Abstract
Surgery for craniosynostosis in children is associated with substantial intraoperative bleeding and the need for blood transfusions. Recent studies have supported the efficacy of tranexamic acid (TXA) in reducing blood loss and transfusion requirements. The records of 72 patients under 18 months of age who had undergone 113 surgeries for non-syndromic craniosynostosis in a single institution for 6 years were retrospectively analyzed. The transfusion requirements in infants with and without TXA administration were compared. The concept of patient blood management before and after TXA implementation in practice was also examined. The rate of intraoperative packed red blood cells (PRBC) transfusion was significantly lower in the TXA than in the non-TXA group (26.7% vs. 83%, p < 0.001). The volume of intraoperatively transfused PRBC was also lower in the TXA group compared to the non-TXA group (mean 68.25 mL vs. 101.91 mL, p < 0.001), as was the weight-adjusted volume (mean 11.2 mL/kg vs. 15 mL/kg, p = 0.002). There were no significant differences in the rate and volumes of postoperative PRBC transfusion between groups. The volume of intraoperatively transfused PRBC was dependent on TXA administration, but not on the affected suture and type of intervention. We found that while in the pre-TXA period all patients were transfused intraoperatively, the frequency of intraoperative transfusions in the post-TXA period was reduced by 40.9% even in the patients who had not received TXA. TXA seems to effectively reduce the intraoperative transfusion requirements in children undergoing craniosynostosis surgery. The optimal blood management in this patient population remains to be further evaluated.
Introduction
Craniosynostosis is a relatively common disorder that affects the membranous portions of the skull. It is defined as the premature closure of one or more cranial sutures, which, in turn, can result in dysmorphic appearance and impaired brain development if left uncorrected. The incidence is approximately 1 in 2100–2500 births [Citation1]. Depending on the affected sutures, craniosynostoses can be classified as sagittal (accounting for ∼60% of all cases), coronal (∼25%), metopic (∼15%) and lambdoid (∼2%) [Citation2]. They are additionally divided into simple and complex disorders according to the number of fused sutures. When they are part of a syndrome, e.g. Apert or Crouzon, they are referred to as syndromic, and in the case of an isolated disorder, they are classified as non-syndromic. The treatment consists of surgical repair, preferably performed within the first year of life. The operative procedures are usually associated with substantial intraoperative bleeding ranging between 20 and 500% of the total blood volume [Citation3]. Numerous techniques for blood loss reduction have been described, including cell savers, acute normovolemic hemodilution, preoperative fresh frozen plasma (FFP) transfusions and the administration of various local and systemic agents [Citation4,Citation5]. Among the medications, tranexamic acid (TXA) is the most commonly used and extensively investigated in the literature. It is a synthetic analog of lysine that reversibly and competitively inhibits the lysin receptor sites on plasminogen, thus leading to the suppression of fibrinolysis.
Our study aimed to compare the rate and amount of packed red blood cells (PRBC) transfusion with and without TXA in infants operated on for craniosynostosis and to evaluate the influence of the TXA protocol implementation in our practice on patient blood management.
Subjects and methods
Ethics statement
This retrospective study was approved (Ref. No. EC78-NS/26.06.2023) by the Institutional Review Board of University Hospital “St. Ivan Rilski” (Sofia). As all data were anonymized, without any personal identification information, no informed consent was required for the study.
Study design
After obtaining approval from our Institutional Review Board (University Hospital “St. Ivan Rilski”, Sofia), we examined the records of all patients under 18 months of age who had undergone surgery for any type of non-syndromic craniosynostosis from January 2017 to December 2022. The endoscopic surgeries were excluded. The study included a total of 113 operations on 72 patients, ASA (American Society of Anesthesiologists) Classes I and II. All except three cases were simple craniosynostoses. The remaining patients had a complex disorder of combined sagittal and metopic fusion. They were treated in three consecutive stages—initially two operations for sagittal synostosis and then a surgery for metopic repair. Thus, each of these three patients had three separate procedures.
The operations were performed by the same neurosurgical team. Several techniques were used, depending on the type of affected suture. Sagittal synostosis patients were managed by a modification of the Pi technique on each side of the cranium in two consecutive stages within 2–4 weeks. The procedure for metopic repair included fronto-orbital reconstruction with bilateral frontal bone advancement. The unicoronal fusion was corrected by fronto-orbital advancement on the affected side, and the bicoronal fusion was managed by the same technique performed on both sides of the cranium.
