Abstract
Non-alcoholic fatty liver disease (NAFLD) is the most common liver disease. The mechanism by which bisphenol A (BPA) promots NAFLD remains unclear. Palmitic acid (PA) and lipopolysaccharide (LPS) were used to simulate NAFLD in HepG2 cells in vitro. Total cholesterol (TC), triglyceride (TG) content, and lipid accumulation were measured to evaluate lipid metabolism. The caspase-1-stained cells and NLRP3 inflammasome-associated proteins were evaluated for pyroptosis. Western blot analysis was used to detect protein levels and co-immunoprecipitation (Co-IP) was used to detect the association between the proteins. Cycloheximide (CHX) treatment combined with western blot was performed to access protein stability. This data have shown that BPA induces lipid metabolism dysfunction and pyroptosis by upregulating O-GlcNAc transferase (OGT) level. NLRP3 directly interacts with OGT, and elevated OGT enhanced the stability of NLRP3 protein. BPA promoted OGT-mediated O-GlcNAcylation to stabilised NLRP3, thus accelerating NAFLD progress in vitro. Our study reveals that BPA, as an environmental factor, may be involved in the promotion of NAFLD, and that targeting NLRP3 and OGT may inhibit BPA’s induction of NAFLD.
Introduction
Non-alcoholic fatty liver disease (NAFLD) is a chronic liver disease characterised by hepatocyte steatosis and lipid accumulation, but without a history of excessive alcohol consumption (Pawlak et al. Citation2015, Ipsen et al. Citation2018). NAFLD is the most common chronic liver disease that is estimated to affect >25% of adults worldwide and more than half of patients with type 2 diabetes, with large variations between regions and by ethnicity (Duell et al. Citation2022). Its incidence is increasing worldwide, which has seriously threatened people’s health. Regional epidemiological patterns of NAFLD indicate that economics, environment, and lifestyle are critical factors in disease progression (Zhou et al. Citation2020).
Recently, bisphenol A (BPA) exposure has been gradually recognised as a risk factor for NAFLD (Dallio et al. Citation2019, An et al. Citation2021). BPA is one of the most common endocrine disruptors that people are exposed to Dallio et al. (Citation2018). BPA is mainly used in the production of products containing polycarbonate and epoxy resins and has been detected in various body fluids and tissues. Previous studies have shown that BPA is not only detectable in the urine of most Americans, but also raises concerns about its reproductive toxicity (Lakind and Naiman Citation2011, Peretz et al. Citation2014). Evidence from experimental animals and human exposure in recent years suggests that BPA below the lowest adverse effect level (50 mg/kg/d) can affect female reproduction and have potentially adverse effects on the male reproductive system (Peretz et al. Citation2014). Recent epidemiological studies have indicated that BPA exposure may potentially be associated with alterations in hormone levels, impairment of ovary and uterine function and reduction of sperm quality (Siracusa et al. Citation2018). A large number of studies have shown that BPA can induce obesity, insulin resistance, type II diabetes mellitus, NAFLD and other chronic metabolic diseases by interfering with the body’s glucose and lipid metabolism (Pjanic Citation2017, Farrugia et al. Citation2021, Long et al. Citation2021, Pérez-Bermejo et al. Citation2021). However, the molecular mechanism of hepatic lipid metabolism disorder induced by BPA exposure remains unclear.
Posttranslational modifications of proteins play an important role in cell signalling, protein-protein interactions and regulation of gene expression (Wei et al. Citation2020). O-GlcNAcylation is a identified post-translational modification found on serine and threonine residues of proteins in the nucleus, cytoplasm, and mitochondria (Duan et al. Citation2018, Wu et al. Citation2019, Chatham et al. Citation2021). O-GlcNAcylation is characterised as a process of adding an O-GlcNAc molecule to the threonine or serine residues of nuclear, cytoplasmic, and mitochondrial proteins under enzymatic modulation (Xie et al. Citation2023). The enzymes mediating O-GlcNAcylation consist of O-GlcNAc transferase (OGT) and O-GlcNAcase (OGA), which add and remove O-GlcNAc from target proteins, respectively (Zhou et al. Citation2022). O-GlcNAcylation participates in the regulation of liver lipogenesis. In vivo and in vitro studies have indicated that under high-glucose stimulation, suppressing OGT and GFAT inhibits lipid accumulation, while an inhibitor of OGA promotes lipid deposition in both HepG2 cells and animal liver (Lee et al. Citation2019). Whether O-GlcNAcylation is involved in the regulation of BPA on hepatic lipid metabolism was discussed in this study. Recent studies have shown that NLRP3 inflammasome, as the core part of the inflammatory response, mediates the occurrence and development of NAFLD (Wan et al. Citation2016, Mridha et al. Citation2017). NLRP3 activation can accelerate the progression of the disease, and inhibiting NLRP3 inflammasome activation can reduce the inflammatory response and delay the progression of NAFLD (Unamuno et al. Citation2021, Wang et al. Citation2021). Pyroptosis, a unique type of programmed cell death, is involved in immune regulation. The assembled NLRP3 inflammasome can activate the protease caspase-1 to induce gasdermin D (GSDMD)-dependent pyroptosis and facilitate the release of IL-1β and IL-18, which contribute to innate immune defense and homeostatic maintenance (Huang et al. Citation2021). Inhibiting NLRP3 inflammasome and pyroptosis can ameliorate NAFLD (Yu et al. Citation2019).
