Abstract
Background
Transthyretin (ATTR) amyloidosis is often diagnosed in an advanced stage, when irreversible cardiac damage has occurred. Lumbar spinal stenosis (LSS) may precede cardiac ATTR amyloidosis by many years, offering the opportunity to detect ATTR already at the time of LSS surgery. We prospectively assessed the prevalence of ATTR in the ligamentum flavum by tissue biopsy in patients aged >50 years undergoing surgery for LSS.
Methods
Ligamentum flavum thickness was assessed pre-operatively on axial T2 magnetic resonance imaging (MRI) slices. Tissue samples from ligamentum flavum were screened centrally by Congo red staining and immunohistochemistry (IHC).
Results
Amyloid in the ligamentum flavum was detected in 74/94 patients (78.7%). IHC revealed ATTR in 61 (64.9%), whereas amyloid subtyping was inconclusive in 13 (13.8%). Mean thickness of ligamentum flavum was significantly higher at all levels in patients with amyloid (p < .05). Patients with amyloid deposits were older (73.1 ± 9.2 vs. 64.6 ± 10.1 years, p = .01). No differences in sex, comorbidities, previous surgery for carpal tunnel syndrome or LSS were observed.
Conclusions
Amyloid, mostly of the ATTR subtype, was found in four out of five patients with LSS and is associated with age and ligamentum flavum thickness. Histopathological work-up of ligamentum flavum might inform future decision making.
Introduction
Systemic amyloidoses are a group of diseases characterized by tissue deposition of fibrils derived from a misfolded circulating precursor protein. The protein type and its properties determine the amyloid subtype and are responsible for the peculiar tissue tropism, and respective organ dysfunction [Citation1,Citation2]. Physiological mechanisms are ineffective in removing amyloid deposits. Thus, for patients affected by systemic amyloidosis, early diagnosis and timely treatment initiation are key determinants for patient outcome.
Immunoglobulin light chain (AL) amyloidosis and wild-type transthyretin (ATTRwt) amyloidosis are the most commonly encountered systemic amyloidoses [Citation1,Citation3]. For AL amyloidosis, a biomarker-based screening program has been proposed for patients at risk [Citation3,Citation4]. Currently, no such recommendation exists regarding ATTRwt amyloidosis despite the prevalence of ATTR in multiple localization in the body, particularly in various ligaments and tendons [Citation2]. The typical and most devastating late manifestation of ATTRwt amyloidosis is myocardial infiltration with organ dysfunction [Citation5,Citation6]. However, according to some studies, up to 60% of patients with ATTRwt amyloidosis have been reported with a personal history of carpal tunnel syndrome (CTS), and 5–14% have a personal history of lumbar spinal stenosis (LSS) [Citation7]. Importantly, such manifestations may precede the occurrence of clinically relevant cardiac disease by 5–10 years, making them a potential tool for screening and detection of ATTRwt amyloidosis [Citation7–9]. Early diagnosis is of interest, given the emergence of several therapeutic disease modifying approaches with proven efficacy in patients with ATTRwt amyloidosis [Citation10,Citation11].
Histological studies are not routinely performed during surgical procedures for LSS, and the occurrence of ATTR in the ligamentum flavum had not been prospectively assessed at the time of study initiation. Thus, the aim of this prospective study was to histologically define the prevalence of ATTR in unselected patients >50 years undergoing surgical decompression for LSS.
Methods
Patients and study design
We offered participation in the prospective, observational IOSI-EMA-007 trial (NCT03966105) to all patients >50 years with LSS scheduled for decompression surgery at our tertiary referral centre between February 2020 and October 2021. A pre-existing diagnosis of systemic amyloidosis was the only exclusion criterion. Clinical data, blood samples and urine samples were collected on the day of the pre-surgical assessment and then stored at −80 °C for biobanking. All patients received a standardized questionnaire including information regarding comorbidities, prior surgery for CTS or LSS, and family history. Routine laboratory tests were performed according to local standards. In March 2020, the hospital policy regarding the management of patients undergoing elective surgery had to be adapted due to the COVID-19 pandemic. In order to reduce the workload for the heavily burdened health care workers, the novel policy did not allow for additional outpatient visits, sample collection and storage. Consequently, more than half of the patients undergoing LSS decompression surgery could no longer be screened, and included in the study, mainly for logistical reasons.
