ABSTRACT
Objective
Abnormal visual processing has been proposed as a mechanism underlying excessive focus on minor appearance flaws in body dysmorphic disorder (BDD). Existing BDD research has not differentiated the various stages of face processing (featural, first-order configural, holistic and second-order configural) that are required for higher-order processes such as emotion recognition. This study investigated a hierarchical visual processing model to examine the nature of abnormalities in face processing in BDD.
Method
Thirty BDD participants and 27 healthy controls completed the Navon task, a featural and configural face processing task and a facial emotion labelling task.
Results
BDD participants performed similarly to controls when processing global and local non-face stimuli on the Navon task, when detecting subtle changes in the features and spacing of a target face, and when labelling emotional faces. However, BDD participants displayed poorer performance when viewing inverted faces, indicating difficulties in configural processing.
Conclusions
The findings only partially support prior work. However, synthesis of results with previous findings indicates that heterogenous task methodologies may contribute to inconsistent findings. Recommendations are provided regarding the task parameters that appear most sensitive to abnormalities in BDD.
Body dysmorphic disorder (BDD) is characterised by preoccupation with a perceived flaw/s in physical appearance (American Psychiatric Association, Citation2013). Distortions in visual perception may underlie excessive focus on minor appearance flaws in BDD (e.g. Beilharz et al., Citation2017; Li et al., Citation2013), with a bias towards detailed visual processing, over holistic or global processing (Feusner, Moody, et al., Citation2010; Feusner et al., Citation2007; Kerwin et al., Citation2014; Li et al., Citation2013). Individuals with BDD describe heightened focus on disliked physical features when looking in the mirror or mentally picturing themselves (Veale, Citation2004; Veale & Riley, Citation2001); and eye-tracking studies find some BDD participants focus excessively on “problem areas’ in visual scanning (Greenberg et al., Citation2014; Toh et al., Citation2015, Citation2017). Further, neurobiological models of BDD propose an imbalance between first and second-order visual processing networks could underlie overreliance on detailed rather than holistic processing (Grace et al., Citation2017).
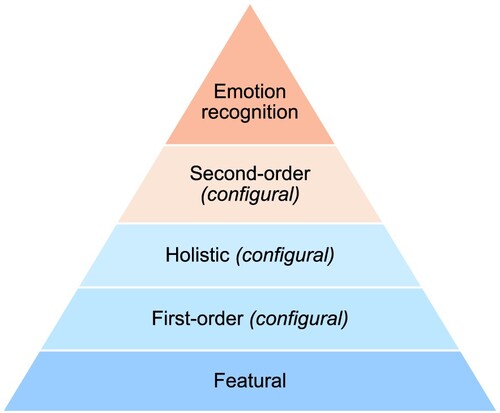
Whereas behavioural studies in BDD have investigated local and global processing in relation to faces specifically (e.g. Feusner et al., Citation2007; Jefferies et al., Citation2012; Monzani et al., Citation2013), this work has arguably not considered the multidimensional nature of human face processing (Maurer et al., Citation2002; Zhen et al., Citation2013). Research in healthy individuals has identified consequential steps in efficient processing of human faces (Maurer et al., Citation2002): featural processing (identifying local facial information, e.g. eyes or nose, in a piecemeal manner; Duchaine & Weidenfeld, Citation2003); first-order configural processing (identifying typical, shared facial configuration of two eyes situated earlier nose and mouth, hence distinguishing faces from other objects); holistic processing (combining individual features into a whole); and second-order configural processing (detecting subtle variation in spatial distances between features; (Maurer et al., Citation2002; Taubert et al., Citation2011). These processes facilitate higher-order facial perception, including accurate identification of faces and emotional expressions (Zhen et al., Citation2013); see ).
Figure 1. Proposed hierarchy of the stages of visual processing of human faces.
Notes: Holistic and first-order configural processes are often challenging to disentangle in existing cognitive tasks; as such, they are considered as separate, but parallel process in the current study.
Intact configural processing is crucial for higher-order visual processes, such as facial identification. This is demonstrated through the face inversion effect, whereby healthy individuals show reduced accuracy and longer reaction times (RT) when processing inverted rather than upright faces (Farah et al., Citation1995). Face inversion disrupts first-order configural information, leading to piecemeal processing of individual features, rather than more efficient holistic processing (Freire et al., Citation2000).Footnote1 Whereas inversion can also disrupt the ability to discern second-order configural information from the face, this has not been found to contribute as strongly to the inversion effect as first-order configural information (Civile et al., Citation2016)
Face inversion studies in BDD have yielded inconsistent results, with some reporting reduced inversion effects relative to controls (Feusner, Moller, et al., Citation2010; Toh et al., Citation2017) and others demonstrating no differences (Monzani et al., Citation2013). Feusner, Moller and colleagues (Citation2010) found reduced inversion effects in BDD participants on long stimulus duration trials (5000 ms), but similar performance to controls on short trials (500 ms). As such, they proposed that individuals with BDD prioritise detailed processing with longer durations, but rely on configural processing when there is insufficient time to process specific details (Feusner, Moller et al., Citation2010).
Second-order configural processing has been interrogated by manipulating spatial frequency of facial stimuli which alters the level of available visual detail. When processing faces at low (global/configural information), normal (configural and featural) and high (local/featural information) spatial frequencies, BDD participants and healthy controls (HC) show no differences in response accuracy or RTs (Feusner et al., Citation2007; Li, Lai, Bohon, et al., Citation2015; Li, Lai, Loo, et al., Citation2015; Moody et al., Citation2015). Similarly, when discriminating between pairs of faces digitally manipulated to display either featural or configural differences, BDD participants exhibit similar accuracy and RTs to HCs (Monzani et al., Citation2013). These findings suggest no second-order configural processing deficits in BDD.