The proinflammatory mechanism of BPA in NAFLD and whether pyroptosis pathway mediates its proinflammatory mechanism are still unclear. Therefore, this study observed the effect of BPA on pyroptosis pathway in NAFLD, and verified whether it is related to the O-GlcNAcylation modification of NLRP3 protein, so as to explore the mechanism of BPA-induced NAFLD. In this work, BPA induced dysfunction of lipid metabolism and pyroptosis by upregulating OGT level. NLRP3 interacted directly with OGT, and elevated OGT enhanced NLRP3 protein stability. BPA promoted OGT-mediated O-GlcNAcylation stabilised NLRP3, thus accelerating NAFLD progress in vitro.
Methods and materials
Cell culture and treatment
HepG2 cell which was sensitive to lipid accumulation was purchased from Shanghai Institute of Cell Biomedicine, and was cultured as previously reported (Lin et al. Citation2017). To establish the in vitro NAFLD cell model, HepG2 cells (1 × 104 cells/mL) were cultured in the presence of 4 mM palmitic acid (PA) – bovine serum albumin (BSA) mixture (Wu et al. Citation2022) for 24 h and exposed to lipopolysaccharide (LPS, 1 μg/mL, L5293, Sigma–Aldrich, St. Louis, MO) (Wu et al. Citation2022) and incubated with BPA with different concentration (1, 2, and 4 μM) for 48 h (Long et al. Citation2021).
Cell transfection
To knock-down OGT, small interference RNA of OGT (si-OGT) and control siRNA (si-nc) purchased from GenePharma (Shanghai, China) were transfected into cells using Lipofectamine® 3000 reagent (Thermo Fisher, Waltham, MA) according to manufacturer’s protocols. Knock-down was confirmed by western blotting of OGT.
Determination of total cholesterol (TC) and triglyceride (TG) content
TC (A111–1-1, Nanjing Jiancheng Bioengineering Institute, Nanjing, China) and TG (10010303, Cayman Chemical, Ann Arbor, MI) levels were measured by the commercial ELISA kit as the protocol described.
Oil Red O staining
The cells were soaked in paraformaldehyde solution for 15 min to reduce oxidation, then in isopropanol solution for 5 min. Afterwards, Oil Red O solution was incubated with samples for 10 min. Haematoxylin was added and stained samples were incubated for 12 min, then the samples were rinsed with water for imaging.
Flow cytometry assay
Of 2 × 105 cells were gathered, caspase-1 activity was identified by a Caspase-1 Assay Kit under the description of manufacturer’s protocol (KTA3020; Abbkine, Atlanta, GA). Then, 5 μL of PI was added and incubated in darkroom for 15 min. Double-stained positive cells were analysed using NovoCyte Advanteon B4 Flow Cytometer on NovoSampler Q software (Agilent Technologies, Santa Clara, CA).
Western blot
Primary antibodies from Abcam (Cambridge, MA) were anti-NLRP3 (ab263899, 1:800), anti-caspase-1 (ab238979, 1:1000), anti-GSDMD-N (ab215203, 1:600), anti-OGT (ab177941, 1:800), and anti-O-GlcNAc RL2 (ab93858, 1:1000). anti-O-GlcNAc (MABS1254, 1 μg/mL), and anti-OGA (SAB4200311, 2 μg/mL) were from Sigma. Protein was extracted from macrophages by RIPA (Sigma–Aldrich) reagents. The total protein concentration in each cell lysate was determined with a BCA Protein Assay Kit (Beyotime, Shanghai, China). Additionally, the proteins were separated by 10% SDS-PAGE and electrotransferred to polyvinylidene fluoride membranes. The membranes were blocked with 5% skim milk for 60 min and incubated with primary antibodies at 4 °C overnight. Next days, the membranes were incubated with secondary antibodies (ab6721, 1:2000) for 1.5 h. Subsequently, the protein expression was determined by an ECL kit using Scion Image version 4.0.2 software (Scion Corporation, Chicago, IL).