The operative procedures were grouped into procedures with and without intraoperative administration of tranexamic acid (TXA group vs. non-TXA group). There was further stratification within the groups depending on the affected suture and the type of surgery: 1st stage sagittal suture repair, 2nd stage sagittal suture repair, metopic suture repair, unilateral coronal suture repair and bilateral coronal suture repair. There were no cases of lambdoid craniosynostosis registered during the study period. Of all 113 procedures, 60 (performed on 44 patients) were in the TXA group, and 53 (performed on 36 patients) were in the non-TXA group. Eight patients with more than one operation were present in both groups, depending on whether they were or were not administered TXA during each of their procedures. The administration of TXA was introduced in our institution in November 2018. The protocol that we used included a bolus dose of 30 mg/kg over 15 minutes given after induction of anesthesia and before skin incision, followed by a continuous infusion of 5 mg/kg/h for the next 5 hours. The decision for an intraoperative PRBC transfusion was made at the discretion of the attending anesthesiologist and neurosurgeon. It was based on either of two criteria: prophylactic transfusion started immediately after bone incision or the development of hemodynamic instability (hypotension and tachycardia). The volume transfused was not calculated by any formula. It was fixed to 1 or 2 pediatric units of PRBC (one pediatric unit is approximately 60 mL) and guided by the subjective estimation of blood loss. The triggers for postoperative PRBC transfusion were hemoglobin levels below 7 g/dL or clinical deterioration (reduced activity, lethargy, refusal to eat, tachypnea).
Statistical analysis
The statistical analysis was performed using SPSS, Version 20 (IBM SPSS Statistics). Continuous variables were expressed as mean (±standard deviation) when they were normally distributed or median (range) in the case of a non-Gaussian distribution. They were analyzed by the two-sample Student’s t-test or the Mann-Whitney nonparametric U test, depending on the distribution. Categorical variables were shown as frequencies and percentages and were analyzed by the Pearson chi-squared test. Finally, a multivariate analysis of variance (MANOVA) was conducted to identify the factors influencing the transfusion requirements in our study population. Differences were considered statistically significant at the level of p < 0.05 for all testing.
Results
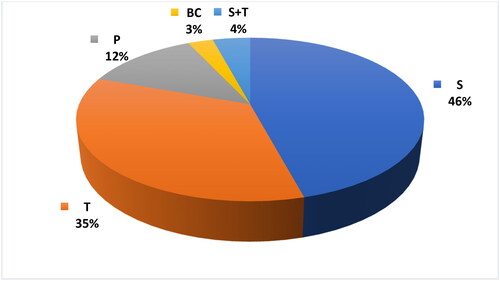
The most frequent disorder in our study population was sagittal craniosynostosis (scaphocephaly), followed by metopic (trigonocephaly), unicoronal (plagiocephaly) and bicoronal craniosynostosis. The data are presented in . The performed surgeries consisted of 37 cases of 1st stage sagittal repair (S1), 37 cases of 2nd stage sagittal repair (S2), 28 corrections of trigonocephaly (T), 9 corrections of plagiocephaly (P) and 2 cases of bicoronal fusion repair.
Figure 1. Distribution of craniosynostoses according to the affected suture. S: scaphocephaly; T: trigonocephaly; P: plagiocephaly; BC: bicoronal fusion; S + T: complex disorder of sagittal and metopic fusion.
There was no significant difference between TXA and non-TXA groups in terms of age, weight, duration of surgery and postoperative length of stay (). The preoperative values of hemoglobin and hematocrit were also comparable.
Table 1. Demographic data and characteristics of patients who underwent craniosynostosis surgery.
The rate of intraoperative PRBC transfusion was significantly lower in the TXA group (16 of 60 procedures, 26.7%) than in the non-TXA group (44 of 53 procedures, 83%, p < 0.001). The reduction was 67.9%. The volume of intraoperatively transfused PRBC was also lower in the TXA group compared to the non-TXA group (mean 68.25 mL vs. 101.91 mL, p < 0.001), as was the weight-adjusted volume (mean 11.2 mL/kg vs. 15 mL/kg, p = 0.002). However, there were no significant differences in the rate, volume or weight-adjusted volume of postoperative PRBC transfusions between groups ().