Histology and immunohistochemistry
During the surgical procedure, the removal of the ligamentum flavum represents the fundamental step in order to decompress the narrow canal at the level of the stenosis. All the material removed during the procedure was sent for the analysis. Serial sections of formalin-fixed and paraffin embedded (FFPE) tissues were cut from each paraffin block and stained with haematoxylin and eosin, and Congo red [Citation12]. The presence of amyloid was confirmed in each case by the typical green–yellow–orange birefringence in cross-polarized light after Congo red staining. Amyloid was classified immunohistochemically as previously published [Citation13]. Immunostaining for complement 9 (C9) was done as described by Lux et al. [Citation14].
The study was conducted in accordance with the Declaration of Helsinki and principles of Good Clinical Practice. The Local Ethic Committees approved the study (ID 2019-00936/CE 3481) and all patients provided written informed consent.
Digital image analysis
Amyloid load was assessed by digital image analysis as described previously [Citation7]. Congo red stained tissue sections were scanned using a Hamamatsu NanoZoomer 2.0 RS scanner (Hamamatsu Photonics Deutschland GmbH, Herrsching am Ammersee, Germany) at 400 times magnification in sequential bright field and fluorescence mode (excitation/emission wavelength 560/607 nm for detection of Congo red fluorescence and 485/526 nm for compensation of unspecific background signal, respectively). ImageJ version 1.52p (National Institutes of Health, Bethesda, MD) was used to select and count all tissue pixels in the bright field image and all pixels showing Congo red signal in the fluorescence image (compensated for unspecific background), respectively. Amyloid load was calculated as the ratio of both pixel counts.
MRI
Pre-operative MRI scans of all enrolled patients were evaluated by an expert neuroradiologist. The short axis of the left and right ligamentum flavum was measured on 3 mm thickness axial T2-weighted images, at each lumbar level from L1 to S1 as previously described [Citation15]. Levels L1–L2, L2–L3, L3–L4, L4–L5 and L5–S1 were not available in N = 41, N = 15, N = 13, N = 14 and N = 12 patients, respectively. Left- and right-side measurements were finally averaged together. Levels with prior surgery were excluded. The analysis of the MRI scans was performed by the expert neuroradiologist blinded to the histopathology results.
Statistical analysis
A descriptive statistical analysis was performed to assess the demographic, clinical and histopathologic characteristics of patients undergoing surgical decompression for LSS. Measures of central tendency and dispersion (mean ± standard deviation, median and interquartile range (IQR)) were used to describe quantitative variables and counts and percentages were applied to report qualitative variables. Comparisons between categorical variables were performed using the Chi-squared or the Fischer exact test, and continuous variables were compared using the Mann–Whitney U-test. The covariates assessed by univariate logistic regression analysis as potential independent factors for amyloidosis incidence included age at intervention, MRI levels (L1–L2, L2–L3, L3–L4, L4–L5 and L5–S1), median body mass index (BMI, kg/m2), comorbidities and separately hypertension, diabetes, chronic kidney disease, autoimmune diseases, previous CTS or previous surgery for LSS. p Values were adjusted for multiple comparisons where indicated and unadjusted p values are also shown [Citation16]. Statistical analyses were performed using SPSS v. 25.0 (SPSS Inc., Chicago, IL).
