Abstract
The present study was carried out to establish the antinociceptive, anti-inflammatory and antipyretic properties of an aqueous extract of jute plant leaves, Corchorus capsularis L. (Tiliaceae), in experimental animals. The antinociceptive activity was measured using the abdominal constriction, hot plate and formalin tests, while the anti-inflammatory and antipyretic activities were measured using the carrageenan-induced paw edema and brewer’s yeast-induced pyrexia tests, respectively. The extract, obtained after 72 h soaking of the air-dried leaves in distilled water, freeze-drying for 72 h and then prepared in dosages of 11.57, 57.85, and 115.7 mg/kg, was administered subcutaneously (10 ml/kg) 30 min prior to subjection to the above mentioned assays. The extract was found to exhibit significant (antinociceptive, anti-inflammatory and anti-pyretic, activities in a dosage-independent manner. In conclusion, the aqueous extract of C. capsularis possesses antinociceptive and antipyretic activities and supports the previous claim of its traditional use to treat various ailments.
Introduction
Corchorus capsularis L. (Tiliaceae) is a jute plant also known to the Malays as kancing baju (CitationPFAF, 2004). C. capsularis leaves have been eaten as vegetables in Africa, the Middle East, and Southeast Asia, including Malaysia, for a long time; in Bangladesh, particularly, C. capsularis is used as a vegetable more than C. olitorius L. due to the bitter taste of the latter (CitationEBPS, 2006). The leaves, which are the most edible part of C. capsularis, are directly added to salad if they are younger leaves or cooked as a pot-herb if they are older leaves. The dried leaves are also used as a thickener in soups but usually prepared as tea (CitationPFAF, 2004). In addition, the Japanese have been using the leaves of C. capsularis as health-food, in which the dry leaves were substituted for coffee or tea. Traditionally, the leaves of C. capsularis are used as demulcent, appetizer, laxative, carminative, stomachic and stimulant, while its infusion is used in the treatment of fevers, dysentery, constipation, dyspepsia and liver disorders (CitationPFAF, 2004). In addition, a decoction of the roots and unripe fruits can also be used to treat dysentery while the seeds, which contain a substance that produced a less intense action on the heart when compared to digitalin, are used in the treatment of heart problems (CitationPFAF, 2004).
Due to lack of scientific studies to support its traditional use as demulcent and pyrexia relieving agent, as well as to establish its other potential pharmacological properties, this study was undertaken to evaluate the antinociceptive, anti-inflammatory and antipyretic properties of an aqueous extract of C. capsularis (AECC) leaves.
Materials and methods
Plant material
The leaves of C. capsularis were collected in CitationJuly–September, 2005 from its natural habitat in Kg. Kuala Kangkong, Simpang Ampat, Alor Setar, Kedah, Malaysia by Mohd. Suhaimi Ismail. It was identified by Shamsul Khamis, a botanist at the Institute of Bioscience (IBS), Universiti Putra Malaysia, (UPM), Serdang, Selangor, Malaysia, and a voucher specimen (SK 856/05) was deposited at the Herbarium of the Laboratory of Natural Products, IBS, UPM, Malaysia.
Preparation of an aqueous extract of C. capsularis
The leaves of C. capsularis were washed and rinsed with water to remove all the dirt and unwanted particles and then air-dried for 1-2 weeks at room temperature (27 ± 2°C). The dried leaves were then ground into powder form, weighed and added with distilled water (dH2O) in a ratio of 1:20 (w/v). This mixture was then left for 72 h and the supernatant was collected and filtered using Whatman No. 1 filter paper while the remaining plant residue was kept in an oven for future use (i.e., extraction using an organic solvent such as chloroform for antimicrobial study). The supernatant obtained, labeled as AECC and considered as stock solution with 100% concentration/strength, was prepared in the concentrations/strengths of 10% and 50% by diluting the stock solution with dH2O for the pharmacological studies described earlier. A 30 ml portion of the obtained supernatant was also subjected to freeze-drying process and yielded 0.347 g of crude dried AECC (23.13% of yield). Based on the amount of crude dried AECC obtained, it was estimated that the 10, 50, and 100% concentrations AECC were approximately equivalent to the dosages of 11.57, 57.85, and 115.7 mg/kg, respectively.