Co-immunoprecipitation (Co-IP)
Co-IP was applied to examine interaction of endogenous OGT and NLRP3 protein. Cells were lysed with Pierce IP buffer (Xu et al. Citation2020). Next, cell lysate with anti-NLRP3 (ab263899, 1:800), anti-OGT (ab177941, 1:800), and anti-IgG (ab7099, 1:100, Abcam) antibody was incubated at 4 °C overnight. The mixture was vortexed with protein G beads (Dynabeads, ThermoFisher) at 4 °C for 8 h, followed by western blot analysis.
Protein stability
Cells were cultured in a 12-well plate to a cell density of 70%, and then divided into four groups: si-nc, si-OGT, Thiamet-G (TMG, OGA inhibitor, 1 μM; Selleck, Shanghai, China) (Rahman et al. Citation2019) and DMSO (reagent control for inhibitor). Afterwards, cycloheximide (CHX, 50 mg/mL; Selleck, China) was added (Wu et al. Citation2019), and the samples were collected at 0, 4, 8, 12, and 24 h, and the cell proteins were extracted. The expression of NLRP3 was detected by western blot.
Immunofluorescence staining
The cells were seeded in small dishes and cultured at a constant temperature. When the growth density of the cells was moderate, the cells were fixed with 4% paraformaldehyde for 15 min and washed with PBS for three times. The cells were permeated with 0. 2% Triton X-100 for 3–5 min, and cultivated with 5% BSA for 30 min. After the blocking solution was discarded, rabbit anti-NLRP3 (ab270449, 1:50) and anti-OGT (ab177941, 1:50) were added and incubated in a wet box for 4 h overnight. The next day, they were rewarmed for 30 min, and then added FITC-labeled goat anti-rabbit secondary antibody was added in the dark, and incubated for 1 h. The nucleus was stained with PI for 4 min in darkness. Images were acquired on a fluorescence Nikon (DIAPHOT, Tokyo, Japan) fluorescence microscope.
RT-qPCR
Cells were mixed with TRIzol® reagent (Invitrogen, Carlsbad, CA) to extract the total RNA. A Reverse Transcription Kit (Takara, Beijing, China) was used to reserve transcription. Primers were synthesised by Genscript Biotech (Nanjing, China). The mRNA expression of OGT was performed by qRT-PCR in 20 μL reaction system containing specific primers and SYBR Green Master Mix (Takara, China) by CFX96 Real-Time PCR Detection System (Bio-Rad, Hercules, CA). GAPDH was used for internal reference. Fold changes of the indicated genes were calculated using 2−ΔΔCt method. Primer sequences are as follows (3′→5′): OGT: forward TCCTGATTTGTACTGTGTTCGC and reverse AAGCTACTGCAAAGTTCGGTT.
Statistical analysis
All experiments were performed with at least three times. The error bars indicated ± standard deviation (SD). All data were in a normal distribution, and variance was similar between the groups that are being statistically compared. Statistical analyses were analysed in GraphPad Prism version 7 (GraphPad Software, Inc, La Jolla, CA). Statistical significance was determined by using unpaired Student t-test for two groups or one-way ANOVA when there are more than two groups. p < 0.05 was considered significant.
Results
Hepatocellular lipotoxicity is induced by BPA
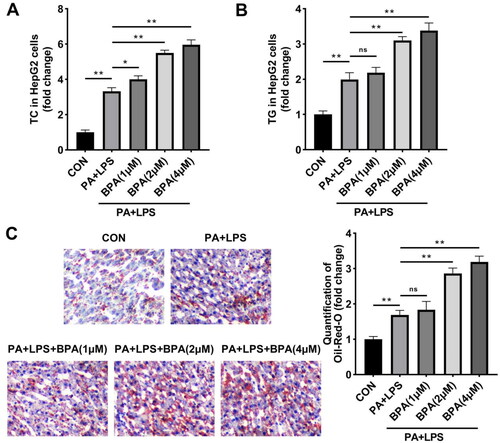
BPA is a class of organic compounds associated with obesity caused by metabolic disorders (). HepG2 cells were selected to study BPA-induced lipid metabolism abnormalities in NAFLD in vitro. First, we evaluated the effects of BPA on lipid metabolism in HepG2 cells by three indexes: TC, TG, and lipid accumulation degree. The results indicated that TC, TG, and lipid accumulation levels of HepG2 cells were prominently increased after PA and LPS treatment (), indicating abnormal lipid metabolism in the cells. Low concentrations of BPA also induced lipid metabolism abnormalities in HepG2 cells, and the abnormalities are markedly aggravated with the increase of BPA concentration.
Figure 1. Hepatocellular lipotoxicity is induced by BPA. (A) TC and (B) TG content of HepG2 cells were accessed by ELISA. (C) Images (200x) and quantitative analysis of lipid accumulation in HepG2 cells. *p < 0.05; **p < 0.01. BPA: bisphenol A; TC: total cholesterol; TG: triglyceride; ns: no significance.
