Abstract
Objectives. This study describes growth, local and remote aortic events, and survival in patients with proximal (root, ascending) aortic diameters just below threshold for operation. Methods. Patients with proximal aortic diameter of 4.5 to 5.4 cm at baseline, were followed with serial computed tomography studies and data collected retrospectively. Aortic growth rate was estimated using mixed effects modelling. Clinical and radiological features associated with outcomes (all-cause death, aortic death, local or remote aortic events (dissection, rupture, intramural hematoma, or intervention)) were assessed with Cox analysis. Survival and freedom from events were estimated using Kaplan-Meier methods. Results. 80 patients underwent 274 CT scans during 265 patient-years. Median proximal aortic growth was 0.2 cm in 3 years. 32 events occurred in 28 patients (35%). Eleven events were local, all elective proximal aortic surgery. Nine events were remote: 5 type B aortic dissections, 3 descending aneurysms undergoing elective repair, and one infrarenal aortic rupture. Twelve patients died, half of type B aortic dissection. Relative survival compared to a matched normal population was 82% (95% confidence limits 55–98%) at 10 years. In Cox analysis, increased descending aortic diameter was an independent predictor of all-cause death (hazard ratio [HR], 1.39) and aortic death (HR 1.96). Conclusions. Descending, but not proximal, aortic growth was predictive of lethal events. The decreased relative survival, the substantial number of remote aortic events and aortic deaths strongly suggest continuous serial CT surveillance of the entire aorta. Other indicators than proximal aortic diameter appear needed to improve management of this patient group.
Introduction
From a surgical standpoint, thoracic aortic disease is mainly managed based on a single variable, the maximum aortic diameter. Previous studies on the natural history of aortic dilatation imply that the risk of aortic dissection (AD) or rupture increases markedly at a diameter >6 cm in the proximal (root or ascending) part of the aorta [Citation1,Citation2]. In effect, guidelines recommend that prophylactic surgical repair of proximal thoracic aortic aneurysm in patients without specific risk features (connective tissue disease, family history of AD, bicuspid aortic valve, rapid aneurysm growth, or symptomatic aneurysm) is considered at a diameter of 5.5 cm [Citation3–5].
However, studies have implied that aortic diameter alone is a poor predictor for AD and insufficient as the sole indicator for preventive surgery. Indeed, pre-dissection aortic diameter <5.5 cm have been reported in 59–96%, with an average pre-dissection diameters at 3.7–5.3 cm [Citation6–9]. Evidence supporting the management of the moderately (4.5–5.4 cm) dilated proximal aorta remain scarce, and the prognosis in this group ill-defined. Reported outcomes are divergent concerning aortic growth rate, aortic events, and use of prophylactic surgery in this group [Citation10–12].
The present study was specifically aimed to evaluate this group of patients with a proximal aortic dilatation just below indication for intervention (regardless of aortic diameters in other segments at the time of inclusion). The purpose was to outline (1) the growth of both the proximal and distal aorta by computed tomography (CT) measurements; (2) the occurrence of aortic events, i.e. death (aortic or non-aortic), AD, rupture, intramural hematoma or intervention (surgical or endovascular) in both the proximal and distal aorta and (3) the prognosis, expressed as relative (observed versus expected) survival.
Knowledge of the outcomes in this patient group is necessary to inform management, ultimately selecting patients to continued surveillance or elective intervention.
Patients and methods
Ethical statement
The study was approved by the regional research ethics committee (No 2008/1771-31). Written informed consent was obtained from all study subjects.
Study population
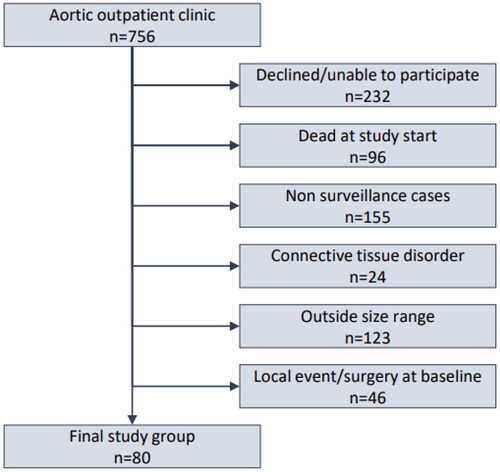
Study inclusion and exclusion criteria were designed to isolate the group of patients defined by a proximal (aortic root, ascending aorta) diameter close to, but not qualifying for prophylactic intervention in the proximal aorta, and therefore reproducing this common clinical scenario. The basis for the present study consisted of all patients referred to a University Hospital Thoracic Aortic outpatient clinic between 1992 and 2011. Patients unwilling or unable to give study consent, including those that were dead at the start of the data collection, were excluded. Patients with connective tissue disorder and those without indication for continued surveillance or otherwise lacking at least one follow-up CT examination were excluded. Patients with baseline proximal (root or ascending) aortic diameter outside the specified size range were excluded as well those that were previously operated on the proximal aorta or had a proximal aortic event at baseline. Hence, the resulting study group included only unoperated patients with baseline proximal aortic diameter 4.5–5.4 cm without other established primary indication for operation on the proximal aorta and subject to longitudinal CT follow-up (). Clinical and radiological data were retrospectively collected from medical records and complete imaging studies. Radiological follow-up ended 2011, vital status and cause of death were recorded in 2016, with 100% coverage.