Reduced accuracy and/or slower RTs in BDD participants on higher-order emotion labelling tasks has been more consistently demonstrated (Buhlmann et al., Citation2006; Buhlmann et al., Citation2004; Feusner, Bystritsky et al., Citation2010; Grace et al., Citation2019), with specific impairments for neutral or negative emotions (Buhlmann et al., Citation2011; Grace et al., Citation2019; Jefferies et al., Citation2012). Others have found BDD participants interpret negative emotions (e.g. disgust and anger) in neutral faces (Buhlmann et al., Citation2006, Citation2011), or mistake other emotions as anger (Toh et al., Citation2015). Conversely, one study found BDD and HCs did not differ in emotion recognition performances (Rossell et al., Citation2014). Discrepancies across studies may be explained by sampling (e.g. medication status, comorbidities, BDD severity) or methodological differences (e.g. task design). Additionally, it remains unclear whether emotion processing alterations in BDD are driven by top-down changes (e.g. attentional biases, emotional dysregulation), or by alterations in lower-level visual processes disrupting efficient facial emotion processing.
Research aims
We aimed to clarify facial processing aberrations in BDD, employing a hierarchical model (). We used tasks which allowed distinct analysis of featural, first-order/holistic, second-order configural face processing, and higher-order facial emotion labelling, to investigate visual processing in BDD compared to healthy participants. Whilst the primary goal was to explore distorted face processing, we included the Navon task (which assesses global versus local processing of letters; Navon, Citation1977) to determine whether visual processing aberrations extend to simple, non-face stimuli, which would implicate lower-level processes on the hierarchy. Our specific research questions were:
Are there behavioural differences between BDD and HC groups when processing local and global features of nonface stimuli?
Do BDD participants demonstrate different face inversion effects to HCs (suggestive of disrupted holistic and/or first-order configural processing)?
How do BDD participants perform when detecting subtle configural and featural changes in upright faces (i.e. featural and second-order configural processing when inversion is not considered)?
How do BDD participants perform when identifying emotional faces?
Are emotion perception deficits related to lower-level (i.e. featural and configural) face processing abnormalities in BDD?
To contextualise the findings, we provide a conceptual review of the BDD visual face processing literature in our discussion. Here, we aimed to identify what task parameters are most sensitive to abnormalities in each of the aforementioned levels of processing in BDD (Research Question 6).
Method
The study was approved by the Alfred Hospital Ethics Committee, Australian Catholic University and Swinburne University of Technology Human Research Ethics Committees and abided by the Declaration of Helsinki. All participants provided written informed consent.
Participants
Thirty adults (18 years and older) with BDD and 27 HCs were recruited through community and outpatient psychiatric services and public advertisements. BDD diagnosis was confirmed using the BDD Diagnostic Module (Phillips, Citation2005) for the Diagnostic and Statistical Manual of Mental Disorders – Fifth Edition (DSM-V; American Psychiatric Association, Citation2013). Thirteen BDD (43.3%) participants were taking stabilised psychiatric medications. Psychiatric comorbidities in the BDD group were assessed using the Mini International Neuropsychiatric Interview 7.0.2 (MINI 7.0.2; Sheehan, Citation2016; see Supplementary Table 1). The MINI was used to confirm the absence of psychiatric disorders in the HC group. All participants were required to speak fluent English, have no history of brain injury or neurological disorders, or uncorrected visual or hearing impairments. They were also required to have an estimated IQ earlier 70 based on the Wechsler Test of Adult Reading, to facilitate meaningful participation and informed consent (Wechsler, Citation2001). BDD participants were administered the Yale-Brown Obsessive-Compulsive Scale – Modified for BDD (BDD-YBOCS; Phillips et al., Citation1997) to assess symptom severity, and the Brown Assessment of Beliefs Scale (Eisen et al., Citation1998) to measure insight.
Procedure and materials
All participants completed three visual processing tasks, presented on a laptop using Presentation® software (Version 18.0, Neurobehavioural Systems, Inc., Berkeley, CA).
Local and global non-face processing
Local and global processing of non-face stimuli was assessed using the Navon task (Navon, Citation1977) to address Research Question 1. Participants were presented with a global letter (“H” or “S”) formed by a configuration of smaller, local letters. Participants completed two counterbalanced blocks of 95 trials each, where they were instructed to identify either the local or global letter by pressing the H or S key on the keyboard. Trials began with a 500 ms fixation cross, followed by the stimulus, which remained on-screen until a response was made. Accuracy and RTs were recorded. Healthy participants generally demonstrate a global precedence effect (i.e. faster RTs and higher accuracy on global rather than local trials) on this task (Navon, Citation1977).
Featural and configural face processing
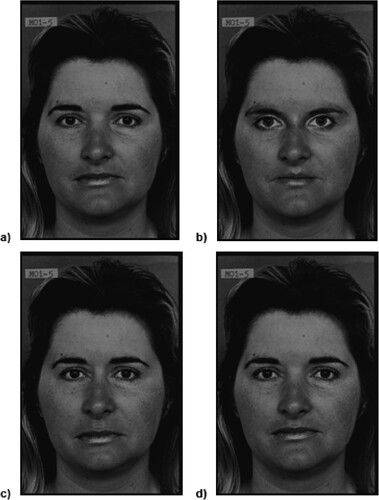
A facial discrimination task (Joshua et al., Citation2016) was used to investigate featural, first-order, and second-order configural processing (Research Questions 2 and 3). A neutral female face from the Pictures of Facial Affect Series (Ekman & Friesen, Citation1976) was manipulated to display either configural or featural changes (detailed description in Joshua et al., Citation2016). Featural manipulations involved replacing the eyes, nose, or mouth on the template face with features from other faces, whereas configural manipulations involved adjusting the spacing between the eyes, nose, and mouth (). Four configural and four featural face manipulations were created. Pairs of face stimuli were presented on-screen for 5000 ms, followed by a 300 ms fixation cross. Participants indicated via two-button press whether the faces were the “same” or “different”. RTs and accuracy rates were recorded. The task was presented in two counterbalanced blocks (configural, featural sets; 160 trials each). To investigate inversion effects, each pair of faces (80 different pairs) was presented in both upright and inverted orientation, randomised within each block. Inversion effects were calculated by subtracting accuracy and RTs on inverted trials from upright trials.