Results
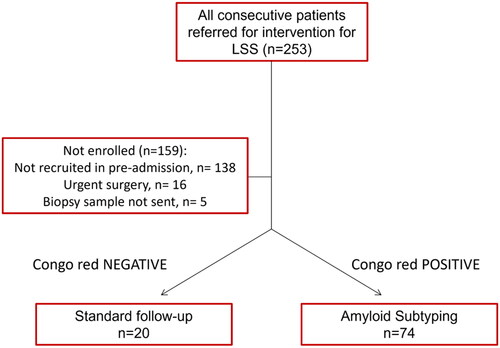
On a total of 253 patients undergoing LSS decompression surgery between February 2020 and October 2021, 94 (37.1%) were enrolled in the study (). Drop-out reasons were altered logistics during the COVID-19 pandemic (N = 138), indication for urgent surgery (N = 16) and shipment issues with the biopsy sample (N = 5). Further information on recruitment is stated in the Methods section. Baseline characteristics of the study cohort are shown in . Median age was 73 years (range 65–79), median BMI was 27.1 kg/m2 (range 23.8–31.0). Comorbidities were reported in 81.9% of patients, with hypertension and diabetes type II being the most prevalent diseases. Thirteen patients had previously been treated for LSS, and eight patients reported prior surgery for CTS.
Table 1. Characteristics of the study cohort, % (N).
Histology
Congo red positive amyloid deposits were present in 78.7% (74/94) patients. Immunohistochemistry (IHC) revealed ATTR in 61 (64.9%) patients, whereas amyloid subtyping was inconclusive in 13 (13.8%) patients (). According to a semi-quantitative assessment, in 23 cases amyloid deposits were described as ‘low’ and in 38 cases as ‘high’. Subsequently, we assessed amyloid load by digital image analysis. The median amyloid load was 0.31% (0–11.8%). No significant difference was found between men (median 0.48%; range 0–11.8%) and women (median 0.28%; range 0–9.4%; p = .530). Median age of the cohort was 71 years. Median amyloid load was lower in the age group ≤71 years (median 0%; range: 0–5.2%) compared with the age group >71 years (median 1.6%; range 0–11.8%; p = .063). In the younger age group, 12 patients had no amyloid compared with four patients in the age group >71 years. These data show that amyloid load increases with age. Complement 9 was assessable in 35 patients with ATTR and detected within the amyloid in each case.
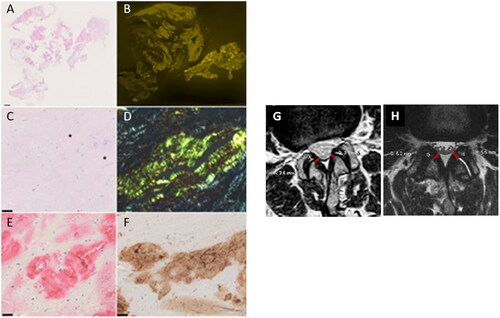
Figure 2. Histology of resection specimens showed fragments of soft tissue and ligamentum flavum (A). Variable amounts of amyloid were found in the ligamentum flavum (B). At higher magnification, a homogenous eosinophilic material was noted after haematoxylin and eosin staining (asterisk; C), which showed a typical yellow-green birefringence in polarized light after Congo red staining (D). These deposits immunoreacted with antibodies directed against transthyretin (E) and complement 9 (F). Haematoxylin and eosin staining (A, C); Congo red staining viewed in fluorescence microscopy (B); Congo red staining viewed in polarization microscopy (D), anti-transthyretin-immunostaining (E); anti-complement 9-immunostaining (F). Barr denotes 1 mm (A) and 50 µm (C, E, F). Original magnifications 0.56-fold (A, B); 200-fold (C–F). (G, H) Exemplary images of ligamentum flavum measurement on axial T2-weighted MR images (L2–L3 level), in a patient without (G) and with (H) proven amyloid deposition at histopathology. The corresponding measurements are given for each side in mm.
MRI
The ligamentum flavum increased progressively in thickness from L1–L2 to L4–L5 and decreased in L5–S1. This trend was present in all patients, independently from the presence of amyloid in the tissue biopsy. However, the mean thickness of the ligamentum flavum was significantly higher at all levels (from L1–L2 to L5–S1) in patients with histologically proven amyloid deposits (p < .05 for all) ( and , and ) and different levels were highly collinear (p < .05 for all) (Supplementary Figure 1).