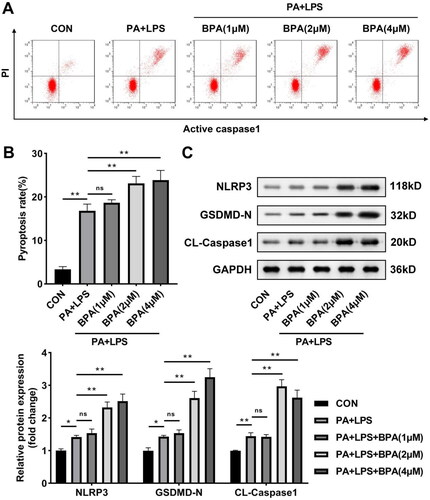
BPA facilitates pyroptosis of NAFLD in vitro
Next, the effects of BPA on pyroptosis of HepG2 cells were investigated. After 24 h of LPS and PA stimulation, active Caspase-1 of HepG2 cells was induced (). Meanwhile, protein levels of NLRP3, GSDMD-N, and cleaved-Caspase-1 were elevated by LPS and PA exposure, suggesting the activation of inflammasome (). Compared with LPS and PA treatment group, pyroptosis rate and NLRP3 inflammasome activation were promoted by BPA in a dose-dependent way.
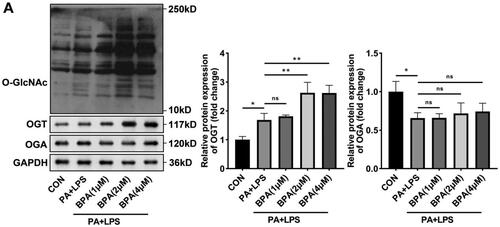
BPA promotes O-GlcNAcylation in NAFLD
Subsequently, we investigated the changes of O-GlcNAcylation in NAFLD after BPA treatment. As shown in , cells with abnormal lipid metabolism induced by PA and LPS showed not only induction of O-GlcNAcylation but also upregulation of OGT expression. BPA treatment also caused all of these levels to rise sharply. OGA expression was not significantly affected by any treatment.
Knockdown of OGT inhibits lipotoxicity and pyroptosis induced by BPA
Considering that OGT was modulated by BPA in HepG2 cells, we speculated that OGT may participate in abnormal lipids metabolism induced by BPA. OGT was successfully downregulated in HepG2 cells evaluated by RT-qPCR (), and si-OGT #1 suppressed OGT expression more potently, and was selected for subsequent experiments. Meanwhile, knockdown of OGT significantly downregulated the total O-GlcNAcylation levels (). TC level, TG level, and number of Oil red O stained cells elevated by PA and LPS exposure treatment were dramatically further elevated by BPA (). Inhibition of OGT prominently relieved the lipid metabolism dysfunction induced by BPA. Furthermore, BPA promoted pyroptosis of HepG2 cells treated on the basis of PA and LPS, knockdown of OGT remarkably suppressed BPA-induced pyroptosis ().
Figure 4. Knockdown of OGT inhibits lipotoxicity and pyroptosis induced by BPA. (A) mRNA of OGT in HepG2 cells determined by RT-qPCR. (B) Protein levels of O-GlcNAcylation in HepG2 cells after knockdown of OGT. (C) TC and (D) TG content of HepG2 cells were accessed by ELISA. (E) Images (200x) and quantitative analysis of lipid accumulation in HepG2 cells after knockdown of OGT. (F) Scatter diagram and quantitative analysis of pyroptosis performed by flow cytometry. *p < 0.05; **p < 0.01. TC: total cholesterol; TG: triglyceride; OGT: O-GlcNAc transferase.
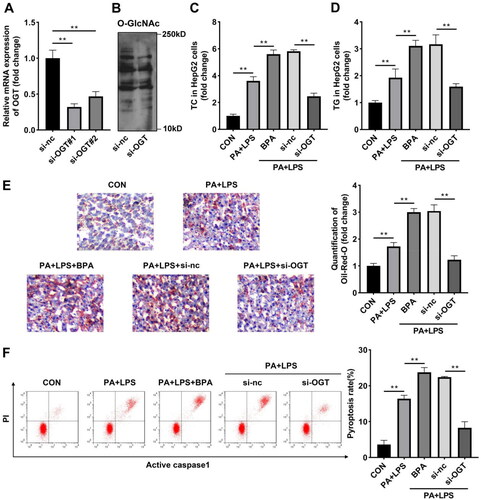
OGT interacts with NLRP3 in vitro
The previous data suggested that BPA can activate the NLRP3 inflammasome, and BPA improved O-GlcNAcylation by increasing the expression of OGT. Therefore, whether OGT can specifically bind NLRP3 was studied. Co-IP analysis results showed that NLRP3 bind with OGT at the endogenous protein level (). Meanwhile, immunofluorescence staining images demonstrated that both NLRP3 and OGT were detected in cytoplasm (). Then the O-GlcNAcyled NLRP3 level was detected in HepG2 cells incubated with the O-GlcNAc RL2 antibody. Knockdown of OGT suppressed both NLRP3 and O-GlcNAcyled NLRP3 protein levels, indicating the binding relationship was weakened by inhibition of OGT ().