Facial emotion labelling
The facial emotion labelling task (Van Rheenen & Rossell, Citation2014) was used to assess Research Questions 4 and 5. It was designed to assess participants’ ability to identify emotional expressions as portrayed by five male and five female greyscale models from the Ekman and Friesen (Citation1976) series. Each model portrayed six basic expressions: happiness, sadness, anger, fear, disgust and neutrality. Individual face stimuli were presented on-screen for 2000ms (1500 ms interstimulus intervals). Participants identified the emotional expression displayed on the target face by pressing one of six corresponding keys. Stimuli were randomised across 160 trials. Accuracy rates (i.e. percentage of correctly identified emotions), RTs and mistakes (i.e. mistaking one emotional expression as another) were recorded.
Statistical analyses
Demographic and clinical group differences were assessed via t-tests or chi-squared analyses. A series of mixed-design analyses of variance (ANOVA) were computed to assess whether the BDD and HC groups differed on visual tasks. Significant interaction effects were followed up with planned post-hoc tests, with Bonferroni corrections for multiple comparisons. To explore non-face visual processing (Research Question 1), two mixed-design ANOVAs (group x trial type) were computed to assess group differences in RT and accuracy on global and local trials of the Navon task. To determine whether first-order/holistic configural processing differed between groups (Research Question 2), inversion effects were calculated from the facial discrimination task, providing accuracy and RT scores for four inversion effect conditions (configural same, configural different, featural same and featural different). Inversion effect scores were entered into a three-way mixed-design ANOVA (group x task type [configural or featural] x face pair [same or different]). To assess featural and second-order configural processing between groups (Research Question 3), RTs and accuracy rates from upright face trials only were entered into mixed-design ANOVAs (group x task type [configural or featural] x face pair [same or different]). To address the Research Question 4, group differences in RTs, accuracy rates and mistakes on the facial emotion labelling task were assessed via two-way mixed-design ANOVAs (group x emotion). Tasks that produced significant group differences were then correlated with one another to identify shared mechanisms (Research Question 5).
Results
There were no significant differences between the BDD and HC groups in age, IQ, educational attainment, or occupational status (). Performance on visual processing tasks did not differ by medication status in the BDD group (Supplementary Table 2).
Table 1. Demographic and clinical features of the BDD and HC groups.
Global and local non-face processing: the Navon task
One outlier in the HC group was removed from Navon task analyses due to extended RTs. RTs were significantly faster on global (rather than local) trials for all participants, F(1, 54) = 47.39, p < .001, η2p = .47. The BDD group responded more slowly overall (to both global and local trials) than HCs, F (1, 54) = 4.51, p = .04, η2p = .08. There was no significant group-by-trial interaction effect for RT, F (1, 54) = 1.12, p = .30, η2p = .02, and no significant main effects or interactions in accuracy rates.
First-order and holistic processing: inversion effects
RT
Mean inversion effects are presented in . There were no significant main effects of group for RT inversion effects (F(1, 53) = .001, p = .96, η2p = .001) or trial type, F(1, 53) = .01, p = .92, η2p = .001. A main effect was observed between “same” and “different” trials, whereby larger inversion effects occurred on “different” face pairs in both groups, F(1, 53) = 29.30, p < .001, η2p = .36. A three-way interaction was observed between group, task type, and same/different trials, F(1, 53) = 4.03, p = .05, η2p = .07. In the BDD group, significant inversion effects were observed on featural and configural “different” trials (faster and more accurate processing of upright faces), but RTs were unaffected by inversion on “same” trials ((a) and (b)). The HC group displayed significant RT inversion effects only on “different” configural trials.
Figure 3. Inversion effects on the featural and configural face processing tasks.
Notes: RTs depicted for (a) featural trials and (b) configural trials. Accuracy rates are shown for (c) featural trials and (d) configural trials. HC = healthy control group. *denotes significant inversion effect at p < .001.
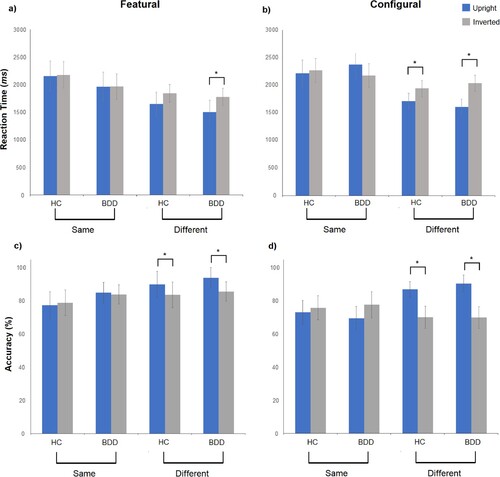
Table 2. Differences in face inversion effects between the BDD and HC groups.
Post-hoc tests are displayed in . Whereas inversion effects were similar between BDD and HCs on featural trials, significant differences emerged on configural trials. For “same” trials, BDD participants displayed a positive inversion effect (slower processing on upright rather than inverted trials), but a negative inversion effect (slower processing on inverted rather than upright trials) on “different” trials. For both “same” and “different” configural trials, BDD participants demonstrated larger inversion effects than controls.
Accuracy
Mixed-design ANOVA results revealed no significant main effect of group, F(1, 53) = .20, p = .65, η2p = .001, or interaction terms. However, a main effect of task condition was observed, F(1, 53) = 6.15, p = .02, η2p = .10, indicating that accuracy inversion effects were significantly larger for all participants on configural rather than featural trials. BDD and HC groups displayed reduced accuracy when faces were inverted on “different” trials ((c) and (d)). Accuracy was unaffected by inversion on “same” trials in both groups.
Second-order configural processing
Accuracy and RTs on upright face trials are presented in . The mixed-design ANOVA revealed a significant main effect of trial type, F(1, 53) = 7.91, p = .007, η2p = .13, with faster RTs overall on featural rather than configural trials. BDD and HC participants performed faster on “different” rather than “same” trials, F(1, 53) = 55.87, p < .001, η2p = .51. There were no significant main effects of group, F(1, 53) = .38, p = .54, η2p = .01, or interactions between group and trial type, F(1, 53) = 2.99, p = .09, η2p = .05. The overall sample also achieved higher accuracy on featural rather than configural trials, F(1, 53) = 8.40, p = .005, η2p = .14, and on “different” rather than “same” trials, F(1, 53) = 38.77, p < .001, η2p = .42. There was no significant main effect of group, F(1, 53) = .88, p = .35, η2p = .02, or interaction between group and trial type, F(1, 53) = 1.60, p = .21, η2p = .03.
