Abstract
Ten methanol extracts from various parts of seven medicinal plants, Angiopteris evecta Hoffm.., (Marattiaceae) Citrus hystrix DC.., (Rutaceae) Laurentia longiflora (L.). Peterm.., (Campanulaceae) Nelumbo nucifera Gaertn.., (Nelumbonaceae), Piper sarmentosum Roxb.., (Piperaceae), Portulaca oleracea Linn.., (Portulacaceae) and Stachytarphera indica (L.). Vahl. (Verbenaceae), commonly used in Thai traditional medicine, were evaluated for their antioxidative and free radical scavenging activities. Among them, only that prepared from sacred lotus (N. nucifera) leaves exhibited a pronounced activity in the 1,1-diphenyl-2-picrylhydrazyl (DPPH) scavenging assay with an IC50 of 90 µg/ml, compared with an IC50 of 30 µg/ml for the butylated hydroxytoluene (BHT) control. The same extract was also found to be the most potent in removing the superoxide anion () radical and in inhibiting the 2,2′-azo-bis.-(2-amidinopropane) dihydrochloride (AAPH)-induced erythrocyte hemolysis and lipid peroxidation in a rat brain homogenate. The extract from leaves and peels of the Kaffir lime (C. hystrix.) exerted the strongest effect on production of the hydroxyl radical (OH·). They conferred a twice greater protection of deoxyribose from OH· than did tannin. However, none of the extracts examined in this study showed a significant pro-oxidant action in the bleomycin-dependent DNA oxidation system.
Introduction
Naturally generated reactive oxygen species (ROS) are molecules that can attack cell components and create several types of biological damage. They play important roles in the pathogenesis of various diseases ranging from carcinogenesis to aging (Ames et al., Citation1993). Thus, substances that scavenge ROS can protect against these diseases. Because the possibility has been raised that some synthetic antioxidants may be toxic (Yang et al., Citation2000), there have been numerous investigations into the possibility that some natural compounds may exist with potent antioxidative activity against free radicals but with low toxicity. So far a number of them, including polyphenolics, flavonoids, and various plants extracts, have been reported to be effective radical scavengers and inhibitors of lipid peroxidation (Abuja et al., Citation1998; Kerry & Abbey, Citation1998; Pearson et al., Citation1999; Zhang et al., Citation2001).
Thailand is located in the tropical region of Southeast Asia. Because innumerable varieties of tropical plants are found there, the Thais have a long ethnomedical knowledge, and have utilized alleged medicinal herbs and plants as cures for many ailments. In this study, various parts of seven plants namely, Angiopteris evecta. Hoffm.., (Marattiaceae) Citrus hystrix. DC.., (Rutaceae) Laurentia longiflora. (L.). Peterm.., (Campanulaceae) Nelumbo nucifera Gaertn..., (Nelumbonaceae), Piper sarmentosum. Roxb.., (Piperaceae), Portulaca. oleracea Linn.., (Portulacaceae) and Stachytarphera indica. (L.). Vahl. (Verbenaceae), selected from those that have appeared most frequently in Thai pharmacopoeia recipes, were investigated. A wide range of pharmacological properties of these plants and some of their chemical constituents have been reported. For example, a unique group of flavonoids, apigenin di–C-glycosyl flavones, have been detected in A. evecta. (Wallace et al., Citation1981). Glyceroglycolipids from C. hystrix. leaves have been demonstrated to have antitumor activities both in vivo. (Murakami et al., Citation1999) and in vitro. (Tiwawech et al., Citation2000), and they also contain a variety of flavonoids (Berhow et al., Citation1996). An alcohol extract of P. sarmentosum. leaves has neuromuscular blocking activity (Ridtitid et al., Citation1998) and antiplasmodic activiities (Rahman, Citation1999). An aqueous extract of P. oleracea. has antimicrobial activities (Ongsakul et al., Citation1992–1993) and contains omega-3 fatty acids along with several antioxidants in its leaves (Simopoulos et al., Citation1992). S. indica. contains an anti-inflammatory agent, ipolamiide (Tantisewie & Sticher, Citation1975). In spite of these reports, none have yet mentioned any inhibitory effect on free radicals, that is the principal mechanism of antioxidants. Therefore, in this study we have screened these plant extracts for their free radical scavenging activities as an attempt to find potential new sources for natural antioxidants.