Preparation of drugs
Acetylsalicylic acid (ASA) (100 mg/kg; Bayer, Singapore) and morphine (5 mg/kg; Sigma, Germany), used for the purposed of comparison, were prepared by dissolving them in dH2O.
Experimental animals
Male Balb-C mice (25-30 g; 5-7 weeks) and Sprague-Dawley rats (180-200 g; 8-10 weeks old), obtained from the Animal Source Unit, Faculty of Veterinary Medicine, Universiti Putra (UPM), Serdang, Selangor, Malaysia, were used in this study. All of the animals were kept at room temperature (27° ± 2°C; 70-80% humidity; 12 h light/darkness cycle) in the Animal Holding Unit, Faculty of Medical and Health Sciences, UPM for at least 48 h before use. Food and water were supplied ad libitum up to the beginning of the experiments. At all times the rats were cared for in accordance with current UPM principles and guidelines for the care of laboratory animals and the UPM ethical guidelines for investigations of experimental pain in conscious animals as adopted from CitationZimmermann (1983).
All mice were equally divided into 10 groups of 7 mice each (n = 7) and received (sc) DH2O, ASA (100 mg/kg) or AECC (11.57, 57.85 or 115.7 mg/kg) 30 min prior to subjection to the abdominal constriction or hot plate tests, respectively. All rats were equally divided into 16 groups of 5 rats each (n = 5). The first batch, consisting of six groups of rats, were used in the formalin test and received (sc) dH2O, 100 mg/kg ASA, 5 mg/kg morphine or AECC (11.57, 57.85, and 115.7 mg/kg), respectively, 30 min prior to subjection to the test. The second and third batches, consisting of five groups of rats each, were used in the anti-inflammatory and antipyretic studies, and received (sc) dH2O, 100 mg/kg ASA or AECC (11.57, 57.85, or 115.7 mg/kg), respectively, 30 min prior to subjection to the said tests. All of the test solutions were administered in the volume of 10 ml/kg body weight.
Antinociceptive assay
Abdominal constriction test
The abdominal constriction test (CitationDambisya & Lee, 1995) was used with slight modifications as described by CitationZakaria et al. (2005) to evaluate the chemically induced peripheral antinociceptive activity of AECC.
Hot plate test
The 50°C hot-plate test (CitationWilson et al., 2003), with slight modification as described by CitationZakaria et al. (2005), was used to assess the thermally induced central antinociceptive activity of AECC.
Formalin test
The formalin test Citation(Hunskaar & Hole, 1987) was used, with slight modifications as described by CitationZakaria et al. (2006a), to assess the non-anti-inflammatory, antinociceptive effects of AECC.
Anti-inflammatory assay
The carrageenan-induced paw edema test (CitationChakraborty et al., 2004) was used, with slight modification as described CitationZakaria et al. (2006a), to assess the anti-inflammatory properties of the AECC.
Antipyretic assay
The antipyretic activity of AECC was measured using the Brewer’s yeast induced pyrexia test (CitationReanmongkol et al., 2002) with slight modifications Citation(Zakaria et al., 2006b).
Statistical analyses
The results are presented as mean ± standard error of mean (SEM). Data for the abdominal constriction and formalin tests were analyzed and compared using the one-way ANOVA with Dunnett post-hoc test while the formalin, paw edema and pyrexia tests were analyzed and compared using the two-way ANOVA, with P < 0.05 as the limit of significance.