Table 3. RTs and accuracy rates for upright featural and configural trials.
Facial emotion labelling
Average RTs and accuracy rates on the facial emotion labelling task are depicted in .
RT
RTs differed across emotions, F(5, 52) = 67.90, p < .001, η2p = .88, with both groups responding fastest to happy faces, and slowest to angry faces (see ). Overall RTs were similar between BDD and HC groups, F(1, 52) = .001, p = .95, η2p = .001, with no significant group-by-emotion interaction, F(5, 52) = .52, p = .76, η2p = .01.
Table 4. RTs and accuracy rates on the facial emotion labelling task.
Accuracy
Results from the mixed-design ANOVA (group x emotion) for accuracy revealed a significant main effect of emotion, F(5, 52) = 83.25, p < .001, η2p = .62, with accuracy being highest for happy faces and lowest for angry faces in both groups. There were no significant group differences in accuracy rates, F(1, 52) = .61, p = .44, η2p = .01, and no group-by-emotion interaction, F(5, 52) = 1.00, p = .41, η2p = .02.
Emotional biases
A significant main effect of emotion, F(5, 52) = 65.26, p < .001, η2p = .56, indicated that all participants were more likely to mistake other emotions as neutral, and least likely to mistake them as happy. There were no significant differences between groups in their mistake biases towards a particular emotion (F(1, 52) = 1.60, p = .21, η2p = .03) or group-by-emotion interaction effects (F(5, 52) = .68, p = .60, η2p = .01).
Discussion
We examined a multidimensional model of facial processing in BDD, to determine whether individuals with BDD display specific or widespread face processing abnormalities. Results revealed that BDD participants performed similarly to HCs on the Navon task, suggesting no differences in global and local processing of simple non-face stimuli (Research Question 1). Further, BDD and HC groups displayed similar accuracy inversion effects, with both groups being significantly less accurate in discriminating between inverted rather than upright faces. The BDD group further displayed slower RTs on both configural and featural inverted trials, whereas HCs were affected by inversion on configural trials only. Moreover, BDD participants displayed larger (rather than smaller) inversion effects than HCs on the configural task, indicating some deficits in first-order configural/holistic processing in BDD (Research Question 2). There were no differences between BDD and HCs in accuracy or RTs when identifying manipulations to details or spacing of facial features, suggesting no difficulties in featural and second-order configural processing (Research Question 3). Contrary to expectation, the BDD and HC groups achieved similar RTs and accuracy rates on the facial emotional labelling task, and BDD participants did not demonstrate any mistake biases (Research Question 4). Due to the lack of group differences on the emotion labelling task, correlations between the visual processing tasks were not computed (Research Question 5).
Our results are consistent with previous studies showing BDD patients perform normally on the Navon task (Monzani et al., Citation2013) and do not differ from HCs in their ability to detect subtle changes in facial features or spacing in upright faces (Feusner et al., Citation2007; Li, Lai, Loo, et al., Citation2015; Monzani et al., Citation2013; Moody et al., Citation2015). However, our findings regarding larger facial inversion effects in BDD contradict prior research (Feusner, Moller, et al., Citation2010; Jefferies et al., Citation2010; Toh et al., Citation2017). Notably, significant inversion effects only emerged on “different” pair trials. Other researchers have not separated these from “same” pair trials, which may require more “visual checks’ and thus produce longer RTs (Sergent, Citation1984). Furthermore, our finding of similar facial emotion recognition results among BDD and HCs contrasts with previous studies which showed poorer performance in BDD participants (Buhlmann et al., Citation2006; Buhlmann et al., Citation2004; Feusner, Bystritsky et al., Citation2010; Grace et al., Citation2019; Toh et al., Citation2015).
The discrepancy with prior results is unlikely due to sampling variability, as our participants exhibited moderate-to-severe symptom severity and poor insight, similar to previous studies. Thus, we suggest that discrepancies between findings of visual processing abnormalities in BDD may be due to methodological heterogeneity within this research domain. Our following synthesis of the existing literature regarding featural, configural, and emotional face processing in BDD within the context of the visual processing hierarchy () indeed suggests that task characteristics may play a role in discrepancies across findings (15 studies; ; two were excluded due to additional complexity or task demands beyond visual processing, see Supplementary Table 3).
Table 5. Summary of results and task methodologies used to assess face processing in BDD.
Featural face processing
Five studies have assessed featural face processing in BDD by digitally manipulating facial features on a target face, akin to our study (Monzani et al., Citation2013), or by presenting high spatial-frequency images (Feusner et al., Citation2007; Li, Lai, Bohon, et al., Citation2015; Li, Lai, Loo, et al., Citation2015; Moody et al., Citation2015). These studies varied in use of male and/or female faces, duration of stimulus presentation (ranging from 250 ms to 5000 ms), and response paradigms (i.e. utilising either a trio of faces requiring matching one of the bottom stimuli with the top face, or discrimination between two faces presented side-by-side). As no significant differences between BDD and HC have emerged in these studies, it is unlikely that stimuli presentation times or response format affect performance. Instead, as processing of facial features is proposed to be the most basic level of the hierarchy, it is possible that these tasks have been too easy, with ceiling effects concealing subtle abnormalities in the BDD group in both our study and previous works.
First-order and holistic configural processing
Inversion effects have been explored in six prior studies, using neutral expression faces (Feusner, Moller, et al., Citation2010; Monzani et al., Citation2013) or Mooney faces (fragmented, black and white faces; Toh et al., Citation2017). Our study and that of Monzani et al. (Citation2013) manipulated neutral human face photographs to display either featural or configural changes, whereas others manipulated both featural and configural elements in the same image (Feusner, Moller, et al., Citation2010). The former approach allows separate exploration of the effects of priming participants to configural or featural differences. Specially, inversion may have a greater effect on configural face processing conditions, as individuals are primed towards spatial information that is disrupted by inversion (Schwaninger & Mast, Citation2005; Sergent, Citation1984). Contrastingly, featural conditions prime participants toward facial features, which is considered a superior strategy for processing inverted faces (Schwaninger & Mast, Citation2005). Such effects were demonstrated in the current study, where larger accuracy inversion effects (i.e. poorer performances) were observed on configural rather than featural trials in both groups.