Materials and Methods
Plant materials
All plant materials were collected locally. Their voucher specimens were identified at the Herbarium of Faculty of Science, Prince of Songkla University, and deposited in the authors' laboratories at the Department of Chemistry.
Preparation of plant extracts
Parts of plants () were cut into small pieces and extracted by three successive macerations in methanol at room temperature. The filtrates were combined and evaporated to dryness under vacuum at 45–55°C. The final residues were then stored at room temperature in a closed container until used.
Table 1 The parts of seven medicinal plants used for preparing crude extracts in this study.
Chemicals
1,1-Diphenyl-2-picrylhydrazyl (DPPH) was a product of Merck (Darmstadt, Germany). Butylated hydroxytoluene (BHT), bleomycin sulfate, DNA (type XIV from Herring testis), nitrobluetetrazolium dihydrochloride (NBT), and tetramethyl murexide (TMM) were purchased from Sigma (St. Louis, MO, USA). 2,2′-Azo-bis.-(2-amidinopropane) dihydrochloride (AAPH), 2-deoxy-D-ribose, and thiobarbituric acid (TBA) were obtained from Fluka (Switzerland). All other chemicals were of analytical grade.
Determination of total phenolics
The amount of total phenolics in extracts was determined using the AOAC (1990) spectrophotometric method. Each sample was dissolved in methanol to give a final concentration of 1 mg/ml. A 40-µl aliquot of sample solution was added to 5 ml of distilled water and then mixed with 0.5 ml of Folin-Danis reagent. After standing at room temperature for 3 min, 1 ml of 35% (w/v) Na2CO3 was added to the reaction mixture and the mixture was diluted to 10 ml with distilled water. After a 20 min incubation period at room temperature, the absorbance was read at 760 nm. The amount of total phenolics in each extract was determined from a standard curve prepared from different amounts of catechin and was expressed as catechin equivalents in μmole per mg of plant extract.
Iron chelation activity
According to the method of Shimada et al. (Citation1992), various concentrations of extract made to 2 ml in 10 mM hexamine, pH 5.0, were mixed with an equal volume of 3 mM FeSO4. An 0.2 ml aliquot of TMM (tetramethyl murexide) was then added and left for 3 min at room temperature. The absorbance was read at 485 nm against the control containing no extract. The percentage of chelation was then calculated from
where Asample is the absorbance of the test sample and Acontrol is the absorbance of the control.
Free radical scavenging assays
DPPH Assay
This was carried out according to Yen and Hsieh (Citation1997). To different concentrations of a sample in methanol (0.5 ml each) was added 1 ml of a methanol solution of 0.2 mM DPPH. After mixing thoroughly, the mixture was allowed to stand in the dark for 30 min and the absorbance at 523 nm was measured using methanol for the baseline correction. The results were then compared with that of the control prepared as above but without added sample. Radical scavenging activity was expressed as a percentage and was calculated using the following formula:
where Asample is the absorbance of the test sample and Acontrol is the absorbance of the control.
For each sample, the result was presented as an IC50 (sample concentration that produced 50% scavenging of the DDPH radical).