Figure 5. OGT interacts with NLRP3 in vitro. (A) Co-IP and western blot were combined to indicate binding relationship between OGT and NLRP3. (B) Immunofluorescence colocalisation in HepG2 cells revealed that OGT can colocalise with NLRP3. (C) NLRP3 protein levels in HepG2 cells after inhibition of OGT. Co-IP: Co-immunoprecipitation; OGT: O-GlcNAc transferase.
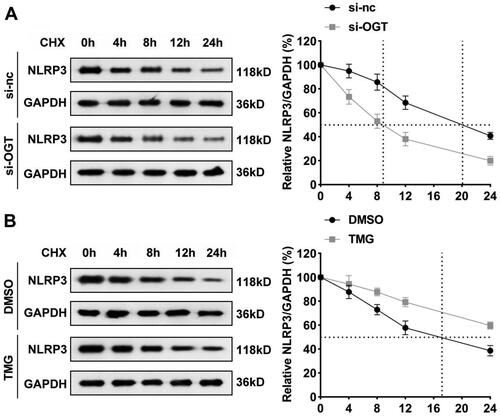
O-GlcNAcylation increases NLRP3 protein stability
Subsequently, how O-GlcNAcylation regulating NLRP3 in BPA-treated HepG2 cells was the next to be studied. Since O-GlcNAcylation is reported to be an important factor affecting protein stability (Shang et al. Citation2021), we therefore investigated whether downregulation of O-GlcNAcylation alters the stability of NLRP3. CHX was used to inhibit protein synthesis of HepG2 cells after knockdown of OGT, and then the remaining NLRP3 was detected. Our data revealed that NLRP3 was reduced to 50% of original amount at 20th hour under the treatment of CHX. OGT silencing dramatically brought this point forward to the 9th hour, demonstrating that OGT knockdown promoted the degradation of NLRP3 protein (). Similar results showed that NLRP3 protein levels were reduced to 50% at 17th hour after DMSO treatment, and after TMG treatment, the degradation of NLRP3 protein was not up to 50% after 24 h of CHX treatment (). Hence, OGT regulates the stability of NLRP3 mainly by modifying the NLRP3 protein through O-GlcNAcylation and increasing protein stability.
Discussion
NAFLD is a metabolic stress liver damage, and abnormal fat metabolism is a risk factor. The global prevalence of NAFLD is much higher than previously estimated and continues to increase at an alarming rate (Riazi et al. Citation2022). Therefore, it is of great significance to study fat metabolism in NAFLD (Deprince et al. Citation2020, Gao et al. Citation2020). In this study, we demonstrated that BPA can promote fat accumulation and inflammation in hepatocytes, and induce pyroptosis. Interestingly, O-GlcNAcylation levels were upregulated in NAFLD cell models; therefore, we focused on the correlation between BPA-induced NAFLD and O-GlcNAcylation.
As the central regulator of lipid homeostasis, liver is an important organ of lipid metabolism. The hallmark of NAFLD is hepatic steatosis (Ipsen et al. Citation2018). Increased liver TC synthesis and TC accumulation are associated with NAFLD. For instance, by knocking out ChREBP in mice with fructose-induced steatosis, TC synthesis can be promoted to induce histological liver damage (Zhang et al. Citation2017). Similarly, reducing fatty acid uptake in hepatocytes and liver TG content can reverse fatty changes (Ipsen et al. Citation2018). In this work, abnormal lipid accumulation was found in steatotic HepG2 cells, and BPA further induced the unbalanced lipid homeostasis which is in line with the previous studies (Chakraborty et al. Citation2023). Pyroptosis has been demonstrated to drive the pathogenesis of NAFLD in mouse models of NAFLD (Zhu et al. Citation2021). BPA has been reported to promote pyroptosis in various studies (Zhang et al. Citation2022, Shi et al. Citation2023). As we speculated, BPA also promoted pyroptosis in steatotic HepG2 cells.