Clinical variables
Chronic obstructive pulmonary disease, diabetes, and hypertension were defined as requiring medical therapy. Smoking status was subdivided into current, former or never. Status of all variables was determined at inclusion, i.e. at the time of baseline CT.
Computed tomography variables and follow-up
All CT scans were studied according to a pre-specified protocol by an independent radiologist blinded to clinical variables and outcome data on the individual level. The aorta was examined at defined segments: the root (mid-sinus of Valsalva), the sinotubular junction, the ascending aorta (between the sinotubular junction and the brachiocephalic trunk take-off), the arch (from the origin of the brachiocephalic trunk to and including the left subclavian take-off), the isthmus (from distal to the subclavian artery and including the proximal descending aorta in the part corresponding to the ligamentum arteriosum), the descending aorta (until the take-off of the celiac trunk), the suprarenal aorta (from the celiac trunk to the main renal arterial take-off) and the infrarenal aorta (until the aortic bifurcation). Aortic size was measured as outer diameter (cm) in the axial plane perpendicular to flow in each segment, and the largest diameter was recorded and used as benchmark for serial comparison. For each segment, the presence of wall-lining thrombus (graded as 0, none; 1, limited (≤20%); 2, moderate (21–49%); 3, severe (≥50%)) and calcified plaques (graded as 0, none; 1, limited (single); 2, moderate (multiple); 3, severe (confluent)), respectively, were recorded. Generally, CT scans were performed annually or by an individualized plan and discontinued either by an event, if the aortic size was judged stable over time, if the patient was judged ineligible for surgical intervention, or by patient preference.
Outcome measures
The outcome measures were aortic growth (diameter increase in mm in largest aortic segment), all-cause mortality, aortic mortality, relative survival, and local and remote aortic events. Local events involved the aortic root or ascending aorta. Remote events involved the aortic arch, descending, thoracoabdominal or infrarenal aorta. Events were defined as new (compared to baseline) aortic dissection, rupture, intramural hematoma or intervention (open surgical repair or endovascular repair). Several events could occur in a single individual, as well as a combination of event(s) and mortality. Interventions are not themselves part of a natural history of aortic disease, but were considered events from a clinical perspective, relevant to the patient.
Statistical analyses
Variables are reported as counts with percentages or medians with ranges. Aortic growth was evaluated using a mixed effects linear regression model. Uni- and multivariable analysis was performed using Cox regression with time-to-event data for the specified outcomes. Cox models included variables with a p-level <.10 in univariate analysis. Survival and event rates were estimated using Kaplan-Meier methods. Relative survival (RS) was calculated as a measure of cause-specific mortality. RS is the ratio between estimated observed mortality and expected mortality, in a normal population matched for age, sex, and study period, using data from the Human Mortality Database (www.mortality.org). RS was calculated using the Ederer II estimator of the Stata strs command [Citation13]. Stata v 16 (Stata Corp., College Station, Texas) was used for data management and statistical analyses, performed by a biostatistician.
Results
From a total of 756 referred patients, 80 remained and formed the study group after application of the inclusion and exclusion criteria (). Baseline characteristics and comorbid conditions are summarized in . Median age was 62 years (range 34–80), the majority were men, and the prevalence of hypertension was high (82.5%). In total, 274 CT scans were performed. Total follow-up time was 265 patient-years with a median of 3 years (range 0.2–11). Median baseline diameter of the largest proximal aortic segment (root, sinotubular junction, or ascending aorta) was 4.6 cm and last median diameter of the same segment was 4.8 cm, for a median growth of 0.2 cm in 3 years (). In a mixed effects linear regression model, annual slope (mm/year) was 0.082 and almost doubled (0.13 vs. 0.077) in individuals with baseline aortic diameter ≥5 vs. <5 cm (Supplement Table 1A). For the descending aorta, the pattern was similar but more pronounced, with annual slope of almost 1 mm for those with an index descending diameter ≥5 cm (Supplement Table 1B).