Hydroxyl radical scavenging assay
The method previously described by Murcia et al. (Citation2001) was modified. Various amounts of test sample were mixed with 134 µl of 30 mM KH2PO4-KOH buffer (pH 7.4), 67 µl of 17 mM deoxyribose, 33 µl of 34 mM H2O2, 33 µl of 1.2 mM EDTA, and 67 µl of 300 µM FeCl3. A 67 µl aliquot of 0.6 mM ascorbic acid was then added to start the reaction. After incubation at 37°C for 1 h, the products of the hydroxyl radical attack on deoxyribose were determined by adding 333 µl of 1% (w/v) TBA (thiobarbituric acid) in 50 mM NaOH, followed by 333 µl of 2.8% (w/v) TCA (trichloroacetic acid). After further incubation at 80°C for 20 min, the reaction mixtures were centrifuged. The absorption of the clear supernatants was then measured at 532 nm. A parallel assay omitting the test sample acted as a control, whereas the normal reaction mixture without deoxyribose was used as a sample blank. The results were expressed as percent inhibition of the deoxyribose attack using the following formula:
where Asample is the absorbance of the test sample, Acontrol is the absorbance of the control, and Asample blank is the absorbance level of the sample blank.
For each sample, the result was presented as an IC50 (sample concentration that produced 50% inhibition of hydroxyl radical).
Superoxide radical scavenging assay
The assay was carried out according to the method for estimating superoxide dismutase activity by Beyer and Fridovich (1987). Test samples were prepared in methanol, and 20 µl of each was added to 1 ml of the reagent composed of 9.9 mM L-methionine, 1.72 mM NBT (nitroblue tetrazolium), 1% (w/v) Triton X-100, and 117µM riboflavin in 50 mM K2HPO4, pH 7.8. After mixing, the reaction mixture was illuminated beneath a 40-W fluorescent light for 7 min. A control tube in which the test sample was replaced by methanol and a sample blank that contained no riboflavin were also run in parallel. The absorbance at 560 nm was then measured. The results were expressed as percent scavenging of the superoxide radical using the following formula:
where Asample is the absorbance of the test sample, Acontrol is the absorbance of the control, and Asample blank is the absorbance level of the sample blank.
For each sample, the result was presented as an IC50 (sample concentration that produced 50% inhibition of the superoxide radical).
Antioxidation of biomolecules
Lipid peroxidation assay
Based on the Liu and Ng method (2000), the brains of normal female albino rats of the Wistar strain (age 3 months) were dissected and homogenized in chilled 20 mM Tris-HCl, pH 7.4, at a ratio of 1:10 (w/v). The homogenate was centrifuged at 20,000 × g. for 15 min at 4°C. An 0.5-ml aliquot of the supernatant was then taken to incubate with 0.3 ml of the test sample, 0.1 ml of 10 µM FeSO4, and 0.1 ml of 0.1 mM ascorbic acid at 37°C for 1 h. The reaction was then stopped by addition of 0.75 ml of 28% (w/v) trichloroacetic acid (TCA) and 0.5 ml of 1% (w/v) thiobarbituric acid (TBA), successively. The mixture was then heated at 100°C for 45 min. After centrifugation, all precipitated proteins were removed and the color of the malondialdehyde (MDA)-TBA complex in the supernatant was detected at 532 nm. The percent inhibition was calculated using the following formula:
where Asample is the absorbance of the test sample and Acontrol is the absorbance of the control.
Erythrocyte hemolysis assay
Human blood was obtained from healthy volunteers by venepuncture and collected in heparinized tubes. Erythrocytes were separated from plasma and washed three-times with 10 volumes of PBS, pH 7.4. During the last wash, the erythrocytes were centrifuged at 1000 × g. for 10 min to obtain a constantly packed cell preparation.
In the test system, following the method of Liu and Ng (Citation2000), erythrocyte hemolysis was mediated by peroxyl radicals. A 10% suspension of erythrocytes in PBS, pH 7.4, was added to the same volume of 0.2 M AAPH in PBS containing the test sample at different concentrations. The reaction mixture was shaken gently while being incubated at 37°C. After 2 h, the reaction mixture was removed, diluted with 8 volumes of PBS, and centrifuged at 1000 × g. for 10 min. The absorbance of the supernatant was then read at 540 nm. In a similar manner, the reaction mixture was diluted with 8 volumes of distilled water to achieve a complete hemolysis, and the absorbance was read at 540 nm. The percentage of hemolysis was calculated compared with that of the control from
where Asample is the absorbance of the test sample and Acontrol is the absorbance of the control.