Figure 3. Box plots showing association between age, mean ligamentum flavum thickness for each level and amyloid detection. *p < .05; **p < .01.
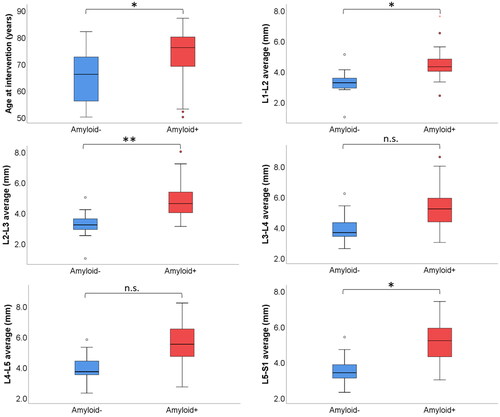
Figure 4. Forest plot showing odds ratio and 95% confidence intervals (CIs) of continuous variables (A) and binary variables (B) for the univariate logistic regression. Bar plot on the right indicates p values after multiple comparison. The red line indicates significance (FDR <0.05). Intervention for previous CTS was excluded for absence of amyloidosis in either group.
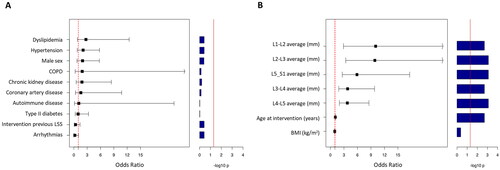
Table 2. Histopathology reports of the amyloid + cohort.
Table 3. Association between age, mean ligamentum flavum thickness and amyloid detection.
Clinical data
Patients with amyloid deposits were older when compared to patients without (73.0 ± 9.2 vs. 64.6 ± 10.1 years, p = .01) (). No difference in sex, comorbidities, previous surgery for CTS or LSS was observed between patients with and without amyloid deposits ( and ). All patients undergoing previous surgery for CTS had ATTR detected on ligamentum flavum compared to 70% with no previous surgery for CTS.
Table 4. Association between comorbidities and amyloid detection.
Discussion
Our prospective single-centre study reveals ATTR deposits in the ligamentum flavum of 64.9% (61/94) of unselected patients with >50 years of age without a personal history of systemic amyloidosis undergoing decompression surgery for LSS. Additional 13.8% (13/94) of patients had Congo red positive amyloid deposits, which could not be further classified by IHC. Thus, local amyloid deposition – mostly of the ATTR subtype – seems to be a major pathophysiological mechanism in LSS, occurring in 78.7% (74/94) of patients with advanced LSS in need of surgery. Amyloid deposition in the ligamentum flavum is associated with age and ligamentum flavum thickness, but is not gender-dependent, suggesting that local amyloid deposition occurs progressively, and in a time-dependent manner.
When we started our study in 2019, no data from prospective trials were available on the prevalence of ATTR in patients with LSS in a geographical region where hereditary ATTR amyloidosis (ATTRv) is not endemic [Citation17–19]. Retrospective data suggested that 14% of patients with proven ATTRwt amyloidosis had a prior history of LSS and case series raised the suspicion that amyloid might be present in virtually all patients with LSS, but subtyping by IHC was often inconclusive, with ATTR detection in only 20–45% of the Congo red positive biopsy specimens [Citation7,Citation18,Citation19]. Our data compare favourably with these reports, as we detected ATTR by IHC in 82.4% (61/74) of Congo red positive tissue biopsies. Technical differences in specimen preparation and IHC are the most likely reason for the excellent results obtained in our reference laboratory [Citation13,Citation20]. Compared to a recent publication by George et al., the divergence in the results is even more resounding: in their retrospective series of 178 patients, only the 13.5% of the total was positive to ATTRwt [Citation21]. Of note, in this study the authors considered the thickness of the ligamentum flavum in ATTRwt positive and ATTRwt negative cases, while we considered the thickness in relation to the presence of amyloid or its absence, irrespective of amyloid type.