Unlike the current study, Toh et al. (Citation2017) used Mooney faces to examine inversion effects, revealing smaller inversion effects in BDD participants compared to healthy controls. Mooney faces include limited visual detail but are recognisable to healthy individuals as human faces when upright (McKone, Citation2004; Schwiedrzik et al., Citation2018). As Mooney stimuli lack detailed first- or second-order facial information, holistic processing becomes the primary mode of perception (Latinus & Taylor, Citation2005). Further, Toh et al. (Citation2017) used the Mooney task as a facial identification task where participants determine whether the target is a face or an object, rather than comparing elements between two face stimuli. The latter is considered a more challenging task (Tsao & Livingstone, Citation2008), and thus may be more sensitive to visual processing abnormalities. However, Mooney faces provide a more robust measure of holistic processing, which could assist in separating abnormalities of first-order and holistic stages of visual processing.
Cross-study differences in task design may also explain discrepancies across findings. For example, our study and Monzani et al. (Citation2013) required participants to determine whether simultaneously presented faces were the same or different. Conversely, Feusner, Moller et al. (Citation2010) required participants to view a target stimulus (for either 500 ms or 5000 ms), then determine which face in a subsequently presented pair of face images matched the target. This task design may have created additional memory and attentional demands (as compared to our design) that could explain study differences. Further, at short stimulus presentations (250-500 ms), neither Monzani et al. (Citation2013) or Feusner, Moller et al. (Citation2010) identified significant inversion effects among BDD participants. At longer stimulus presentations (5000 ms), Feusner, Moller et al. (Citation2010) reported significantly smaller inversion effects in the BDD group, though the opposite was found in our present study. This suggests that investigations of first-order configural processing in BDD should use presentation lengths of 5000 ms or longer. Yet, given these inconsistencies in inversion effect results, further research is needed to investigate first-order configural and/or holistic processing in BDD.
Finally, Monzani et al. (Citation2013) utilised the composite faces task, which asks participants to determine whether the top or bottom halves of two composite faces are the same or different (Monzani et al., Citation2013; Young et al., Citation1987). When the composite face halves are aligned, it is harder to attend to one half whereas ignoring the other, suggesting a reliance on holistic processing (for a review and illustration of the task, see Murphy et al., Citation2017). Misaligned composites facilitate better discrimination by disrupting the holistic template. Thus, individuals with BDD, who are purported to engage in detailed (over holistic) processing should perform better than controls when faces are aligned (Monzani et al., Citation2013). However, this was not supported by Monzani et al.’s (Citation2013) results, albeit the 200 ms presentation time might have been too brief. Future research should include both composite and inversion face tasks to disentangle effects of holistic and first-order configural processing.
Second-order configural processing
Five studies have assessed second-order configural processing using discrimination tasks involving digitally manipulated faces (Monzani et al., Citation2013) or by presenting low spatial-frequency images (Feusner et al., Citation2007; Li, Lai, Bohon, et al., Citation2015; Li, Lai, Loo, et al., Citation2015; Moody et al., Citation2015). Some have used matching paradigms, or same/different discrimination between two faces (Monzani et al., Citation2013). Varying stimuli, presentation times, and discrimination or matching designs have all failed to reveal significant differences between BDD and HCs. Taken together with our findings, second-order configural processing does not seem to be impaired in BDD.
Facial emotion recognition
Prior facial emotion recognition studies in BDD (n = 8) have typically used 6–10 faces from the Ekman and Friesen (Citation1976) series, half male and half female, assessing 4–7 basic emotions (Buhlmann et al., Citation2006; Buhlmann et al., Citation2004; Feusner, Bystritsky et al., Citation2010; Grace et al., Citation2019; Jefferies et al., Citation2012; Rossell et al., Citation2014; Toh et al., Citation2015). However, stimulus presentation times have varied widely (200 ms up to 15s, or no time limit). The present study and Rossell et al. (Citation2014) presented stimuli for 2000ms and did not observe significant differences between BDD and HC groups. Studies which presented stimuli >2000ms found BDD participants performed less accurately than controls (Buhlmann et al., Citation2006; Buhlmann et al., Citation2004; Toh et al., Citation2015). However, Grace et al. (Citation2019) directly compared 200 ms versus 2000ms stimulus presentations and found poorer performances in BDD (versus HC) across both durations. As our task design was similar to Grace et al. (Citation2019), our lack of group differences is unexpected but could reflect sampling of different neurocognitive subtypes in BDD. One of the largest investigations of cognition in BDD identified subgroups of individuals with “broadly impaired” or “selectively impaired” cognition, indicating significant cognitive heterogeneity within BDD samples which may be masked in aggregate data like the current study (Malcolm et al., Citation2020).
Furthermore, findings of group difference have been inconsistent in showing reduced accuracy overall, or reduced accuracy or RTs for specific emotions only (i.e. angry, neutral, sad). Moreover, Buhlmann et al. (Citation2006) found no differences between BDD and HC groups in “other-referent” situations, but BDD participants were less accurate in identifying emotions during self-referent scenarios (Buhlmann et al., Citation2006). The lack of group differences in our study could be influenced by participants not finding the stimuli personally relevant. Available evidence suggests that people with BDD have alterations in emotion processing, yet further research is needed to determine if stimulus presentation times (<2000ms to >2000ms) and self- or other-referent contexts impact processing performances.
General findings and limitations
Across all domains of face processing in prior BDD research, task instructions and randomisation strategies have varied. Methodological factors related to priming and stimulus presentation duration may explain inconsistent results among these studies and our current study. Firstly, the current study and studies which manipulated spatial frequency of faces (Li, Lai, Loo, et al., Citation2015; Moody et al., Citation2015) presented featural and configural stimuli in separate blocks, rather than interspersing these conditions randomly across trials. Block presentations may prompt participants to focus on either featural or configural elements, creating priming and attentional effects which could override natural visual processing tendencies (Monzani et al., Citation2013). Accordingly, Monzani et al.’s (Citation2013) study using separate global and local Navon task blocks did not report significant group differences, but Kerwin et al. (Citation2014), who randomised global and local Navon trials, found a greater fixation on visual detail and difficulty shifting away from local processing in BDD. Secondly, a similar argument could explain the finding that shorter trial durations do not produce significant visual processing abnormalities in BDD, but longer trials do. Allowing more time to respond may create greater opportunity to selectively attend to most salient visual aspects. As such, top-down influences may underlie difficulties on visual processing tasks in BDD, rather than a visual processing abnormality per se (Johnson et al., Citation2018). Thus, visual processing tasks should be designed to minimise demands on working memory or attention (e.g. randomising task conditions and minimising priming effects), to facilitate more direct assessment of visual system distortions. If group differences are not demonstrated on “pure” visual processing tasks, selective attention may be more central to the phenomenology of BDD, than perceptual abnormalities (Johnson et al., Citation2018; Kollei et al., Citation2017; Lambrou et al., Citation2011).