Bleomycin-dependent DNA damage
The assay was performed according to Liu and Ng (Citation2000). A 100 µl aliquot of sample to be tested at different concentrations was added to a mixture containing 50 μl of 0.5 mg/ml DNA, 50 µl of 0.05 mg/ml bleomycin sulfate, 200 µl of 5 mM MgCl2, and 100 µl of 50 µM FeCl2. After mixing, the reaction mixture was incubated at 37°C for 1 h. The reaction was then terminated by addition of 0.1 M EDTA (50 µl). The color was developed by adding 0.5 ml each of 1% (w/v) TBA and 25% (v/v) HCl followed by heating at 80°C for 10 min. After centrifugation, the extent of DNA damage was indicated by an increase in absorbance at 532 nm. L-Ascorbic acid was used as a positive control in this assay.
Statistical analysis
All experiments were performed in triplicate. The results are presented as the mean ± standard deviation (SD). The IC50 values were determined by non-linear regression analysis using Microsoft Excel 2002. Bivariate correlations were analyzed by Spearman's test using SPSS 10.0 on Windows. Statistically significant differences in radical scavenging activity among plant extracts were performed using unpaired one-way analysis of variance (ANOVA) followed by Scheffe's test; p < 0.05 was considered statistically significant.
Results and Discussion
Ten methanol extracts of the seven selected plants were screened for their free radical scavenging activities by using DPPH, hydroxyl radical (OH·), and superoxide anion () assays. Their IC50 values were calculated and compared with those of the standard compounds as shown in . Of all the extracts examined, the one prepared from lotus leaves was shown to have the best antioxidant activity. It exhibited the highest potency in both DPPH and
inhibition at concentrations comparable with those of the standards and also possessed strong scavenging activity for OH·.
Figure 1 Comparison of IC50 values of the plant extracts against (A) DPPH, (B) , and (C)
free radicals. Each bar represents the mean ±SD of triplicate values. Those bars with the same letter are not statistically different from each other (p > 0.05).
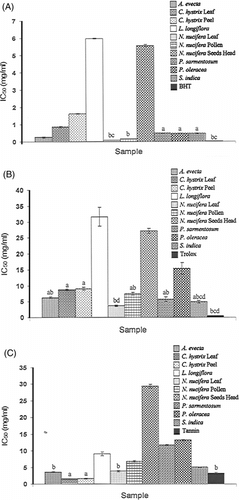
In the DPPH assay, widely used as a screening test for the proton-donating ability of plant extracts (Zhu et al., Citation2002), most of the extracts in the current study were effective in inhibiting DPPH·. Lotus leaf extract had the strongest activity with an IC50 value of 90 µg/ml. The BHT control had an IC50 of 30 µg/ml (i.e., three-times more active). The extracts from lotus seed heads and Laurentia longiflora. were not so effective in this system. The samples were also measured for their OH· and free radical scavenging abilities. In cellular oxidation reactions,
is normally formed first, and its effects can be magnified because it produces other kinds of cell-damaging free radicals including the most destructive radical, OH· (Decker, Citation1998). The crude extracts that showed the best activity in the hydroxyl assay were those from lime leaves and lime peels with IC50 values of 155 µg/ml and 164 µg/ml, respectively, about half that of the standard tannin (IC50 = 325 µg/ml) ( C). Lotus seeds head extract had very little activity in any of these scavenging tests (). When the relative inhibitory potency of the ten extracts on each free radical type was compared, some differences did exist. The rankings of the IC50 values in the DPPH assay (N. nucifera. leaf > N. nucifera. pollen > A. evecta. > P. oleracea. > S. indica. > P. sarmentosum. > C. hystrix. leaf > C. hystrix. peel > N. nucifera. seeds head > L. longiflora.) () and the O2ċ− assay (N. nucifera. leaf > S. indica. > P. sarmentosum. > A. evecta. > N. nucifera. pollen > C. hystrix. leaf > C. hystrix. peel > P. oleracea. > N. nucifera. seeds head > L. longiflora.) () were similar but considerably different from those for the OH· assay (C. hystrix. leaf > C. hystrix. peel > A. evecta. and N. nucifera. leaf > S. indica. > N. nucifera. pollen > L. longiflora. > P. sarmentosum. > P. oleracea. > N. nucifera. seeds head) (). With the exception of lime leaf, lime peel, and L. longiflora. extracts, most of them became less effective at inhibiting OH· than scavenging for DPPH·. The results imply that the OH· scavenging activities of these plant extracts may not be solely attributable to their proton-donating abilities.