In 2015, Yanagisawa et al. demonstrated the clinical, radiological and pathological correlations of ATTR deposits of the ligamentum flavum in a series of 56 patients with LSS and 19 patients with lumbar disc herniation. In this study, amyloid was found in the total of the specimens of the patients with LSS, but IHC revealed the presence of ATTR in less than half of the samples. Nevertheless, they noted a positive significant correlation with age and thickness of the ligamentum flavum [Citation19]. In comparison to this study, our analysis includes a larger and more homogeneous population, excluding lumbar disc herniation.
Most studies report a heterogeneous prevalence of amyloid deposits in patients with LSS [Citation22–24]. A large study with 324 patients from Tufts Medical Center found Congo red positive amyloid deposits in only 19% of the patients, and ATTR was confirmed by mass spectrometry in 13% of the patients [Citation24]. The much lower ATTR prevalence, when compared to our study, can only in part be explained by differences in patient age (median age 66 years, and 73 years, respectively). A Swedish group found a prevalence of amyloid in ligamentum flavum of 88.4% in patients with a mean age of 67.8 years, which is in line with our findings. However, in contrast to our study, ATTR typing by IHC was positive in only 37% of patients, which is almost two-times less, when compared to our patient population [Citation23]. We can only speculate about the reasons for the lower overall amyloid detection rate in the Tufts Medical Center population, and the lower positivity for ATTR in the Swedish cohort. Given the challenges for both Congo red staining as well as IHC, we suppose that technical differences rather than a real difference in prevalence are the reasons for the observed incongruities between group variations [Citation22–24]. Another reason for the lower prevalence in the Tufts Medical Center series might be the inclusion of the cervical and thoracic levels, which seem rather atypical regions of amyloid deposition [Citation25].
In our cohort, the gender distribution of patients with ATTR deposition in the ligamentum flavum is noteworthy and almost even. This is in contrast with studies among patients with ATTRwt amyloidosis, where a striking male predominance of up to 86.9% is reported [Citation26]. Interestingly, this rate demonstrated a 30% drop when considering exclusively patients older than 80 years of age. In a study considering sex differences in patients with ATTRwt amyloidosis from the Transthyretin Amyloidosis Outcomes Survey (THAOS), females with ATTRwt amyloidosis presented at a later age and showed signs of more severe walking impairment in comparison with male patients [Citation27]. It is therefore likely that there are strong gender-based differences in the occurrence of cardiac ATTRwt amyloidosis and peripheral neuropathy in patients with stenosis of the lumbar spine.
Taken together, our data support the hypothesis that amyloid deposition is a major reason for LSS development, with ATTR type being the most common subtype. Nevertheless, the mechanism of ligamentum flavum hypertrophy is still unclear [Citation28]. Recently, it was shown that C9 is commonly found in diverse types of amyloid, including ATTR in CTS [Citation14]. Complement 9, in fact, is a pivotal element of the membrane attack complex (MAC) and given its role in cell disruption, further studies are warranted to clarify whether C9 could be a pathogenetic trigger in the development of amyloid fibrils or a result of cell modifications occurring after the deposition of the altered protein [Citation14]. This now also applies to ATTR in the ligamentum flavum. Complement 9 was present within ATTR in our patient population, lending support to the hypothesis that ATTR in LSS is also associated with an activation of the complement cascade, as it was shown for ATTR in CTS [Citation29]. Further studies on C9 detection are warranted to better characterize the role of patterns and grade of C9 staining. Other hypotheses for ligamentum flavum thickening include mechanical stress, inflammatory cytokines, growth factors and microRNAs [Citation22]. MRI data from our cohort suggest that ligamentum flavum thickness increases, particularly at the L1–L2 and L2–L3 levels where relatively stronger associations were found (), might represent a putative biomarker of LSS development related to amyloid deposition. However, longitudinal multicentre investigations, including cohort stratification according to patients’ demographic and LSS clinical characteristics, are warranted to generalize our findings and validate their potential predictive value.