Results
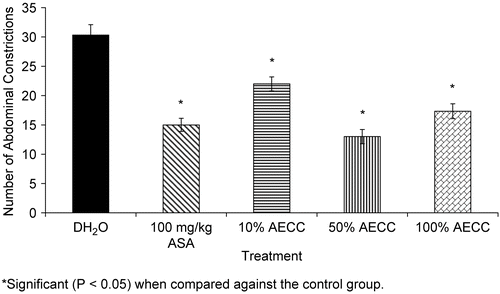
shows the antinociceptive profile of AECC assessed using the acetic acid-induced abdominal constriction test in mice. The extract exhibited a dosage-dependent antinociception as seen in the first two dosages used followed by a significant (P < 0.05) decreased but still retained activity. Interestingly, the 57.85 mg/kg AECC produced an antinociception that was equipotent to that of 10 mg/kg ASA.
Table 1. Phytochemical constituents of C. capsularis.
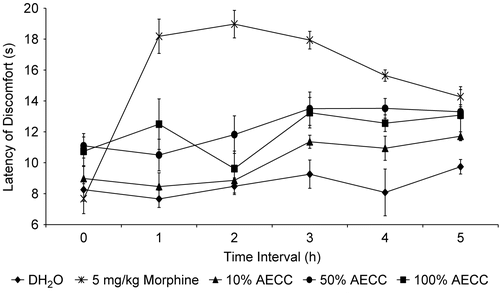
shows the antinociceptive profile of AECC assessed using the hot plate test in mice. The AECC was also found to show a dosage-dependent antinociception for the first two dosages used. However, the 115.7 mg/kg AECC showed an inconsistent activity, at least for the first two hours of the time interval. Unexpectedly, the 115.7 mg/kg AECC did not produce significant effect on the latency of discomfort after 30 min of its administration when compared to the other two dosages of AECC. Furthermore, the AECC-produced antinociception lasted until the end of the experiment.
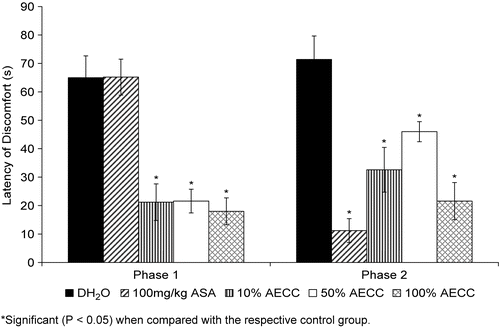
shows the antinociceptive profile of AECC assessed using the formalin test in rats. The extract was found to produce significant (P < 0.05) antinociception in the early and late phases of the test in a non-dosage-dependent manner. The AECC, at all of the dosages used, produced an equieffective activity in the early phase. However, in the late phase, the extract’s antinocicepetion decreased slightly as the dosages of AECC increased from 11.57 to 57.85 mg/kg before significantly (P <0.05) increasing as the dosage of AECC reached 115.7 mg/kg.
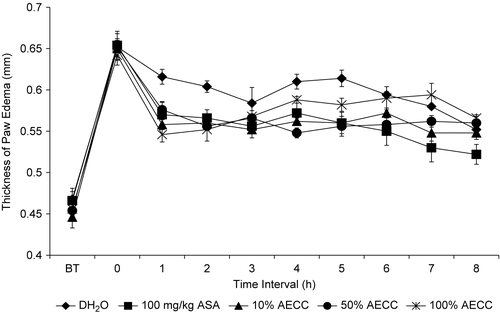
shows the anti-inflammatory profile of AECC assessed using the carrageenan-induced paw edema test in rats. The extract produced a significant (P <0.05) anti-inflammatory activity in a dosage-independent manner with the 115.7 mg/kg AECC exhibiting a less remarkable activity as indicated by its low reduction between the interval time of 4-5 h when compared to the other dosages of AECC. Interestingly, all dosages of the AECC lost their anti-inflammatory activity at the end of the experiment.
Figure 4. The anti-inflammatory profile of AECC assessed by the carrageenan-induced paw edema test in rats.
shows the antipyretic profile of AECC assessed using the Brewer’s yeast (BY)-induced pyrexia in rats. The extract produced an antipyretic activity only for the first four hours regardless of the dosages of AECC used. The dosage-dependent antipyretic activity was observed between the 57.85 and 115.7 mg/kg of the AECC. The 11.57 mg/kg AECC was found to produce an activity that was as effective as that of the 57.85 mg/kg AECC. Interestingly, the AECC antipyretic activity started to diminish after 4 h of its administration regardless of the dosage used.