It is generally known that the OH· generated from an Fe2+-catalyzed Fenton's reaction can be effectively suppressed not only by trapping the radicals but also by chelating free iron and thereby preventing the redox cycle of this transition metal ion. Based on such a concept, the crude extracts were then evaluated for their Fe2+ chelating abilities. The results are presented in . The ten extracts displayed a concentration-dependent effect. Among them, lotus leaf and Angiopteris evecta. rhizome extracts were by far the most effective chelators in this assay producing 99.6% and 71.2% binding, respectively, at 2 mg/ml. Binding by extracts from other plants at 2 mg/ml ranged from 11% to 38%. Comparison of these results with the data in C also reveals a high correlation between the metal binding capacity of the tested extracts and their OH· scavenging activities [Spearman's correlation coefficient (ρS) = 0.624]. With the exception of lotus leaf extract, they depend on each other.
Table 2 The Fe2+-chelating activities of the plant extracts.
Phenolics are the most widespread secondary metabolites in the plant kingdom. This diverse group of compounds has received much attention as potential natural antioxidants in terms of their abilities to act as both efficient radical scavengers and metal chelators (Sanchez-Moreno et al., Citation2000). We have therefore determined the total phenolics present in these extracts using the modified Folin-Ciocalteau method. Values are expressed as catechin equivalents per mg of dried plant extract (). The results obtained seem to follow those in the metal binding assay (ρS = 0.745). The findings that the lotus leaf and A. evecta. extracts contained the most abundant phenolics while those exhibiting rather weak metal binding activity such as the lotus pollen, L. longiflora., and Piper sarmentosum. were not so rich in these compounds, also confirm the contribution of phenolics in the tested extracts to their metal chelation activities. According to the above results, it is likely that the OH· scavenging capability of the plant extracts could be predicted on the basis of their total phenolic contents. However, both radical scavenging and metal binding properties of phenolics are also related to their structural features (Arora et al., Citation1998; Brown et al., Citation1998; Croft, Citation1998; Morel et al., Citation1998). Hence, in addition to the amounts, the structural variations of phenolic constituents in extracts from different plants would have some effects on their antioxidant activities.
Table 3 Total amounts of phenolics in the plant extracts.