Table 2. Intraoperative and postoperative PRBC transfusions in patients with and without TXA.
Analyzing the data in regards to the affected suture and the corresponding type of operative procedure, we found that there was a significant reduction in the rate of intraoperative PRBC transfusion when using TXA for the subgroups of 1st and 2nd stages of sagittal repair and metopic repair. There was a non-significant difference for the subgroup of plagiocephaly (). The bicoronal craniosynostoses were excluded from this analysis, as there were only two cases in our study population. The postoperative PRBC transfusion rates were not influenced by TXA administration in any of the subgroups (). Using the MANOVA test, it was estimated that the volume of intraoperatively transfused PRBC, both total and weight-adjusted, was dependent on TXA administration, but not on the affected suture or type of operative procedure. On the other hand, neither TXA nor the type of suture nor the operative procedure affected the volume (both total and weight-adjusted) of postoperatively transfused PRBC.
Table 3. PRBC transfusions in relation to the affected suture and type of operation.
After the introduction of TXA in our practice in 2018, the agent was administered in 60 out of a total of 82 cases. The remaining 22 cases were not contraindicated for its use, but the patients did not receive it either because of the reluctance of the attending anesthesiologist or because of a temporary shortage of supply. We compared the rates and volumes of PRBC transfusions in those 22 cases and in the patients operated on in the period before TXA introduction. We found that while in the pre-TXA period, all patients were transfused intraoperatively, the frequency of intraoperative transfusions in the post-TXA period was reduced by 40.9%. There was no significant difference between the frequency of the postoperative transfusions: 6.5% in the pre-TXA period vs. 13.6% in the post-TXA period (p = 0.378). The volumes (both total and weight-adjusted) of transfused PRBC were significantly lower in the post-TXA period, both intra- and post-operatively (). Hence, even without TXA administration, there was a clear trend toward a reduction in PRBC transfusions after the agent was introduced in our practice.
Table 4. Intraoperative and postoperative PRBC transfusions in patients without TXA.
Discussion
Craniosynostosis surgery has been historically associated with extensive blood loss and the need for transfusions of allogeneic blood products. In this retrospective cohort study, we analyzed the effect of TXA administration on PRBC requirements, both intra- and postoperatively. We did not include surgical blood loss evaluations due to insufficient data. There was no standardization of hematocrit examination in our cases, which made calculations by formula inappropriate. On the other hand, the gauze counts and measurements of operative suction volumes were not recorded, as they were considered inaccurate in this patient population [Citation6]. The findings from our study were consistent with the results from previous reports, suggesting that the utilization of TXA reduces the need for PRBC transfusions. Some authors also found a reduction in FFP requirements when TXA was administered [Citation6,Citation7]. However, according to the conclusions of a recent meta-analysis, the use of TXA was not associated with any significant difference in the incidence of FFP transfusion in open craniosynostosis surgery [Citation8]. In our study, FFP was extremely rarely needed (only in two cases in the non-TXA group), so we did not include it in the analysis.
The existing data about the influence of TXA administration on the frequency of PRBC transfusions is controversial. Several studies reported that all patients needed to be transfused during surgery, despite the beneficial effects of the antifibrinolytic agent on blood loss [Citation9–11]. Our results, on the other hand, demonstrated a significant reduction in the percentage of intraoperative PRBC transfusions: 87% without TXA vs. 26.7% with TXA. A similar trend was observed by Crantford et al. [Citation12] in their retrospective study: 100% vs. 76%. Dadure et al. [Citation13] showed a reduction in PRBC transfusion rates from 45% without TXA to 11% with TXA. The most pronounced decline in the number of transfused children was reported by Escher et al. [Citation14], 100% vs. 14.3%. It is important to point out that all participants in the latter two studies also had preoperative applications of erythropoietin.