Discussion
The present study has proven the potential of AECC as antinociceptive, anti-inflammatory, and antipyretic agents, and confirmed the traditional use of C. capsularis leaves infusion in the treatment of fever. The AECC was found to exhibit a dosage-independent antinociceptive activity when assessed using the abdominal constriction, hot plate and formalin tests indicating its effectiveness in blocking the nociception induced by chemical and thermal stimuli. The ability to directly stimulate the nociceptor and inhibit the inflammatory mediators releases are suggested since the AECC was also found to exhibit an antinociceptive activity in both phases of the formalin test.
The abdominal constriction test is a very sensitive test since it was able to detect antinociception of compounds/dose levels that may be inactive in other tests like the hot plate or tail-flick tests (CitationBentley et al., 1981). The assay involved the local peritoneal receptor system stimulation (CitationBentley et al., 1983) and is due to the prolonged acetic acid-induced irritation of the peritoneal cavity (CitationDeraedt et al., 1980). This will lead to an increase in the prostaglandins levels of peritoneal fluid, which will, in turn, enhance inflammatory pain by increasing the capillary permeability (CitationVogel & Vogel, 1997). According to CitationChan et al. (1995), this assay is not specific since it did not specify the involvement of peripheral or central activity. The hot plate and formalin assays are usually performed together with the abdominal constriction test before a final conclusion could be made on the type of mechanism involved in any antinociceptive agents under investigation.
The hot plate test, which measures the complex response to a non-inflammatory, acute nociceptive input, is usually used to indicate the involvement of central antinociceptive mechanism (CitationPini et al., 1997). Drugs acting centrally inhibit the abdominal constriction and hot plate tests (CitationHosseinzadeh & Younesi, 2002) while those acting peripherally inhibit only the abdominal constriction test (CitationAmanlou et al., 2005). The activity seen with the hot plate test suggested the ability of AECC to affect the central nociceptive mechanism.
According to CitationHeapy et al. (1987), the injection of formalin into the paw of rat causes an immediate and intense increase in the spontaneous activity of C fiber afferent and evokes a distinct quantifiable behavior indicative of pain (i.e., licking of the injected paw). The formalin test produced a distinct biphasic nociceptive response labeled as the early and late phases (CitationMalmberg & Yaksh, 1992). The early phase is an acute response observed immediately after the administration of formalin and persists for 5 min while the late phase appears between 15 and 60 min after the formalin administration. According to CitationTjølsen et al. (1992), the acute early phase appeared as a result of direct stimulation of nociceptors by formalin, while the late tonic phase involved the inflammatory processes and activation of the neurons located in the dorsal horns of the spinal cord. Due to their different properties, the early and late phases have been regarded as very useful tools for assessing the effectiveness of pain relieving compounds and expounding the actual mechanism(s) involved. In terms of this activity, drugs that act centrally (i.e., opioids) have been demonstrated to affect both phases while drugs that act peripherally (i.e., NSAIDs) only influence the late phase (CitationChan et al., 1995). CitationCowan (1990) has also suggested the use of formalin test in the assessment of pain relieving agents potential in combating chronic pain due to inflammation. The AECC was demonstrated to affect both phases of the said test which is concomitant with the activity shown by centrally acting analgesic drugs (CitationChan et al., 1995).