In addition to their free radical scavenging activities, we also evaluated the plant extracts for their abilities to protect biomolecules from oxidative damage. In the lipid peroxidation system, all extracts were able to decrease the oxidation of rat brain homogenate initiated by OH·. Oxidation of unsaturated fatty acids in biological membranes leads to the formation and propagation of lipid radicals, uptake of oxygen, rearrangement of the double bonds in unsaturated lipids, and eventual destruction of membrane lipids, with production of breakdown products such as malondialdehydes (Slater et al., Citation1987). At the testing concentrations of 250 and 500 µg/ml, all were inhibitory (). The extracts of Portulaca oleracea., lime leaf, lime peel, and L. longiflora. that produced less than 50% inhibition at 250 µg/ml produced more inhibition at 500 µg/ml. The maximum inhibition achieved was just less than 80% so those extracts that produced more than 60% inhibition gave only a small or no increase at 500 µg/ml. The most active extracts at 250 µg/ml from A. evecta. and lotus leaf and pollen were those that gave the best activity in the DPPH assay. Therefore, those active materials in the tested extracts that can donate protons to peroxyl radicals and terminate the radical chain reaction may play a major role in this case. Surprisingly, the anti-lipid peroxidation activities of the plant extracts were not dependent on their OH· scavenging activities. Lime leaf and lime peel extracts that previously demonstrated the highest and the next highest potency with respect to their OH· scavenging assay () were less effective in this system, whereas those from the lotus seeds head and lotus pollen were among the most active ones. The reasons for such differences are not clear. In contrast with the inhibition of OH· in the deoxyribose system, the protection of lipids from metal ion–mediated oxidation is also dependent on the solubility characteristics of the antioxidants. The more lipid-soluble they are, the better they may reach the site of oxidation and act on the reactive radicals.
Table 4 Effect of the plant extracts on lipid peroxidation in a rat brain homogenate.
In a study employing RBC hemolysis mediated by the peroxyl radicals from APPH thermolysis, the abilities of the ten extracts to inhibit non-metal-dependent lipid peroxidation on the RBC membrane were investigated (). The results that differ from those using the brain homogenate assay may arise from the different mechanisms involved in the two assays. The inhibitory process in the Fenton-type lipid peroxidation system is dependent on a number of factors such as metal chelation and proton or electron donation to the affecting radicals, whereas the hemolysis assay is less complex. While the same mode of inhibition by donating protons to the peroxyl radicals was expected in both the DPPH and AAPH-induced RBC hemolysis assays, the potencies of these extracts to protect RBC membrane lipids from the AAPH-induced oxidation still varied from their DPPH· scavenging activities (). This could be due to the antioxidant materials of the plant extracts possessing differences in their lipid solubilities.
Table 5 Effects of the plant extracts on hemolysis of human erythrocytes.
Although vitamin C is a strong antioxidant, a high intake of this vitamin may, in some situations, lead to a pro-oxidant activity in the body especially when free transition metals are available at the same time (Herbert et al., Citation1996). In this study, the pro-oxidant activity of vitamin C was more than ten times higher than that of all plant extracts when tested using the bleomycin-dependent DNA damage as a parameter. Bleomycin is an antibiotic that is also known for its capability to degrade DNA upon reacting with Fe2+ in the presence of O2 (Gutteridge et al., Citation1981). In the method used, the resulting DNA fragments were detected as a pink chromogen of base propenals upon heating with thiobarbiturate at low pH. Increased absorbance at 532 nm indicating a stimulation of DNA degradation was then adopted as the pro-oxidant properties of test materials. At both concentrations tested, only lotus leaf extract displayed a slight pro-oxidant effect while the rest were very low (). All extracts also displayed a concentration-dependent pro-oxidation, except that of P. oleraceae. and BHT, which showed inhibition in response to their increased concentrations under the same test system.
Table 6 Pro-oxidant effects of the plant extracts on ferric bleomycin induced DNA damage.
In the current study, we have demonstrated that methanol extracts of seven Thai medicinal plants contained constituents that could serve as natural sources to develop antioxidants. Among these plants, which are common and abundant in Thailand, the sacred lotus leaf seems to be most worthy of further studies because it showed remarkable activities in comparison with the others. Currently, efforts are underway to isolate and identify the active components responsible for its relatively high antioxidative activity.
Acknowledgments
The authors wish to thank the Graduate School and the Natural Products Research Unit of the Faculty of Science, Prince of Songkla University, for the financial support of this study. Appreciation is also extended to Dr. Kitja Sawangjaroen of Department of Pharmacology for providing all rat brains used in the lipid peroxidation assay and to Mr. Decha Pinkaew and Ms. Pratum Ritthisunthorn for their assistance in manuscript preparation. We thank Dr. Brian Hodgson for his kind revision of the manuscript.