Figure 2. Diagram (“spaghetti plot”) of proximal aortic diameter (y-axis) development over time (x-axis) in each patient (individual measure points not shown). Patients dying during the study period highlighted in red. Note logarithmic scale on x-axis.
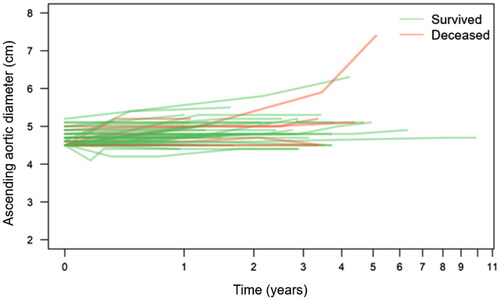
Table 1. Clinical characteristics of 80 patients with moderately (45–54 mm) proximal thoracic aorta.
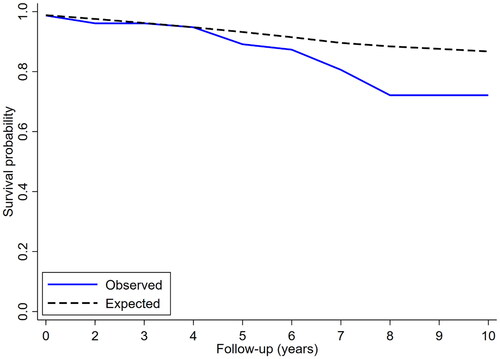
Of the 80 patients, 52 (65%) remained event-free for their entire study period. In all, 32 events occurred in 28 patients (35%): 12 deaths, 11 local (proximal) aortic events and 9 remote (distal) aortic events, respectively. The estimated (Kaplan-Meier) overall survival at five years was 80% (95% confidence limits, 67–96%), (). The estimated freedom from aortic-related death at five years was 92% (81–100%), (). The estimated freedom from local () and remote () events at five years was 68% (55–86%) and 86% (76–97%), respectively. Relative survival, expressed as the percentage of expected survival in an age-, sex-, and time-period matched normal population, was comparable at 96% (85–101%) at five years but significantly reduced to 82% (55–98%) at ten years ().
Figure 3. Kaplan-Meier survival curves (with 95% confidence interval) for (panel A) freedom from all-cause death; (panel B) freedom from aortic death; (panel C) freedom from local (proximal aorta) event; (panel D) freedom from remote (distal aorta) event. Of local events, n = 11 (100%) were elective surgical repair. Of distal events, n = 6 (66%) were acute type B dissections.
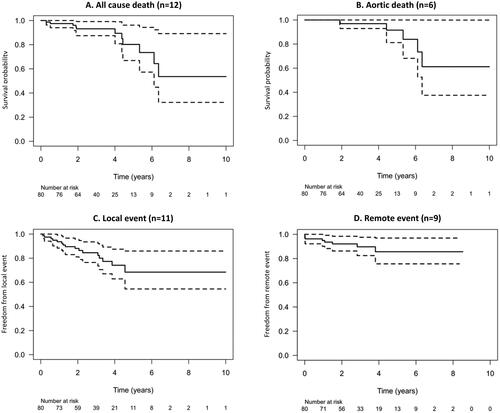
Figure 4. Relative survival (observed versus expected) for patients with proximal aortic dilatation (solid line) versus age-, sex- and time period-matched normal population (cross-hatched line). At ten years of follow-up, relative survival was 82%.