Several limitations are worth noting. The scope of this study was to investigate hierarchical visual processing difficulties on behavioural tasks. However, some studies described here may not have revealed behavioural group differences but identified unusual patterns in eye-tracking and neural activation (Beilharz et al., Citation2017). Incorporating behavioural, neuroimaging and eye-tracking studies into a future review may elucidate whether individuals with BDD experience altered visual processing patterns, which do not necessarily translate into differences in task performance. Further, the current research could be enhanced by multi-level modelling of the interrelationships between visual processing domains in BDD, though this requires larger samples. Additionally, we used a face inversion paradigm to explore holistic and first-order configural processing simultaneously. Future research should include other tasks that directly probe holistic visual processes (e.g. a composite or Mooney face task; Monzani et al., Citation2013; Toh et al., Citation2017), which would further increase the utility of a multidimensional model of face processing.
Conclusions
In summary, we found no widespread deficits in featural, configural and emotion processing in BDD. Given the discrepancy between these findings and previous literature, a synthesis and review of the task designs of previous studies was conducted, revealing wide variation in methodology. This variation emphasises the need for a unified consensus on the cognitive difficulties present in BDD, and the behavioural tasks which are most sensitive to these differences. A cognitive consensus battery could reinforce existing evidence regarding cognitive and visual difficulties in BDD, and may facilitate further exploration of the “cognitive subtype” hypothesis where some individuals with BDD present with cognitive difficulties whereas others do not (Malcolm et al., Citation2020). Further, a cognitive consensus battery could aid the development, monitoring and evaluation of visual and attention retraining programmes (e.g. Beilharz et al., Citation2018) to enhance clinical outcomes in BDD.
Supplemental Material
Download MS Word (28 KB)Acknowledgements
The views expressed herein are the authors’ opinions and do not reflect any official positions of the institutional affiliations or funding bodies. The authors do not have any conflicts to declare in relation to this manuscript.
Disclosure statement
No potential conflict of interest was reported by the author(s).
Data availability statement
The data that supports the findings of this study are available from the corresponding author, TP, upon reasonable request.
Additional information
Funding
Notes on contributors
Toni D. Pikoos
Toni D. Pikoos is a postdoctoral researcher at Swinburne University, and a clinical psychologist working primarily with body image conditions such as body dysmorphic disorder in Melbourne, Australia.
Amy Malcolm
Amy Malcolm is a postdoctoral researcher at Swinburne University, and is currently completing her Masters in Clinical Psychology in Melbourne, Australia.
David J. Castle
David J. Castle is a Professor of Psychiatry at the University of Tasmania and Co-Director of the Centre for Mental Health Service Innovation in Tasmania, Australia.
Susan L. Rossell
Susan L. Rossell is a Professor and Director of Clinical Trials at Swinburne University in Melbourne, Australia. She is also an Adjunct Research Professor in Psychiatry at St Vincent's Hospital, Melbourne.
Notes
1 First-order and holistic visual processes are often difficult to decouple, as they are simultaneously affected in inversion paradigms and are both required for efficient discrimination of second-order facial configuration (Piepers & Robbins, Citation2012; Taubert et al., Citation2011; Tsao & Livingstone, Citation2008). As such, first-order configural and holistic processing are considered as separate, but parallel processes within the current study.
References
- American Psychiatric Association. (2013). Diagnostic and statistical manual of mental disorders DSM-V.
- Beilharz, F., Castle, D. J., Grace, S., & Rossell, S. L. (2017). A systematic review of visual processing and associated treatments in body dysmorphic disorder. Acta Psychiatrica Scandinavica, 136(1), 16–36. https://doi.org/10.1111/acps.12705
- Beilharz, F., Castle, D. J., Phillipou, A., & Rossell, S. L. (2018). Visual training program for body dysmorphic disorder: Protocol for a novel intervention pilot and feasibility trial. Pilot and Feasibility Studies, 4(1), 189. https://doi.org/10.1186/s40814-018-0384-3
- Buhlmann, U., Etcoff, N., & Wilhelm, S. (2006). Emotion recognition bias for contempt and anger in body dysmorphic disorder. Journal of Psychiatric Research, 40(2), 105–111. https://doi.org/10.1016/j.jpsychires.2005.03.006
- Buhlmann, U., Gleiss, M. J., Rupf, L., Zschenderlein, K., & Kathmann, N. (2011). Modifying emotion recognition deficits in body dysmorphic disorder: An experimental investigation. Depression and Anxiety, 28(10), 924–931. https://doi.org/10.1002/da.20887
- Buhlmann, U., McNally, R. J., Etcoff, N. L., Tuschen-Caffier, B., & Wilhelm, S. (2004). Emotion recognition deficits in body dysmorphic disorder. Journal of Psychiatric Research, 38(2), 201–206. https://doi.org/10.1016/S0022-3956(03)00107-9
- Civile, C., McLaren, R., & McLaren, I. P. (2016). The face inversion effect: Roles of first- and second-order configural information. The American Journal of Psychology, 129(1), 23–35. https://doi.org/10.5406/amerjpsyc.129.1.0023
- Duchaine, B. C., & Weidenfeld, A. (2003). An evaluation of two commonly used tests of unfamiliar face recognition. Neuropsychologia, 41(6), 713–720. https://doi.org/10.1016/S0028-3932(02)00222-1
- Eisen, J. L., Phillips, K. A., Baer, L., Beer, D. A., Atala, K. D., & Rasmussen, S. A. (1998). The brown assessment of beliefs scale: Reliability and validity. American Journal of Psychiatry, 155(1), 102–108. https://doi.org/10.1176/ajp.155.1.102
- Ekman, P., & Friesen, W. (1976). W. Pictures of facial affect. Human interaction laboratory. Univ. California Medical Center.