O-GlcNAcylation regulates hepatic glucose and lipid metabolism by modifying key transcription factors that regulate glucose metabolism, such as peroxisome proliferator-activated receptor (PPARγ), PPARγ coactivator (PGC1α), glucose response element-binding protein (ChREBP), and liver X receptor (LXRα/β), etc. (Ji et al. Citation2012, Benhamed et al. Citation2014, Fan et al. Citation2017, Brainard and Facundo Citation2021). Furthermore, inhibition of O-GlcNAcylation has been reported to suppress lipid accumulation (Lee et al. Citation2019). Along with lipid accumulation, O-GlcNAcylation was significantly increased in NAFLD. Free fatty acids and glucose have been reported to increase O-GlcNAcylation in hepatocytes and cancer cell lines (Kumar et al. Citation2021, Tan et al. Citation2021). Here, we demonstrated that O-GlcNAcylation is increased in steatotic HepG2 cells after BPA treatment and that OGT is also upregulated. In addition, BPA induced NLRP3 inflammasome activation.
NRLP3 can be involved in the deposition of lipid and adipose tissue in the liver of mice (Han et al. Citation2021). High fat diet can increase the expression of NLRP3 inflammasome-related genes in liver tissue of mice (Han et al. Citation2021). In vitro experiments suggested that NLRP3 expression in saturated fatty acid-treated hepatocytes was increased, which promoted local liver inflammation and the progression of NAFLD (Sheik Abdul et al. Citation2019). High fat diet-induced NAFLD can be ameliorated by inhibiting NLRP3 inflammasome activation. NLRP3 inhibitors have also been shown to reduce oxidative stress, apoptosis and inflammation in rat liver (Sheik Abdul et al. Citation2019). In this study, we investigated how O-GlcNAcylation and OGT regulate NLRP3 expression. OGT specifically binds to NLRP3. BPA can significantly increase the expression of OGT and thus improve the protein stability of NLRP3.
Conclusion
In summary, BPA induced abnormal lipid accumulation and pyroptosis of HepG2 cells; meanwhile, O-GlcNAcylation and OGT were elevated by BPA exposure. Knockdown of OGT reversed the damage induced by BPA. BPA-mediated O-GlcNAcylation stabilised NLRP3, thus accelerating NAFLD progression.
Disclosure statement
The authors report there are no competing interests to declare.
Data availability statement
Not applicable.
Additional information
Funding
References
- An, S.J., et al., 2021. The association between urinary bisphenol A levels and nonalcoholic fatty liver disease in Korean adults: Korean National Environmental Health Survey (KoNEHS) 2015–2017. Environmental Health and Preventive Medicine, 26 (1), 91.
- Benhamed, F., et al., 2014. O-GlcNAcylation links ChREBP and FXR to glucose-sensing. Frontiers in endocrinology, 5, 230. doi: 10.3389/fendo.2014.00230.
- Brainard, R.E., and Facundo, H.T., 2021. Cardiac hypertrophy drives PGC-1α suppression associated with enhanced O-glycosylation. Biochimica et biophysica acta molecular basis of disease, 1867 (5), 166080. doi: 10.1016/j.bbadis.2021.166080.
- Chakraborty, S., et al., 2023. Ancestral BPA exposure caused defects in the liver of medaka for four generations. The science of the total environment, 856 (Pt 1), 159067. doi: 10.1016/j.scitotenv.2022.159067.
- Chatham, J.C., Zhang, J., and Wende, A.R., 2021. Role of O-linked N-acetylglucosamine protein modification in cellular (Patho)physiology. Physiological reviews, 101 (2), 427–493. doi: 10.1152/physrev.00043.2019.
- Dallio, M., et al., 2019. Chemical effect of bisphenol A on non-alcoholic fatty liver disease. International journal of environmental research and public health, 16 (17), 3134.
- Dallio, M., et al., 2018. Role of bisphenol A as environmental factor in the promotion of non-alcoholic fatty liver disease: in vitro and clinical study. Alimentary pharmacology & therapeutics, 47 (6), 826–837. doi: 10.1111/apt.14499.
- Deprince, A., Haas, J.T., and Staels, B., 2020. Dysregulated lipid metabolism links NAFLD to cardiovascular disease. Molecular metabolism, 42, 101092. doi: 10.1016/j.molmet.2020.101092.
- Duan, F., et al., 2018. O-GlcNAcylation of RACK1 promotes hepatocellular carcinogenesis. Journal of hepatology, 68 (6), 1191–1202. doi: 10.1016/j.jhep.2018.02.003.
- Duell, P.B., et al., 2022. Nonalcoholic fatty liver disease and cardiovascular risk: a scientific statement from the American heart association. Arteriosclerosis, thrombosis, and vascular biology, 42 (6), e168–e185. doi: 10.1161/ATV.0000000000000153.