References
- Abuja PM, Murkovic M, Pfannhauser W (1998): Antioxidant and prooxidant activities of elderberry (Sambucus nigra.) extract in low density lipoprotein oxidation. J Agric Food Chem 46: 4091–4096, [CSA]
- Ames BN, Shigenaga MK, Hagen TM (1993): Oxidants, antioxidants, and the degenerative disease of aging. Proc Natl Acad Sci USA 90: 7915–7922, [INFOTRIEVE], [CSA]
- Arora A, Nair MG, Strasburg GM (1998): Structure-activity relationships for antioxidant activities of a series of flavonoids in a liposome system. Free Radic Biol Med 24: 1355–1363, [INFOTRIEVE], [CROSSREF], [CSA]
- AOAC (1990): Determination Method of Polyphenols: Official Method of Analysis, 15th ed. Washington, DC, Association of Official Analytical Chemists.
- Berhow MA, Fong CH, Hasegawa S (1996): Limonoid and flavonoid composition in varieties of Papeda and Papedocitrus. Biochem Sys Ecol 24: 237–242, [CROSSREF], [CSA]
- Beyer Jr. WF, Fridovich I (1978): Assaying for superoxide dismutase activity: some large consequences of minor changes in conditions. Anal Biochem 161: 559–566, [CROSSREF], [CSA]
- Brown JE, Khodr H, Hider RC, Rice-Evans CA (1998): Structure dependence of flavonoid interactions with Cu2+ ions; implications for their antioxidant properties. Biochem J 330: 1173–1178, [INFOTRIEVE], [CSA]
- Croft KD (1998): The chemistry and biological effects of flavonoid and phenolic acids. Ann NY Acad Sci 854: 435–442, [INFOTRIEVE], [CROSSREF], [CSA]
- Decker E (1998): Antioxidants. In: Akoh, CC, Min BD, eds., Food Lipids: Chemistry, Nutrition and Biotechnology, New York: Marcel Dekker, pp. 423–448.
- Gutteridge JMC, Rowley DA, Halliwell B (1981): Superoxide-dependent formation of hydroxyl radical in the presence of iron salts. Detection of free iron in biological system using bleomycin-dependent degradation of DNA. Biochem J 199: 263–265, [INFOTRIEVE], [CSA]
- Herbert V, Shaw S, Jayatilleke E (1996): Vitamin C driven free radical generation from iron. J Nutr 126: 1213S–1220S, [INFOTRIEVE], [CSA]
- Kerry N, Abbey M (1998): The isoflavone genistein inhibits copper and peroxyl radical mediated low density lipoprotein oxidation in vitro.. Atherosclerosis 140: 341–347, [INFOTRIEVE], [CROSSREF], [CSA]
- Lui F, Ng TB (2000): Antioxidative and free radical scavenging activities of selected medicinal herbs. Life Sci 66: 725–735, [CROSSREF], [CSA]
- Morel L, Cillard P, Cillard J (1998): Flavonoid-metal interactions in biological system. In: Rice-Evans CA, Packer L, eds., Flavonoids in Health and Diseases, 3rd ed., New York, Marcel Dekker, pp. 163–177.
