischemia. Survival was compared between patients with SCI vs. no SCI patients; overall survival of patients with any SCI was not statistically different (p = 0.4725) ().
Table 9. Mortality.
Table 10. Relation between mortality and SCI.
7. Discussion
SCI, as one of the major complications after TEVAR, impacts on patients’ quality of life seriously and had always been a concern for clinicians; and despite advancements in risk stratification and management, the incidence of this complication still ranges between 2% and 15%. Indeed, despite a heightened awareness, liberal CSF drainage, and judicious use of adjuncts such as subclavian revascularization and intensive monitoring, the rate of SCI has been consistent over time at 9%, with a permanent deficit rate of 4.3%. Although lower than the usual reported rate of this complication in open aortic repair, this is certainly not insignificant given the devastating impact of SCI [Citation2].
This retrospective study reviewed records of 137 patients who underwent TEVAR and TAEVAR successfully between January 2012 and June 2022. In this study, the incidence of SCI was 10.95% (). The duration of the procedure is the most critical risk factor affecting the outcome (). However, prolonged procedure duration cannot be treated as a single factor, as it represents several other factors, such as complex stent deployment technique and extended segment coverage.
As the procedure duration increases, the patient is exposed to higher levels of anesthetics, leading to increased hemodynamic fluctuations, potential hypotension, and the need for vasopressors. Additionally, more IV contrast and blood loss should be anticipated. Prolonged exposure to cold temperatures, may have a protective effect on the spinal cord. While the majority of these factors were examined individually and lacked statistical significance in relation to SCI, the interaction of all these factors could potentially worsen the risk and account for the findings.
A variety of previously identified risk factors have been reported to be associated with SCI after TEVAR and TAEVAR, including advanced age, male gender, a history of renal insufficiency, presence (or previous repair) of an abdominal aneurysm, acute dissection, lumbar/hypogastric artery patency, urgency of TEVAR, aortic coverage length, and left subclavian artery coverage [Citation2]. However, most of those associations have not been corroborated in this analysis.
MAP is the main factor affecting spinal cord perfusion. During TEVAR and TAEVAR procedures, spinal perfusion changes, and collateral circulation is activated. Higher mean arterial pressure (MAP) increases perfusion to the spine to prevent ischemia [Citation4]. Improved recovery from SCI has been reported after rapidly elevating MAP to 80–85 mmHg [Citation5].
Elevating MAP can be achieved by administering intravenous fluids, pressors, or inotropes. Spinal cord perfusion pressure is calculated as the difference between MAP and either CSF pressure or CVP, whichever is greater [Citation6]. General guidelines for minimizing SCI include elevating MAP and draining CSF <10 mmHg) in order to maintain spinal cord perfusion pressure at levels of 80 mmHg [Citation2].
Our data showed that 12 (14.8%) patients who had functioning spinal drains developed paralysis (). In two of them, the drain was not inserted even after paralysis occurred as one patient was coagulopathic, and the other one had very mild paresthesia in both lower limbs on day 9 postoperative and the decision was made to elevate MAP and follow-up. Three patients had a spinal drain, and paralysis developed after drain removal at day 6, day 5 and day 1 postoperatively. Subsequently, the spinal drain was reintroduced, leading to an improvement in symptoms. One case did not have a drain initially, and a drain was inserted after developing paralysis, but the paralysis persisted.
Unfortunately, despite the use of spinal drainage, and the positive impact on short-term neurologic recovery in some patients, drain placement did not appear to affect the rate of SCI.
Naloxone infusion is another method used for neuroprotection. The potential protective mechanisms of naloxone have been researched but not definitively proven. Glutamate inhibition could explain naloxone’s neuroprotective abilities [Citation7]. Endogenous opioids may also reduce microcirculatory blood flow following injury. Animal model indicated improved blood flow and outcome in the naloxone group during an ischemic event. Evidence suggested the role of kappa receptors in SCI, with minimal delta receptor involvement [Citation8]. Three patients in our study were administered a naloxone drip upon experiencing paralysis. The symptoms of paralysis improved in all three patients. However, naloxone was not administered alone, as these patients also underwent CSF drainage and had their blood pressure elevated.
Nine patients of SCI group had spinal cord imaging (MRI or CT spine) only three of them (33.3%) showed positive findings for ischemia, and one patient did not tolerate the MRI machine, so the study was discontinued. Spinal cord MRI findings can be classified into three types: focal (asymmetrical focal high intensity on axial T2-weighted images involving two or fewer segments of the spinal cord), sporadic (asymmetrical multiple high intensity on axial T2-weighted images involving more than three segments of the spinal cord), and diffuse (symmetrical high intensity on axial T2-weighted images). According to the causative pathology, athero-embolism mostly causes focal and sporadic findings, while spinal cord hypoperfusion causes the diffuse finding [Citation9].