The carrageenan-induced rat paw edema test has been widely used to study the potential anti-inflammatory properties of newly developed drugs or newly discovered natural products (CitationWinter et al., 1962; CitationChan et al., 1995). The release of kinins, polymorphonuclear leucocytes as well as their proinflammatory factors (i.e., prostaglandins) have been suggested as contributing to the development of edema (CitationDamas et al., 1986). The inflammation caused by carrageenan administration has been categorized into the early and late phases (CitationVineger et al., 1969; CitationSüleyman et al., 2004). The former was thought to be caused by serotonin and histamine release and observed around 60 min after the irritant administration (CitationCrunkhon & Meacock, 1971) while the latter was thought to be caused by the release of inflammatory mediators (i.e., prostaglandin-like compounds) (CitationVineger et al., 1969). In line with the claim made by CitationVineger et al. (1969), the steroidal and non-steroidal anti-inflammatory agents were very effective in inhibiting the late phase (CitationDi Rosa et al., 1971). The significant reduction of paw edema seen with all dosages of the AECC does indicate the presence of anti-inflammatory activity in the said extract. This finding has scientifically confirmed the folklore use of C. capsularis leaves as a demulcent. Furthermore, this finding was in line with the claim that compounds with anti-inflammatory activity also possess antinociceptive activity (CitationAttaway & Zaborsky, 1993).
The AECC was also found to possess antipyretic activity when assessed using the BY-induced pyrexia test. This finding has scientifically confirmed the Malay traditional practice of using C. capsularis leaves in the treatment of pyrexia or to lower the body temperature. The ability to reduce pyrexia indicates the ability of the extract to cross the blood-brain barrier (BBB) (CitationBegley et al., 2000) since the pyrexia mechanism involved the stimulation of a region that controls the body temperature within the hypothalamus by the centrally synthesized prostaglandin (CitationUzcátegui et al., 2004). The ability to cross the BBB could also be the key factor that leads to the observed antinociceptive activity that was assessed using the hot plate test.
The term ‘therapeutic window’ described by CitationTripathi (2001) could be used to explain the concentration-independent effect of AECC seen in most of the assays. It is a phenomenon in which a certain desired therapeutic effect of a drug is exerted only within a narrow drug dosages or plasma drug concentrations range. According to CitationTripathi (2001), drugs with therapeutic window phenomenon will produce suboptimal beneficial effects or even decline in effects if the concentrations are below or above this narrow therapeutic range.
Although extensive study on the pharmacological potential of C. capsularis active constituents has never been carried out, previous studies have revealed the presence of dammarane triterpene (CitationHasan et al., 1984), glycoside (CitationQuader et al., 1990), phenolic acids (CitationMosihuzzaman et al., 1986), xylans Citation(Vignon & Gey, 1998), proteins and amino acids (CitationGhosh & Raksit, 1994) and carbohydrates (CitationMosihuzzaman et al., 1989) in various parts of the plant. Of these compounds, dammarane triterpene glycoside (3-glucoside of 20,24-epoxy-3β,12β,25,30-tetrahydroxy-dammarane) and glycoside (25,30-O-β-digluco-pyranoside) have been reported to be present in the leaves of C. capsularis. A study carried out by CitationYoshikawa et al. (1997) has demonstrated the presence of dammarane-type triterpene oligoglycosides in the seeds of Zizyphus jujube, which were also found to inhibit the release of histamine from rat peritoneal exudate cells induced by antigen-antibody-reaction. Although it has never been proven with the dammarane-type triterpene glycoside of C. capsularis, it is plausible to suggest that the ability of the AECC to produce the anti-inflammatory and antinociceptive activities could be due, partly, to the ability of this compound to inhibit the histamine release as mentioned earlier. Finally, we conclude that the AECC possessed antinociceptive, anti-inflammatory, and antipyretic activities and, thus, confirmed its traditional uses in the treatment of various ailments.
Acknowledgements
This study was supported by a research grant from the Universiti Industri Selangor, Malaysia (Project Code Number: 03013; Project Vote Number: 3090103013). The authors would like to thank Universiti Putra Malaysia for their facilities.
Declaration of interest: The authors report no conflicts of interest. The authors alone are responsible for the content and writing of the paper.
References
- Amanlou M, Dadkhah F, Salehnia A, Farsam H, Dehpour AR (2005): Anti-inflammatory and anti-nociceptive effects of hydrochloric extract of Satureja khuzistanica Jamzad extract. J Pharm Pharmaceut Sci 8: 102–106.
- Attaway DH, Zaborsky OR (1993): Marine Biotechnology: Pharmaceutical and Bioactive Natural Products, Vol 1. New York, Plenum Press, pp. 1–23.