Limitations of the study are represented by the relatively small sample size due to the single-centre setting of the study. The study inclusion rate of 37.1% must be attributed to the COVID-19 pandemic, and the cancellation of the pre-surgical assessment visits, in line with the institutional policy against the diffusion of SARS-CoV-2. Furthermore, the histopathological and IHC work-up in our reference pathology laboratory might not be generally reproducible, as we used non-commercially available antibodies for amyloid subtyping [Citation13]. Another limitation is the lack of genetic information regarding transthyretin gene (TTR) mutations and the lack of a systematic cardiac assessment at baseline, which does not allow to draw conclusions regarding the presence of systemic ATTR amyloidosis in our patients with ATTR detected in ligamentum flavum biopsies.
Based on our experience, we support the idea of a histological work-up of ligamentum flavum biopsies in patients undergoing decompression surgery for LSS. Our data, together with the data of the Swedish group, suggest that the vast majority of patients with LSS present with amyloid deposits in the ligamentum flavum, mostly of the ATTR subtype [Citation23].
Identifying patients with LSS at risk for systemic ATTR amyloidosis will be a challenge that has to be addressed in future studies. Following study conclusion, all our patients with evidence of amyloid deposition were offered multidisciplinary evaluation at the Amyloidosis Centre Ticino. These patients will undergo a comprehensive cardiological investigation including cardiac MRI and genetic testing for TTR mutations. In a second step, these patients will undergo longitudinal observation and screening if no signs for ATTR amyloidosis are detected. First data from other groups indicate that systemic ATTRwt amyloidosis might be present in a low subset of patients with ATTR in LSS and CTS [Citation23,Citation30]. However, so far no predictive markers for patients at risk of developing cardiac involvement could be established.
Conclusions
Amyloid deposition, mostly of the ATTR subtype, is present in four out of five patients undergoing interventional LSS decompression surgery. Older age and thickness of the ligamentum flavum, but not sex correlate with the presence of amyloid deposits. Based on our experience, we support the idea of a histological work-up of ligamentum flavum biopsies in patients undergoing decompression surgery for LSS. Even though the information might not yet have immediate consequences, it might proof useful for future decision making, including, e.g. a cardiac assessment to rule out myocardial involvement. Longitudinal observational studies are needed to identify LSS patients at risk of developing cardiac ATTR amyloidosis at a later stage.
Author contributions
Adalgisa Condoluci and Bernhard Gerber designed the study, interpreted data and wrote the manuscript. Francesco Marchi performed neurosurgical interventions and biopsies and wrote the manuscript. Chiara Kessler managed sample logistics and biobanking and contributed to manuscript revision. Lodovico Terzi di Bergamo performed bioinformatic analysis, interpreted data and contributed to manuscript preparation. Emanuele Crupi and Fabio Bergamini managed clinical data collection and contributed to manuscript revision. Georg Stussi and Claudio Gobbi provided key scientific insights, contributed to data interpretation, and manuscript revision. Pietro Scarone, Dominique E. Kuhlen and Luca Fumagalli performed neurosurgical interventions and biopsies and contributed to data interpretation and manuscript revision. Christoph Röcken performed pathology studies and contributed to data interpretation and manuscript revision. Daniela Distefano and Emanuele Pravatà performed neuro-radiological assessment, interpreted data results and contributed to manuscript revision.
| Abbreviations | ||
| AL | = | light chain |
| ATTR | = | transthyretin amyloid |
| ATTRv | = | variant transthyretin amyloid |
| ATTRwt | = | wild-type transthyretin amyloid |
| BMI | = | body mass index |
| CIs | = | confidence intervals |
| CTS | = | carpal tunnel syndrome |
| FDR | = | false discovery rate |
| HR | = | hazard ratio |
| IQR | = | interquartile range |
| LSS | = | lumbar spinal stenosis |
| MRI | = | magnetic resonance imaging |
| TTR | = | transthyretin gene |
Supplemental Material
Download MS Power Point (176.2 KB)Supplemental Material
Download MS Word (13.9 KB)Disclosure statement
All the authors declare no conflicts of interest.