- Fan, Q., et al., 2017. LXRalpha regulates hepatic ChREBPalpha activity and lipogenesis upon glucose, but not fructose feeding in mice. Nutrients, 9 (7), 678. doi: 10.3390/nu9070678.
- Farrugia, F., et al., 2021. Bisphenol A and type 2 diabetes mellitus: a review of epidemiologic, functional, and early life factors. International journal of environmental research and public health, 18 (2), 716.
- Gao, Y., et al., 2020. Exercise and dietary intervention ameliorate high-fat diet-induced NAFLD and liver aging by inducing lipophagy. Redox biology, 36, 101635. doi: 10.1016/j.redox.2020.101635.
- Han, M., Li, S., and Li, L., 2021. Verapamil inhibits early acute liver failure through suppressing the NLRP3 inflammasome pathway. Journal of cellular and molecular medicine, 25 (13), 5963–5975. doi: 10.1111/jcmm.16357.
- Huang, Y., Xu, W., and Zhou, R., 2021. NLRP3 inflammasome activation and cell death. Cellular & molecular immunology, 18 (9), 2114–2127. doi: 10.1038/s41423-021-00740-6.
- Ipsen, D.H., Lykkesfeldt, J., and Tveden-Nyborg, P., 2018. Molecular mechanisms of hepatic lipid accumulation in non-alcoholic fatty liver disease. Cellular and molecular life sciences, 75 (18), 3313–3327. doi: 10.1007/s00018-018-2860-6.
- Ji, S., et al., 2012. O-GlcNAc modification of PPARgamma reduces its transcriptional activity. Biochemical and biophysical research communications, 417 (4), 1158–1163. doi: 10.1016/j.bbrc.2011.12.086.
- Kumar, V., et al., 2021. Therapeutic targets, novel drugs, and delivery systems for diabetes associated NAFLD and liver fibrosis. Advanced drug delivery reviews, 176, 113888. doi: 10.1016/j.addr.2021.113888.
- Lakind, J.S., and Naiman, D.Q., 2011. Daily intake of bisphenol A and potential sources of exposure: 2005–2006 National Health and Nutrition Examination Survey. Journal of exposure science & environmental epidemiology, 21 (3), 272–279. doi: 10.1038/jes.2010.9.
- Lee, D.E., et al., 2019. Curcumin ameliorates nonalcoholic fatty liver disease through inhibition of O-GlcNAcylation. Nutrients, 11 (11), 2702. doi: 10.3390/nu11112702.
- Lin, Y., et al., 2017. Downregulation of miR-192 causes hepatic steatosis and lipid accumulation by inducing SREBF1: Novel mechanism for bisphenol A-triggered non-alcoholic fatty liver disease. Biochimica et biophysica acta molecular and cell biology of lipids, 1862 (9), 869–882. doi: 10.1016/j.bbalip.2017.05.001.
- Long, Z., et al., 2021. Gestational bisphenol A exposure induces fatty liver development in male offspring mice through the inhibition of HNF1b and upregulation of PPARgamma. Cell biology and toxicology, 37 (1), 65–84. doi: 10.1007/s10565-020-09535-3.
- Mridha, A.R., et al., 2017. NLRP3 inflammasome blockade reduces liver inflammation and fibrosis in experimental NASH in mice. Journal of hepatology, 66 (5), 1037–1046. doi: 10.1016/j.jhep.2017.01.022.
- Pawlak, M., Lefebvre, P., and Staels, B., 2015. Molecular mechanism of PPARalpha action and its impact on lipid metabolism, inflammation and fibrosis in non-alcoholic fatty liver disease. Journal of hepatology, 62 (3), 720–733. doi: 10.1016/j.jhep.2014.10.039.
- Peretz, J., et al., 2014. Bisphenol a and reproductive health: update of experimental and human evidence, 2007-2013. Environmental health perspectives, 122 (8), 775–786. doi: 10.1289/ehp.1307728.
- Pérez-Bermejo, M., Mas-Pérez, I., and Murillo-Llorente, M.T., 2021. The role of the bisphenol A in diabetes and obesity. Biomedicines, 9 (6), 666. doi: 10.3390/biomedicines9060666.
- Pjanic, M., 2017. The role of polycarbonate monomer bisphenol-A in insulin resistance. PeerJ, 5, e3809. doi: 10.7717/peerj.3809.
- Rahman, M.A., et al., 2019. Modulation of O-GlcNAcylation regulates autophagy in cortical astrocytes. Oxidative medicine and cellular longevity, 2019, 6279313–6279313. doi: 10.1155/2019/6279313.
- Riazi, K., et al., 2022. The prevalence and incidence of NAFLD worldwide: a systematic review and meta-analysis. The lancet gastroenterology & hepatology, 7 (9), 851–861. doi: 10.1016/S2468-1253(22)00165-0.