- Begley DJ, Bradbury MWB, Kreuter J (2000): The Blood-Brain Barrier and Drug Delivery to the CNS. New York, Marcel Dekker, pp. 1–8.
- Bentley GA, Newton SH, Starr J (1981): Evidence for an action of morphine and the enkephalins on sensory nerve endings in the mouse peritoneum. Br J Pharmacol 73: 325–332.
- Bentley GA, Newton SH, Starr J (1983): Studies on the antinociceptive action of α-agonist drugs and their interactions with opioid mechanisms. Br J Pharmacol 79: 125–134.
- Chan TF, Tsai HY, Tian-Shang W (1995): Anti-inflammatory and analgesic activities from the roots of Angelica pubescens. Planta Med 61: 2–8.
- Cowan A(1990): Modern Methods in Pharmacology: Testing and Evaluation of Drugs Abuse, Vol. 6. New York, Wiley Liss Inc, pp. 33–42.
- Crunkhon P, Meacock SER (1971): Mediators of the inflammation induced in the rat paw by carrageenan. Br J Pharmacol 42: 392–402.
- Damas J, Remacle-Volon G, Deflandre E (1986): Further studies of the mechanism of counter irritation by turpentine. Arch Pharmacol 332: 196–200.
- Dambisya YM, Lee TL (1995): Effects of l-NAME, l-NMMA and l-arginine on the antinociceptive effects of morphine in mice. Meth Find Exp Clin Pharmacol 17: 577–582.
- Deraedt R, Jougney S, Delevalcee F, Falhout M (1980): Release of prostaglandins E and F in an algogenic reaction and its inhibition. Eur J Pharmacol 51: 17–24.
- Di Rosa M, Giroud J, Willoughby DA (1971): Studies of the mediators of the acute inflammatory response induced in rat in different sites by carrageenan and turpentine. J Path 104: 15–29.
- EBPS (Encyclopaedia Britannica Premium Service) (2006): Encyclopædia Britannica: Corchorus. Available at http://www.britannica.com/eb/article?tocId=9026249 Accessed on March 20, (2006).
- Ghosh PK, Rakshit S (1994): Protein and amino acids in mutated and hybrid plants of white jute (Corchorus capsularis). Indian J Agric Sci 64: 558–561.
- Hasan CM, Islam A, Ahmed M, Ahmed MD, Waterman PG (1984): Capsugenin, a dammarane triterpene from Corchorus capsularis. Phytochemistry 23: 2583–2587.
- Heapy CG, Jamieson A, Russell NJW (1987): Afferent C-fiber and A-delta activity in models of inflammation. Br J Pharmacol 90: 164.
- Hosseinzadeh H, Younesi HM (2002): Antinociceptive and anti-inflammatory effects of Crocus sativus L. stigma and petal extracts in mice. BMC Pharmacol 2: 7–14.
- Hunskaar S, Hole K (1987): The formalin test in mice: Dissociation between inflammatory and non-inflammatory pain. Pain 30: 103–104.
- Malmberg AB, Yaksh TL (1992): Antinociceptive actions of spinal nonsteroidal anti-inflammatory agents on the formalin test in the rat. J Pharmacol Exp Ther 263: 136–146.
- Mosihuzzaman M, Chowdhury TA, Mollah AH, Theander O, Lundgren LN (1986): Phenolic acids in the jute plant (Corchorus capsularis). J Sci Food Agric 37: 955–960.
- Mosihuzzaman M, Quddus A, Nahar N, Theander O (1989): Comparative study of carbohydrates in the two major species of jute (Corchorus capsularis and Corchorus olitorius). J Sci Food Agric 48: 305–310.
- PFAF(2004): Plants for the future: Edible, medicinal and useful plants for a healthier world (Corchorus olitorius L). Available at http://www.pfaf.org/database/plants Accessed on March 20, (2006).
- Pini LA, Vitale G, Ottani A, Sandrini M (1997): Naloxone-reversible antinociception by paracetamol in the rat. J Pharmacol Exp Ther 280: 934–940.