- Murakami A, Gao G, Kim, OK, Omura M, Yano M, Ito C, Furakawa H, Jiwajinda S, Koshimizu K, Oshigashi H (1999): Identification of coumarins from the fruit of Citrus hystrix. DC. As inhibitors of nitric oxide generation in mouse macrophage Raw 264.7 cells. J Agric Food Chem 47: 333–339, [INFOTRIEVE], [CROSSREF], [CSA]
- Murcia MA, Jime'nez AM, Marti'nez-Tome', M (2001): Evaluation of the antioxidant properties of Mediterranean and tropical fruits compared with common food additives. J Food Protect 64: 2037–2046, [CSA]
- Ongsakul M, Sawangjaroen N, Thaina P, Sunbhanich M, Chatbenjarong J, Thongnurung A (1992–1993): Antimicrobial activities of Portulaca oleracea. Linn. in vitro.. Thai J Pharmacol 7: 305–310, [CSA]
- Pearson DA, Tan CH, German JB, Davis PA, Gershwin ME (1999): Apple juice inhibits human low density lipoprotein oxidation. Life Sci 64: 1913–1920, [INFOTRIEVE], [CROSSREF], [CSA]
- Rahman NN, Takahisa F, Kojima S, Takane K, Alimohd M (1999): Antimalarial activity of extracts of Malaysian medicinal plants. J Ethnopharmacol 64: 249–254, [CROSSREF], [CSA]
- Ridttitid W, Rattanaprom W, Thaina P, Chittrakarn S, Sunbhanich S (1998): Neuromuscular blocking activity of methanolic extract of Piper sarmentosum. leaves in the rat phrenic nerve-hemidiaphragm preparation. J Ethnopharmacol 61: 135–142, [CROSSREF], [CSA]
- Sanchez-Moreno C, Jimenez-Escria A, Saura-Calixto J (2000): Study of low density lipoprotein oxidizability indexes to measure the antioxidant activity of dietary polyphenols. Nutr Res 20: 941–953, [CROSSREF], [CSA]
- Shimada K, Fujilawa K, Yahara K, Nakamura T (1992): Antioxidative properties of xanthan on the autoxidation of soybean oil in cyclodextrin emulsion. J Agric Food Chem 40: 945–948, [CROSSREF], [CSA]
- Simopoulos AP, Norman HA, Gillaspy JE, Duke JA (1992): Common purslane, a source of omega-3 fatty acid and antioxidant. J Am Coll Nutr 11: 374–382, [INFOTRIEVE], [CSA]
- Slater TF, Cheeseman KH, Davies MJ, Xin W (1987): Free radical mechanism in relation to tissue injury. Proc Nutr Soc 46: 1–12, [INFOTRIEVE], [CROSSREF], [CSA]
- Tantisewie B, Sticher O (1975): Isolation of ipolamiide from Stachytarpheta indica.. Phytochemistry 14: 1462–1463, [CROSSREF], [CSA]
- Tiwawech D, Hirose M, Futakuchi M, Lin C, Thamavit W, Ito N, Shirai T (2000): Enhancing effects of Thai edible plants on 2-amino-3,8-dimethylimidazo (4,5-f) quinoxaline-hepatocarcinogenesis in a rat medium-term bioassay. Cancer Lett 158: 195–201, [INFOTRIEVE], [CROSSREF], [CSA]
- Wallace JW, Yopp DL, Besson E, Chopin J (1981): Apigenin di-C-glycosylflavones of Angiopteris. (Marattiales). Phytochemistry 20: 2701–2703, [CROSSREF], [CSA]
- Yang JH, Mau JL, Ko PT, Haung LC (2000): Antioxidant properties of fermented soybean broth. J Food Chem 71: 249–254, [CROSSREF], [CSA]
- Yen GC, Hsieh GL (1997): Antioxidant effects of dopamine and related compounds. Biosci Biotech Biochem 61: 1646–1649, [CSA]
- Zhang Z, Chang Q, Zhu M, Huang Y, Walter KK, Chen Z (2001): Characteristic of antioxidants present in Hawthorn fruits. J Nutr Biochem 12: 144–152, [INFOTRIEVE], [CROSSREF], [CSA]
- Zhu QY, Hackman RM, Ensunsa JL (2002): Antioxidative activities of oolong tea. J Agric Food Chem 50: 6929–6934, [INFOTRIEVE], [CROSSREF], [CSA]