Data availability statement
Single patient data available upon motivated request by email to the corresponding author.
Additional information
Funding
References
- Benson MD, Buxbaum JN, Eisenberg DS, et al. Amyloid nomenclature 2020: update and recommendations by the International Society of Amyloidosis (ISA) Nomenclature Committee. Amyloid. 2020;27(4):217–222. doi: 10.1080/13506129.2020.1835263.
- Sueyoshi T, Ueda M, Jono H, et al. Wild-type transthyretin-derived amyloidosis in various ligaments and tendons. Hum Pathol. 2011;42(9):1259–1264. doi: 10.1016/j.humpath.2010.11.017.
- Solomon A. Management of AL amyloidosis in 2021+. Amyloid. 2022;29(1):66. doi: 10.1080/13506129.2021.1974831.
- Rahel S, Flammer Andreas J, Sabine G, et al. Expert recommendation from the Swiss Amyloidosis Network (SAN) for systemic AL-amyloidosis. Swiss Med Wkly. 2020;150:w20364.
- Gilstrap LG, Dominici F, Wang Y, et al. Epidemiology of cardiac amyloidosis-associated heart failure hospitalizations among fee-for-service Medicare beneficiaries in the United States. Circ Hear Fail. 2019;12:e005407.
- Maurer MS, Bokhari S, Damy T, et al. Expert consensus recommendations for the suspicion and diagnosis of transthyretin cardiac amyloidosis. Circ Heart Fail. 2019;12(9):e006075. doi: 10.1161/CIRCHEARTFAILURE.119.006075.
- Aus Dem Siepen F, Hein S, Prestel S, et al. Carpal tunnel syndrome and spinal canal stenosis: harbingers of transthyretin amyloid cardiomyopathy? Clin Res Cardiol. 2019;108(12):1324–1330. doi: 10.1007/s00392-019-01467-1.
- Westin O, Fosbøl EL, Maurer MS, et al. Screening for cardiac amyloidosis 5 to 15 years after surgery for bilateral carpal tunnel syndrome. J Am Coll Cardiol. 2022;80(10):967–977. doi: 10.1016/j.jacc.2022.06.026.
- Sperry BW, Reyes BA, Ikram A, et al. Tenosynovial and cardiac amyloidosis in patients undergoing carpal tunnel release. J Am Coll Cardiol. 2018;72(17):2040–2050. doi: 10.1016/j.jacc.2018.07.092.
- Maurer MS, Schwartz JH, Gundapaneni B, et al. Tafamidis treatment for patients with transthyretin amyloid cardiomyopathy. N Engl J Med. 2018;379(11):1007–1016. doi: 10.1056/NEJMoa1805689.
- APOLLO-B. A study to evaluate patisiran in participants with transthyretin amyloidosis with cardiomyopathy (ATTR amyloidosis with cardiomyopathy). ClinicalTrials.gov; 2022 [cited 2022 Oct 21]. Available from: https://clinicaltrials.gov/ct2/show/NCT03997383
- Puchtler H, Sweat F, Levine M. On the binding of Congo red by amyloid. J Histochem Cytochem. 1962;10(3):355–364. doi: 10.1177/10.3.355.
- Hahn K, Nilsson KPR, Hammarström P, et al. Establishing and validating the fluorescent amyloid ligand h-FTAA (heptamer formyl thiophene acetic acid) to identify transthyretin amyloid deposits in carpal tunnel syndrome. Amyloid. 2017;24(2):78–86. doi: 10.1080/13506129.2017.1316711.
- Lux A, Gottwald J, Behrens HM, et al. Complement 9 in amyloid deposits. Amyloid. 2021;28(3):199–208. doi: 10.1080/13506129.2021.1932799.