- Quader MA, Gray AI, Waterman PG, Lavaud C, Massiot G, Hasan CM, Ahmed MD (1990): Capsugenin 25,30-O-β-diglucopyranoside: A new glycoside from the leaves of Corchorus capsularis. J Nat Prod 53: 527–530.
- Reanmongkol W, Subhadhirasakul S, Pairat C, Poungsawai C, Choochare W (2002): Analgesic activity of Dyera costulata extract in experimental animals. Songklanakarin J Sci Tech 24: 227–234.
- Süleyman H, Demircan B, Karagöz Y, öztas¸an N, Süleyman B(2004): Anti-inflammatory effects of selective COX-2 inhibitors. Pol J Pharmacol 56: 775–780.
- Tjølsen A, Berge OG, Hunskaar S, Rosland JH, Hole K (1992): The formalin test: An evaluation of the method. Pain 51: 5–17.
- Tripathi KD(2001): Essentials of Medical Pharmacology, fourth edition. New Delhi, Jaypee Brothers Medical Publishers, pp. 52–53.
- Uzcátegui B, ávila D, Suárez-Roca H, Quintero L, Ortega J, González B (2004): Anti-inflammatory, antinociceptive, and antipyretic effects of Lantana trifolia Linnaeus in experimental animals. Invest Clín 45: 317–322.
- Vignon MR, Gey C(1998): Isolation, 1H and 13C NMR studies of (4-O-methyl-d-glucurono)-d-xylans from luffa fruit fibres, jute bast fibres and mucilage of quince tree seeds. Carbohydrate Res 307: 107–111.
- Vineger R, Schreiber W, Hugo R (1969): Biphasic development of carrageenan edema in rats. J Pharmacol Exp Ther 166: 96–103.
- Vogel HG, Vogel WH (1997): Drug Discovery and Evaluation: Pharmacological Assays. Lewisville, J.A.Majors Co., pp. 360–418.
- Wilson SG, Bryant CD, Lariviere WR, Olsen MS, Giles BE, Chesler EJ, Mogil JS (2003): The heritability of antinociception II: Pharmacogenetic mediation of three over-the-counter analgesics in mice. J Pharmacol Exp Ther 305: 755–764.
- Winter CA, Risley EA, Nuss GW (1962): Carrageenan-induced edema in hind paw of the rat as an assay for anti-inflammatory drugs. Proc Soc Exp Biol Med 111: 544–547.
- Yoshikawa M, Murakami T, Ikebata A, Wakao S, Murakami N, Matsuda H, Yamahara J (1997): Bioactive saponins and glycosides. X. On the constituents of zizyphi spinosi semen, the seeds of Zizyphus jujuba Mill. var. spinosa Hu (1): Structures and histamine release-inhibitory effect of jujubosides A1 and C and acetyljujuboside B. Chem Pharm Bull (Tokyo) 45: 1186–1192.
- Zakaria ZA, Safarul M, Valsala R, Sulaiman MR, Fatimah CA, Mat Jais AM (2005): Influence of temperature on the opioid-mediated antinociceptive activity of Corchorus olitorius L. in mice. Naunyn-Schmiedeberg’s Arch Pharmacol 372: 55–62.
- Zakaria ZA, Raden Mohd. Nor RNS, Abdul Ghani ZDF, Hanan Kumar G, Sulaiman MR, Fatimah CA (2006a): Antinociceptive and anti-inflammatory properties of Melastoma malabathricum leaves chloroform extract in experimental animals. J Pharmacol Toxicol 1: 337–345.
- Zakaria ZA, Reezal I, Mat Jais AM, Marmin AHI, Sidek H, Husin SH, Rahim MHA, Sabtu L, Somchit MN, Sulaiman MR (2006)b. The anti-inflammatory, antipyretic and wound healing activities of Cocos nucifera (MATAG Types) fresh juice and kernel extracts in experimental animals. J Pharmacol Toxicol 1: 516–526.
- Zimmermann M (1983): Ethical guidelines for investigations of experimental pain in conscious animals. Pain 16: 109–110.