The volume of PRBCs that our patients received intraoperatively was significantly lower when TXA was administered. This finding corroborates the results of several previous studies. Four randomized controlled trials [Citation7,Citation11,Citation13,Citation15] and three retrospective studies [Citation6,Citation9,Citation10] have shown a significant reduction in the amount of transfused blood. Our results are most similar to those of Fenger-Eriksen et al. [Citation7], who demonstrated a drop in the intraoperative red blood cell transfusions from a mean of 14 mL/kg in the control group to 8.2 mL/kg in the treatment group.
A few studies, however, have questioned the benefits of intraoperative TXA administration in craniosynostosis surgery. Hansen et al. [Citation16] did not find any difference in the PRBCs transfused perioperatively, while Danforth et al. [Citation17] reported that in patients with fronto-orbital advancement, TXA was even associated with increased transfusion requirements. In our study, the positive effects of TXA did not extend beyond the intraoperative period. We found relatively low postoperative transfusion requirements, with no significant difference in the rate and amount of PRBC use with and without TXA. Contrary to our findings, in a recent meta-analysis, Lu et al. [Citation8] stated that the overall incidence of PRBC transfusions after surgery was decreased by the use of TXA. In a large retrospective study published in 2022, Varidel et al. [Citation18] demonstrated that there was a statistically significant reduction in postoperative but not in intraoperative transfusion incidence, and suggested that this may represent a relative over-transfusion intraoperatively in the TXA group or under-transfusion in the control group. In our series, intraoperative over-transfusion was observed rather in the non-TXA than in the TXA group, which may explain the opposite results that we obtained.
Whether the dose regimen and infusion duration also had an impact on our findings, remains to be further investigated. There are no strict TXA dosage recommendations for craniosynostosis surgery in the current literature. The limits vary widely between 10 and 50 mg/kg loading dose, followed by 5–10 mg/kg/h infusion [Citation19]. The highest initial dose was proposed by Maugans et al. [Citation20], 100 mg/kg over 20 minutes. In a large meta-analysis including 398 individuals who received TXA for craniosynostosis surgery, O’Donnell et al. [Citation21] concluded that there was no difference in outcomes between high- and low-dose regimens. Very rarely was TXA continued postoperatively. In one of the earliest studies on the topic, Duran de la Fuente et al. [Citation22] reported repeated doses every 8 hours for 48 hours after surgery. More recently, Kurnik et al. [Citation9] described the administration of continuous infusion in the first 24 postoperative hours, basing their choice on the observation that this is the time frame during which children have the highest drain output and potential decrease in hemoglobin levels. Our protocol consisted of an initial dose of 30 mg/kg, which was an average value among those reported in the literature, followed by a 5 mg/kg/h infusion with a fixed duration of 5 hours. Thus, we covered the intraoperative and the very early postoperative periods.
Future analyses are needed to fully evaluate the effect of TXA and define its exact role in our transfusion practices. What we found in this study was that the type of affected suture and the corresponding surgical technique did not influence the intraoperative transfusion requirements. The multivariate analysis demonstrated that the only factor that significantly reduced the PRBC needs was TXA. This conclusion is arguable in the literature. Bonfield et al. [Citation4] reported that surgery for metopic and multisuture craniosynostosis was associated with higher rates of transfusion. In an earlier study, before the era of TXA, Eaton et al. [Citation23] suggested that the anesthesiologist rather than the neurosurgeon or the suture affected is the most critical factor in determining the magnitude of intraoperative transfusion. Han et al. [Citation24] emphasized that the final decision to perform transfusions is highly subjective and varies among surgeons, ICU staff and anesthesiologists. In our series, all patients treated before the introduction of TXA in practice were prophylactically transfused at the beginning of surgery, this being a consensus between the neurosurgeons and anesthesiologists. A similar technique was described by Cortellazzi et al. [Citation25], who used in their center a protocol of early preemptive blood transfusion at the onset of the skin opening based on an estimated blood loss from prior similar procedures. The advantages of this approach were earlier resumption of nutrition and shorter ICU and hospital length of stay. We no longer support such a liberal transfusion strategy in our institution. We rather believe that efforts should be directed toward implementing methods to reduce blood loss and the need for transfusion, thus minimizing the risks of both anemia and blood product use, in a word, implementing patient blood management (PBM) based on the three pillars [Citation26]. Interestingly, after the introduction of TXA in our practice, we lowered the frequency and amount of intraoperative PRBC administration in all patients, not only in those who received the antifibrinolytic agent. Most probably, this is due to the stricter transfusion criteria that we started to apply and the avoidance of mandatory use of blood products during surgery.