- Sakamaki T, Sairyo K, Sakai T, et al. Measurements of ligamentum flavum thickening at lumbar spine using MRI. Arch Orthop Trauma Surg. 2009;129(10):1415–1419. doi: 10.1007/s00402-009-0849-1.
- Bender R, Lange S. Adjusting for multiple testing – when and how? J Clin Epidemiol. 2001;54(4):343–349. doi: 10.1016/s0895-4356(00)00314-0.
- D'Agostino AN, Mason MS, Quinn SF. Lumbar spinal stenosis and spondylosis associated with amyloid deposition in the ligamentum flavum. Clin Neuropathol. 1992;11(3):147–150.
- Westermark P, Westermark GT, Suhr OB, et al. Transthyretin-derived amyloidosis: probably a common cause of lumbar spinal stenosis. Ups J Med Sci. 2014;119(3):223–228. doi: 10.3109/03009734.2014.895786.
- Yanagisawa A, Ueda M, Sueyoshi T, et al. Amyloid deposits derived from transthyretin in the ligamentum flavum as related to lumbar spinal canal stenosis. Mod Pathol. 2015;28(2):201–207. doi: 10.1038/modpathol.2014.102.
- Benson MD, Berk JL, Dispenzieri A, et al. Tissue biopsy for the diagnosis of amyloidosis: experience from some centres. Amyloid. 2022;29(1):8–13. doi: 10.1080/13506129.2021.1994386.
- George KM, Hernandez NS, Breton J, et al. Increased thickness of lumbar spine ligamentum flavum in wild-type transthyretin amyloidosis. J Clin Neurosci. 2021;84:33–37. doi: 10.1016/j.jocn.2020.11.029.
- Wang AY, Saini H, Tingen JN, et al. The relationship between wild-type transthyretin amyloid load and ligamentum flavum thickness in lumbar stenosis patients. World Neurosurg. 2022;164:e113–e118. doi: 10.1016/j.wneu.2022.04.008.
- Eldhagen P, Berg S, Lund LH, et al. Transthyretin amyloid deposits in lumbar spinal stenosis and assessment of signs of systemic amyloidosis. J Intern Med. 2021;289(6):895–905. doi: 10.1111/joim.13222.
- Godara A, Riesenburger RI, Zhang DX, et al. Association between spinal stenosis and wild-type ATTR amyloidosis. Amyloid. 2021;28(4):226–233. doi: 10.1080/13506129.2021.1950681.
- George KM, Dowd RS, Nail J, et al. Wild-type transthyretin amyloidosis occurring in the ligamentum flavum of the cervicothoracic spine. World Neurosurg. 2020;142:e325–e330. doi: 10.1016/j.wneu.2020.06.228.
- Kroi F, Fischer N, Gezin A, et al. Estimating the gender distribution of patients with wild-type transthyretin amyloid cardiomyopathy: a systematic review and meta-analysis. Cardiol Ther. 2021;10(1):41–55. doi: 10.1007/s40119-020-00205-3.
- Campbell CM, LoRusso S, Dispenzieri A, et al. Sex differences in wild-type transthyretin amyloidosis: an analysis from the transthyretin amyloidosis outcomes survey (THAOS). Cardiol Ther. 2022;11(3):393–405. doi: 10.1007/s40119-022-00265-7.
- Tasaki M, Okada M, Yanagisawa A, et al. Apolipoprotein AI amyloid deposits in the ligamentum flavum in patients with lumbar spinal canal stenosis. Amyloid. 2021;28(2):107–112. doi: 10.1080/13506129.2020.1858404.
- Treitz C, Gottwald J, Gericke E, et al. Quantitative proteome profiling provides evidence of an activation of the complement cascade in ATTR amyloidosis. Amyloid. 2022;29(2):102–109. doi: 10.1080/13506129.2021.2015316.
- Maurer MS, Smiley D, Simsolo E, et al. Analysis of lumbar spine stenosis specimens for identification of amyloid. J Am Geriatr Soc. 2022;70(12):3538–3548. doi: 10.1111/jgs.17976.