- Shang, M., et al., 2021. The folate cycle enzyme MTHFD2 induces cancer immune evasion through PD-L1 up-regulation. Nature communications, 12 (1), 1940. doi: 10.1038/s41467-021-22173-5.
- Sheik Abdul, N., Nagiah, S., and Chuturgoon, A.A., 2019. Fusaric acid induces NRF2 as a cytoprotective response to prevent NLRP3 activation in the liver derived HepG2 cell line. Toxicology in vitro, 55, 151–159. doi: 10.1016/j.tiv.2018.12.008.
- Shi, X., et al., 2023. ROS mediated pyroptosis-M1 polarization crosstalk participates in inflammation of chicken liver induced by bisphenol A and selenium deficiency. Environmental pollution, 324, 121392. doi: 10.1016/j.envpol.2023.121392.
- Siracusa, J.S., et al., 2018. Effects of bisphenol A and its analogs on reproductive health: A mini review. Reproductive toxicology, 79, 96–123. doi: 10.1016/j.reprotox.2018.06.005.
- Tan, W., et al., 2021. Posttranscriptional regulation of de novo lipogenesis by glucose-induced O-GlcNAcylation. Molecular cell, 81 (9), 1890–1904.e7. doi: 10.1016/j.molcel.2021.02.009.
- Unamuno, X., et al., 2021. NLRP3 inflammasome blockade reduces adipose tissue inflammation and extracellular matrix remodeling. Cellular & molecular immunology, 18 (4), 1045–1057. doi: 10.1038/s41423-019-0296-z.
- Wan, X., et al., 2016. Role of NLRP3 Inflammasome in the Progression of NAFLD to NASH. Canadian journal of gastroenterology & hepatology, 2016, 6489012. doi: 10.1155/2016/6489012.
- Wang, X., et al., 2021. NLRP3 inflammasome inhibitor CY-09 reduces hepatic steatosis in experimental NAFLD mice. Biochemical and biophysical research communications, 534, 734–739. doi: 10.1016/j.bbrc.2020.11.009.
- Wei, X., et al., 2020. Posttranslational modifications in ferroptosis. Oxidative medicine and cellular longevity, 2020, 8832043.
- Wu, J., et al., 2022. Corydalis saxicola Bunting Total Alkaloids ameliorate diet-induced non-alcoholic steatohepatitis by regulating hepatic PI3K/Akt and TLR4/NF-kappaB pathways in mice. Biomedicine & pharmacotherapy = biomedecine & pharmacotherapie, 151, 113132. doi: 10.1016/j.biopha.2022.113132.
- Wu, N., et al., 2019. O-GlcNAcylation promotes colorectal cancer progression by regulating protein stability and potential catcinogenic function of DDX5. Journal of cellular and molecular medicine, 23 (2), 1354–1362. doi: 10.1111/jcmm.14038.
- Xie, Z., et al., 2023. Emerging role of protein O-GlcNAcylation in liver metabolism: implications for diabetes and NAFLD. International journal of molecular sciences, 24 (3), 2142. doi: 10.3390/ijms24032142.
- Xu, T.H., et al., 2020. OGT-mediated KEAP1 glycosylation accelerates NRF2 degradation leading to high phosphate-induced vascular calcification in chronic kidney disease. Frontiers in physiology, 11, 1092. doi: 10.3389/fphys.2020.01092.
- Yu, X., et al., 2019. Liraglutide ameliorates non-alcoholic steatohepatitis by inhibiting NLRP3 inflammasome and pyroptosis activation via mitophagy. European journal of pharmacology, 864, 172715. doi: 10.1016/j.ejphar.2019.172715.
- Zhang, D., et al., 2017. Lipogenic transcription factor ChREBP mediates fructose-induced metabolic adaptations to prevent hepatotoxicity. The journal of clinical investigation, 127 (7), 2855–2867. doi: 10.1172/JCI89934.
- Zhang, Y., et al., 2022. Bisphenol A induces pyroptotic cell death via ROS/NLRP3/Caspase-1 pathway in osteocytes MLO-Y4. Food and chemical toxicology, 159, 112772. doi: 10.1016/j.fct.2021.112772.
- Zhou, J., et al., 2020. Epidemiological features of NAFLD from 1999 to 2018 in China. Hepatology, 71 (5), 1851–1864. doi: 10.1002/hep.31150.
- Zhou, Y., et al., 2022. O-GlycNacylation remission retards the progression of non-alcoholic fatty liver disease. Cells, 11 (22), 3637. doi: 10.3390/cells11223637.
- Zhu, Y., et al., 2021. Caspase-11-mediated hepatocytic pyroptosis promotes the progression of nonalcoholic steatohepatitis. Cellular and molecular gastroenterology, 12 (2), 653–664.