There are several limitations to this study. It is retrospective. The dose of TXA and the duration of the infusion were chosen arbitrarily because of the lack of strict guidelines in the literature. The blood loss was not estimated in our patients either by measurement or by calculations due to insufficient data available in the records. Nevertheless, we assume that the evaluation of the TXA effect on the PRBC transfusions was feasible, as the surgical team and the surgical techniques remained unchanged over time.
Conclusions
TXA appears to effectively reduce the intraoperative, but not the postoperative transfusion requirements, in children undergoing surgical correction for non-syndromic craniosynostosis. Further evaluation is needed for the optimization of the TXA regimen and the implementation of a comprehensive blood management program in this patient population.
Author contributions
RT: analysis and interpretation of data; conceptualization and design of the work; drafting the manuscript; PV, DF: analysis and interpretation of data; literature research; critical review of the manuscript; DY: analysis and interpretation of data; layout of figures and tables; critical review of the manuscript; SG: design of the work; review and editing of the manuscript; NL: design of the work; analysis and interpretation of data; critical review and final revision of the manuscript. All authors have read and approved the final version of the manuscript and have agreed to be accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved.
Disclosure statement
No potential conflict of interest was reported by the authors.
Data availability statement
The data that support the findings of this study are available from the corresponding author, RT, upon reasonable request.
Additional information
Funding
References
- Governale LS. Craniosynostosis. Pediatr Neurol. 2015;53(5):1–8. doi: 10.1016/j.pediatrneurol.2015.07.006.
- Kajdic N, Spazzapan P, Velnar T. Craniosynostosis: recognition, clinical characteristics, and treatment. Bosn J Basic Med Sci. 2018;18(2):110–116. doi: 10.17305/bjbms.2017.2083.
- Park C, Wormald J, Miranda BH, et al. Perioperative blood loss and transfusion in craniosynostosis surgery. J Craniofac Surg. 2018;29(1):112–115. doi: 10.1097/SCS.0000000000004098.
- Bonfield CM, Sharma J, Cochrane DD, et al. Minimizing blood transfusions in the surgical correction of craniosynostosis: a 10-year single-center experience. Childs Nerv Syst. 2016;32(1):143–151. doi: 10.1007/s00381-015-2900-6.
- White N, Bayliss S, Moore D. Systematic review of interventions for minimizing perioperative blood transfusion for surgery for craniosynostosis. J Craniofac Surg. 2015;26(1):26–36. doi: 10.1097/SCS.0000000000001108.
- Martin JP, Wang JS, Hanna KR, et al. Use of tranexamic acid in craniosynostosis surgery. Plast Surg (Oakv). 2015;23(4):247–251. doi: 10.4172/plastic-surgery.1000946.
- Fenger-Eriksen C, D’Amore Lindholm A, Nørholt SE, et al. Reduced perioperative blood loss in children undergoing craniosynostosis surgery using prolonged tranexamic acid infusion: a randomised trial. Br J Anaesth. 2019;122(6):760–766. doi: 10.1016/j.bja.2019.02.017.
- Lu VM, Goyal A, Daniels DJ. Tranexamic acid decreases blood transfusion burden in open craniosynostosis surgery without operative compromise. J Craniofac Surg. 2019;30(1):120–126. doi: 10.1097/SCS.0000000000004875.
- Kurnik NM, Pflibsen LR, Bristol RE, et al. Tranexamic acid reduces blood loss in craniosynostosis surgery. J Craniofac Surg. 2017;28(5):1325–1329. doi: 10.1097/SCS.0000000000003731.
- Ongun EA, Dursun O, Kazan MS. Tranexamic acid utilization in craniosynostosis surgery. Turk Neurosurg. 2020;30(3):407–415. doi: 10.5137/1019-5149.JTN.27644-19.1.
- Goobie SM, Meier PM, Pereira LM, et al. Efficacy of tranexamic acid in pediatric craniosynostosis surgery: a double-blind, placebo-controlled trial. Anesthesiology. 2011;114(4):862–871. doi: 10.1097/ALN.0b013e318210fd8f.