The overall incidence of mortality (both intraoperatively and 1 year postoperatively) was 11.1% among patients who underwent TEVAR and TEAVAR. Interestingly, despite the higher expected complications in patients with SCI, the in-hospital mortality rate was not different between patients with and without SCI. Additionally, using this analysis, we were not able to determine whether any of the available preventative maneuvers affect the long-term outcomes of SCI.
7.1. Limitation to our study
Some potential limitations of our study need to be underscored. This is mainly a single institutional retrospective study; we recruited a small number of patients; hence, it might limit the generalization of the conclusions of the study to multi-institutional experience because of difference in patient population and selection criteria. Also, because of the lack of a control group of high-risk patients without prophylactic measures, we could not tell whether our current prophylactic strategy reduces the risk of SCI.
8. Conclusion
Paralysis is a severe complication associated with TEVAR and TAEVAR. Research indicates that its occurrence is more closely related to the duration of the procedure rather than factors such as lumbar drain insertion, urgency of the procedure, number of stents inserted, extent of aortic coverage, Hb level, and degenerative aneurysmal pathology. Typical neuroimaging did not detect spinal cord injury in 50% of paralysis cases. Paralysis reversibility is uncertain. Our intervention protocol involved maintaining spinal cord perfusion by increasing mean blood pressure and keeping cerebrospinal fluid pressure below 10 mmHg through lumbar CSF drainage, and naloxone administration. Full reversibility occurred only in 73.3% of cases despite applying the intervention protocol.
List of abbreviation
| CHF | = | Congestive heart failure |
| COPD | = | Chronic obstructive pulmonary disease |
| GFR | = | Glomerular filtration rate |
| HB | = | Hemoglobin |
| IHD | = | Ischemic heart disease |
| MAP | = | Mean arterial pressure |
| TAEVAR | = | Thoraco-abdominal endovascular aortic repair |
| TEVAR | = | Thoracic endovascular aortic repair |
Acknowledgments
We express our gratitude to all subjects involved in the current study.
Disclosure statement
The authors report no competing interests to declare.
References
- Xue L, Luo S, Ding H, et al. Risk of spinal cord ischemia after thoracic endovascular aortic repair. J Thorac Dis. 2018;10(11):6088–6096.
- Fairman AS, Wang GJ. spinal cord ischemia management: Current indications and timing for drainage. Endovascular Today November. 2020;19:NO. 11.
- DeSart K, Scali ST, Feezor RJ. et al. Fate of patients with spinal cord ischemia complicating thoracic endovascular aortic repair. J Vasc Surg. 2013 Sep;58(3):635–42.e2. doi: 10.1016/j.jvs.2013.02.036 Epub 2013 Apr 13. PMID: 23591190; PMCID: PMC4143904.
- Etz CD, Weigang E, Hartert M, et al. Contemporary spinal cord protection during thoracic and thoracoabdominal aortic surgery and endovascular aortic repair: a position paper of the vascular domain of the European Association for cardio-thoracic surgery. Eur J Cardiothorac Surg. 2015;47(6):943–957. doi: 10.1093/ejcts/ezv142. PMID: 25991554.
- Matsuda H, Ogino H, Fukuda T. et al. Multidisciplinary approach to prevent spinal cord ischemia after thoracic endovascular aneurysm repair for distal descending aorta. Ann Thorac Surg. 2010 Aug;90(2):561–565. doi: 10.1016/j.athoracsur.2010.04.067 PMID: 20667350.
- Carroccio A, Marin ML, Ellozy S. Hollier LH. Pathophysiology of paraplegia following endovascular thoracic aortic aneurysm repair. J Card Surg. 2003 Jul-Aug;18(4):359–366. doi: 10.1046/j.1540-8191.2003.02076.x PMID: 12869184.
- Kunihara T, Matsuzaki K, Shiiya N. et al. Naloxone lowers cerebrospinal fluid levels of excitatory amino acids after thoracoabdominal aortic surgery. J Vasc Surg. 2004 Oct;40(4):681–690. doi: 10.1016/j.jvs.2004.07.005 PMID: 15472595.
- Faden AI, Jacobs TP. Zivin JA. Comparison of naloxone and a delta-selective antagonist in experimental spinal stroke. PMID: 6664246. Life Sci. 1983;33(Suppl 1):707–710. doi: 10.1016/0024-3205(83)90600-8
- Tanaka H, Minatoya K, Matsuda H. et al. Embolism is emerging as a major cause of spinal cord injury after descending and thoracoabdominal aortic repair with a contemporary approach: magnetic resonance findings of spinal cord injury. Interact Cardiovasc Thorac Surg. 2014 Aug;19(2):205–210. doi: 10.1093/icvts/ivu148 Epub 2014 May 14. PMID: 24827461.