- Farah, M. J., Tanaka, J. W., & Drain, H. M. (1995). What causes the face inversion effect? Journal of Experimental Psychology: Human Perception and Performance, 21(3), 628. https://doi.org/10.1037/0096-1523.21.3.628
- Feusner, J. D., Bystritsky, A., Hellemann, G., & Bookheimer, S. (2010). Impaired identity recognition of faces with emotional expressions in body dysmorphic disorder. Psychiatry Research, 179(3), 318–323. https://doi.org/10.1016/j.psychres.2009.01.016
- Feusner, J. D., Moller, H., Altstein, L., Sugar, C., Bookheimer, S., Yoon, J., & Hembacher, E. (2010). Inverted face processing in body dysmorphic disorder. Journal of Psychiatric Research, 44(15), 1088–1094.
- Feusner, J. D., Moody, T., Hembacher, E., Townsend, J., McKinley, M., Moller, H., & Bookheimer, S. (2010). Abnormalities of visual processing and frontostriatal systems in body dysmorphic disorder. Archives of General Psychiatry, 67(2), 197. http://dx.doi.org/10.1001/archgenpsychiatry.2009.190
- Feusner, J. D., Townsend, J., Bystritsky, A., & Bookheimer, S. (2007). Visual information processing of faces in body dysmorphic disorder. Archives of General Psychiatry, 64(12), 1417–1425. https://doi.org/10.1001/archpsyc.64.12.1417
- Freire, A., Lee, K., & Symons, L. A. (2000). The face-inversion effect as a deficit in the encoding of configural information: Direct evidence. Perception, 29(2), 159–170. https://doi.org/10.1068/p3012
- Grace, S. A., Labuschagne, I., Kaplan, R. A., & Rossell, S. L. (2017). The neurobiology of body dysmorphic disorder: A systematic review and theoretical model. Neuroscience & Biobehavioral Reviews, 83, 83–96. https://doi.org/10.1016/j.neubiorev.2017.10.003
- Grace, S. A., Toh, W. L., Buchanan, B., Castle, D. J., & Rossell, S. L. (2019). Impaired recognition of negative facial emotions in body dysmorphic disorder. Journal of the International Neuropsychological Society, 25(8), 884–889. https://doi.org/10.1017/S1355617719000419
- Greenberg, J. L., Reuman, L., Hartmann, A. S., Kasarskis, I., & Wilhelm, S. (2014). Visual hot spots: an eye tracking study of attention bias in body dysmorphic disorder. Journal of Psychiatric Research, 57, 125–132. https://doi.org/10.1016/j.jpsychires.2014.06.015
- Jefferies, K., Laws, K., Hranov, G., & Fineberg, N. (2010). P. 1. g. 003 cognitive and perceptual processing in body dysmorphic disorder. European Neuropsychopharmacology, 20, S309–S310. doi:10.1016/S0924-977X(10)70416-8
- Jefferies, K., Laws, K. R., & Fineberg, N. A. (2012). Superior face recognition in body dysmorphic disorder. Journal of Obsessive-Compulsive and Related Disorders, 1(3), 175–179. https://doi.org/10.1016/j.jocrd.2012.03.002
- Johnson, S., Williamson, P., & Wade, T. D. (2018). A systematic review and meta-analysis of cognitive processing deficits associated with body dysmorphic disorder. Behaviour Research and Therapy, 107, 83–94. https://doi.org/10.1016/j.brat.2018.05.013
- Joshua, N., Van Rheenen, T. E., Castle, D. J., & Rossell, S. L. (2016). Taking it at “Face Value”: The use of face processing strategies in bipolar disorder and schizophrenia. Journal of the International Neuropsychological Society, 22(6), 652–661. https://doi.org/10.1017/S1355617716000412
- Kerwin, L., Hovav, S., Hellemann, G., & Feusner, J. D. (2014). Impairment in local and global processing and set-shifting in body dysmorphic disorder. Journal of Psychiatric Research, 57, 41–50. https://doi.org/10.1016/j.jpsychires.2014.06.003
- Kollei, I., Horndasch, S., Erim, Y., & Martin, A. (2017). Visual selective attention in body dysmorphic disorder, bulimia nervosa and healthy controls. Journal of Psychosomatic Research, 92, 26–33. https://doi.org/10.1016/j.jpsychores.2016.11.008
- Lambrou, C., Veale, D., & Wilson, G. (2011). The role of aesthetic sensitivity in body dysmorphic disorder. Journal of Abnormal Psychology, 120(2), 443–453. https://doi.org/10.1037/a0022300
- Latinus, M., & Taylor, M. J. (2005). Holistic processing of faces: Learning effects with Mooney faces. Journal of Cognitive Neuroscience, 17(8), 1316–1327. https://doi.org/10.1162/0898929055002490
- Li, W., Arienzo, D., & Feusner, J. D. (2013). Body dysmorphic disorder: Neurobiological features and an updated model. Z Klin Psychol Psychother (Gott), 42(3), 184–191. https://doi.org/10.1026/1616-3443/a000213
- Li, W., Lai, T. M., Bohon, C., Loo, S. K., McCurdy, D., Strober, M., Bookheimer, S., & Feusner, J. (2015a). Anorexia nervosa and body dysmorphic disorder are associated with abnormalities in processing visual information. Psychological Medicine, 45(10), 2111–2122. https://doi.org/10.1017/S0033291715000045
- Li, W., Lai, T. M., Loo, S. K., Strober, M., Mohammad-Rezazadeh, I., Khalsa, S., & Feusner, J. (2015b). Aberrant early visual neural activity and brain-behavior relationships in anorexia nervosa and body dysmorphic disorder. Frontiers in Human Neuroscience, 9, 301. https://doi.org/10.3389/fnhum.2015.00301
- Malcolm, A., Brennan, S. N., Grace, S. A., Pikoos, T. D., Toh, W. L., Labuschagne, I., Buchanan, B., Kaplan, R. A., Castle, D. J., & Rossell, S. L. (2020). Empirical evidence for cognitive subgroups in body dysmorphic disorder. Australian & New Zealand Journal of Psychiatry, 55(4), 381–390. https://doi.org/10.1177/0004867421998762