- Crantford JC, Wood BC, Claiborne JR, et al. Evaluating the safety and efficacy of tranexamic acid administration in pediatric cranial vault reconstruction. J Craniofac Surg. 2015;26(1):104–107. doi: 10.1097/SCS.0000000000001271.
- Dadure C, Sauter M, Bringuier S, et al. Intraoperative tranexamic acid reduces blood transfusion in children undergoing craniosynostosis surgery: a randomized double-blind study. Anesthesiology. 2011;114(4):856–861. doi: 10.1097/ALN.0b013e318210f9e3.
- Escher PJ, Tu AD, Kearney SL, et al. A protocol of situation-dependent transfusion, erythropoietin and tranexamic acid reduces transfusion in fronto-orbital advancement for metopic and coronal craniosynostosis. Childs Nerv Syst. 2021;37(1):269–276. doi: 10.1007/s00381-020-04654-y.
- Kim EJ, Kim YO, Shim KW, et al. Effects of tranexamic acid based on its population pharmacokinetics in pediatric patients undergoing distraction osteogenesis for craniosynostosis: rotational thromboelastometry (ROTEMTM) analysis. Int J Med Sci. 2018;15(8):788–795. doi: 10.7150/ijms.25008.
- Hansen JK, Lydick AM, Wyatt MM, et al. Reducing postoperative bleeding after craniosynostosis repair utilizing a low-dose transexamic acid infusion protocol. J Craniofac Surg. 2017;28(5):1255–1259. doi: 10.1097/SCS.0000000000003711.
- Danforth RM, Cook JA, Bennett WE, et al. Tranexamic acid in infantile craniosynostosis surgery: friend or foe? Plast Reconstr Surg. 2020;146(5):1119–1127. doi: 10.1097/PRS.0000000000007245.
- Varidel A, Cooper M, Loughran J, et al. Intravenous tranexamic acid is associated with a clinically significant reduction in blood loss in craniosynostosis surgery. J Craniofac Surg. 2022;33(2):636–641. doi: 10.1097/SCS.0000000000008234.
- Zapata-Copete JA, Gómez-Ospina JC, García-Perdomo HA, et al. Role of tranexamic acid in craniosynostosis surgery: systematic review and meta-analysis. J Plast Reconstr Aesthet Surg. 2022;75(4):1389–1398. doi: 10.1016/j.bjps.2021.11.064.
- Maugans TA, Martin D, Taylor J, et al. Comparative analysis of tranexamic acid use in minimally invasive versus open craniosynostosis procedures. J Craniofac Surg. 2011;22(5):1772–1778. doi: 10.1097/SCS.0b013e31822e6283.
- O’Donnell DB, Vazquez S, Greisman JD, et al. Tranexamic acid dosing in craniosynostosis surgery: a systematic review with meta-analysis. Plast Reconstr Surg Glob Open. 2022;10(10):e4526. doi: 10.1097/GOX.0000000000004526.
- Durán de la Fuente P, García-Fernández J, Pérez-López C, et al. Utilidad del ácido tranexámico en la cirugía de remodelación craneal [usefulness of tranexamic acid in cranial remodeling surgery]. Rev Esp Anestesiol Reanim. 2003;50(8):388–394 (Spanish).
- Eaton AC, Marsh JL, Pilgram TK. Transfusion requirements for craniosynostosis surgery in infants. Plast Reconstr Surg. 1995;95(2):277–283. doi: 10.1097/00006534-199502000-00007.
- Han RH, Nguyen DC, Bruck BS, et al. Characterization of complications associated with open and endoscopic craniosynostosis surgery at a single institution. J Neurosurg Pediatr. 2016;17(3):361–370. doi: 10.3171/2015.7.PEDS15187.
- Cortellazzi P, Caldiroli D, Lamperti M, et al. Early transfusion and crystalloid infusion strategy in infants undergoing cranioplasty surgery. Paediatr Anaesth. 2009;19(12):1251–1252. doi: 10.1111/j.1460-9592.2009.03167.x.
- Patient Blood Management Guidelines: Module 6 | Neonatal and Paediatrics. Available from: https://blood.gov.au/system/files/14523_NBA-Module-6-Neonat_Paediatrics_internals_5_updated_14_May_2020.pdf