- Maurer, D., Grand, R. L., & Mondloch, C. J. (2002). The many faces of configural processing. Trends in Cognitive Sciences, 6(6), 255–260. https://doi.org/10.1016/S1364-6613(02)01903-4
- McKone, E. (2004). Isolating the special component of face recognition: peripheral identification and a Mooney face. Journal of Experimental Psychology: Learning, Memory, and Cognition, 30(1), 181–197. https://doi.org/10.1037/0278-7393.30.1.181
- Monzani, B., Krebs, G., Anson, M., Veale, D., & Mataix-Cols, D. (2013). Holistic versus detailed visual processing in body dysmorphic disorder: Testing the inversion, composite and global precedence effects. Psychiatry Research, 210(3), 994–999. https://doi.org/10.1016/j.psychres.2013.08.009
- Moody, T. D., Sasaki, M. A., Bohon, C., Strober, M. A., Bookheimer, S. Y., Sheen, C. L., & Feusner, J. D. (2015). Functional connectivity for face processing in individuals with body dysmorphic disorder and anorexia nervosa. Psychological Medicine, 45(16), 3491–3503. https://doi.org/10.1017/S0033291715001397
- Murphy, J., Gray, K. L., & Cook, R. (2017). The composite face illusion. Psychonomic Bulletin & Review, 24(2), 245–261. https://doi.org/10.3758/s13423-016-1131-5
- Navon, D. (1977). Forest before trees: The precedence of global features in visual perception. Cognitive Psychology, 9(3), 353–383. https://doi.org/10.1016/0010-0285(77)90012-3
- Phillips, K. A. (2005). The broken mirror: Understanding and treating body dysmorphic disorder. Oxford University Press.
- Phillips, K. A., Hollander, E., Rasmussen, S. A., Aronowitz, B. R., DeCaria, C., & Goodman, W. K. (1997). A severity rating scale for body dysmorphic disorder: development, reliability, and validity of a modified version of the Yale-Brown Obsessive Compulsive Scale. Psychopharmacology Bulletin, 33(1), 17–22. https://www.ncbi.nlm.nih.gov/pubmed/9133747.
- Piepers, D., & Robbins, R. (2012). A review and clarification of the terms “holistic,” “configural,” and “relational” in the face perception literature. Frontiers in psychology, 3, 35864.
- Rossell, S. L., Labuschagne, I., Dunai, J., Kyrios, M., & Castle, D. J. (2014). Using theories of delusion formation to explain abnormal beliefs in Body Dysmorphic Disorder (BDD). Psychiatry Research, 215(3), 599–605. https://doi.org/10.1016/j.psychres.2013.12.030
- Schwaninger, A., & Mast, F. W. (2005). The face-inversion effect can be explained by the capacity limitations of an orientation normalization mechanism 1. Japanese Psychological Research, 47(3), 216–222. https://doi.org/10.1111/j.1468-5884.2005.00290.x
- Schwiedrzik, C. M., Melloni, L., & Schurger, A. (2018). Mooney face stimuli for visual perception research. PLoS One, 13(7), e0200106. https://doi.org/10.1371/journal.pone.0200106
- Sergent, J. (1984). An investigation into component and configural processes underlying face perception. British Journal of Psychology, 75(2), 221–242. https://doi.org/10.1111/j.2044-8295.1984.tb01895.x
- Sheehan, D. (2016). The MINI international neuropsychiatric interview, (Version 7.0.2) for DSM–5.
- Taubert, J., Apthorp, D., Aagten-Murphy, D., & Alais, D. (2011). The role of holistic processing in face perception: Evidence from the face inversion effect. Vision Research, 51(11), 1273–1278. https://doi.org/10.1016/j.visres.2011.04.002
- Toh, W. L., Castle, D. J., & Rossell, S. L. (2015). Facial affect recognition in body dysmorphic disorder versus obsessive-compulsive disorder: An eye-tracking study. Journal of Anxiety Disorders, 35, 49–59. https://doi.org/10.1016/j.janxdis.2015.08.003
- Toh, W. L., Castle, D. J., & Rossell, S. L. (2017). How individuals with body dysmorphic disorder (BDD) process their own face: A quantitative and qualitative investigation based on an eye-tracking paradigm. Cognitive Neuropsychiatry, 22(3), 213–232. https://doi.org/10.1080/13546805.2017.1300090
- Tottenham, N. (1998). Macbrain face stimulus set. In D. John, & T. Catherine (Eds.), Macarthur foundation research network on early experience and brain development.
- Tsao, D. Y., & Livingstone, M. S. (2008). Mechanisms of face perception. Annual Review of Neuroscience, 31(1), 411–437. https://doi.org/10.1146/annurev.neuro.30.051606.094238
- Van Rheenen, T. E., & Rossell, S. L. (2014). Let's face it: Facial emotion processing is impaired in bipolar disorder. Journal of the International Neuropsychological Society, 20(2), 200–208. https://doi.org/10.1017/S1355617713001367
- Veale, D. (2004). Advances in a cognitive behavioural model of body dysmorphic disorder. Body Image, 1(1), 113–125. https://doi.org/10.1016/S1740-1445(03)00009-3
- Veale, D., & Riley, S. (2001). Mirror, mirror on the wall, who is the ugliest of them all? The psychopathology of mirror gazing in body dysmorphic disorder. Behaviour Research and Therapy, 39(12), 1381–1393. https://doi.org/10.1016/S0005-7967(00)00102-9
- Wechsler, D. (2001). Wechsler test of adult Reading: WTAR. Psychological Corporation.
- Young, A. W., Hellawell, D., & Hay, D. C. (1987). Configurational information in face perception. Perception, 16(6), 747–759. https://doi.org/10.1068/p160747
- Zhen, Z., Fang, H., & Liu, J. (2013). The hierarchical brain network for face recognition. PLoS One, 8(3), e59886. https://doi.org/10.1371/journal.pone.0059886