Half (6/12) of the patients who died during the study period suffered a fatal aortic event, in all cases an acute type B aortic dissection. Only one of twelve patients displayed proximal aortic growth beyond the cut-off for operation, but declined surgery and succumbed from dissection of an even more enlarged descending aorta. In all others, the proximal aorta remained relatively stable and at below-indication diameter. In contrast, for those suffering lethal type B aortic dissection, a pattern of descending aortic growth was observed (). All of the local events consisted of elective surgical repair, none of the local events consisted of AD, rupture or intramural hematoma. Surgical repair was prompted either by progression of an associated aortic valve pathology or after re-evaluation of clinical and radiological findings. In no case, a significant growth of the proximal aorta was observed pre-operatively (). Surgical (30-day) mortality was zero and there were no late aortic events in these patients. Of the 9 remote events, most (6/9) were acute, type B aortic dissection or rupture, while three corresponded to development of descending aortic dilatation with indication for surgical or endovascular repair. Again, in the proximal aortic segment, no significant growth was observed in the pre-event phase. Notably, patients with a confirmed growth in the distal aorta underwent elective repair, while those suffering an acute event did not display a significant growth before the event (). Of note, median proximal aortic diameter at baseline was 5.1 cm and approaching the indication threshold in those with a local event (i.e. proximal aortic repair) while it was 4.7 cm in the mortality group and 4.6 cm in the remote event group, i.e. towards the lower end of the study inclusion limit.
Table 2. Deaths (n = 12) in 80 patients with moderately (45–54 mm) dilated proximal thoracic aorta, including aortic diameters at baseline and pre-event for both proximal and descending (i.e. affected) aortic segments.
Table 3. Local events: prophylactic surgical procedures (n = 11) on the proximal thoracic aorta.
Table 4. Remote events (n = 9): type of primary event, associated procedure (if any), and diameters of proximal and affected aortic segments at baseline and pre-event.
Increasing aortic descending diameter was the sole independent predictor in Cox multivariable regression analysis for the outcomes all-cause death (HR 1.39) and aortic death (HR 1.96), as detailed in Supplement Tables 2-3. No independent predictors were identified for local or remote events, respectively (Supplement Tables 4-5).
Discussion
In this study of 80 patients – carefully selected eligible surgical candidates under periodic surveillance at a dedicated aortic out-patient clinic – no acute aortic events (AD, rupture, intramural hematoma) occurred in the moderately (4.5–5.4) dilated proximal aorta during up to 11 years (median 3 years) clinical and radiological follow-up. However, only 65% of patients remained free from any kind of event during the study period: 12 patients died, half of whom suffered an aortic death, 9 events occurred in the distal aorta, and 11 patients underwent elective proximal aortic surgical repair. For the descending aorta, TBAD in general occurred essentially without preceding diameter increase () but lethal TBAD or rupture was more often heralded by significant growth (), making descending aortic growth an independent predictor of all-cause and aortic death (Supplemental tables 2-3) but not other aortic events (Supplemental tables 4-5). The overall relative survival (i.e. in comparison to an age-, sex- and date-matched normal population) was significantly reduced, suggesting optimization in the management in this group was warranted.
The observed crude median growth-rate in this study was a modest 0.2 cm in 3 years. On the other hand, expected growth rate in the normal aorta is considerably lower at 0.15–0.17 cm in 10 years [Citation14,Citation15]. Previous studies also found limited growth rates in similar patient groups: 0.42 ± 0.82 mm/year in 251 patients with proximal aortic diameter 40–50 mm (median 46 mm) at baseline [Citation16] and 0.3 mm/year in 110 patients with proximal aortic diameter <5 cm (median 45 mm) [Citation17]. In the present study, both ascending and descending aortic growth rate was clearly higher in individuals with baseline diameter ≥5 cm. Importantly, increased descending aortic diameter conferred an increased hazard ratio for all-cause as well as aortic death.
In a recently published study with 968 unoperated patients with ascending thoracic aortic aneurysm reported that the yearly risk of adverse aortic events (dissection, rupture and aortic death) was relatively flat (<0.3%) until 5.0 cm of aortic size, thereafter with a rapid increase in risk. With the conclusion that an aortic size of 5.0 cm may be the intervention criterion for prophylactic surgery [Citation18]. This is conditionally implemented in the new guidelines for the diagnosis and management of aortic disease from the American College of Cardiology Joint Committee and the American Heart Association published in 2022 [Citation19]: ≥5 cm by experienced surgeons in a multidisciplinary aortic team. The findings of this study could support such an adaption since operative mortality was zero, but on the other hand no other spontaneous aortic events in the proximal aorta occurred even at the studied baseline diameters of up to 5.4 cm. Whether this approach prevented any event in the downstream aorta remains speculative.
A substantial number of patients (35%) experienced an event in this study (26% if excluding elective surgical repairs); 7.5% suffered death due to acute type B aortic dissection. In contrast, Gagnér-Loranger et al. reported 99.5% five-year freedom from aortic event (excluding elective surgical repair) and 97.6% survival in their study of similar patients [Citation16]. Geisbüsch et al. reported no aortic events (3 elective surgical repairs) in 232 patients with “small-to-moderate” (average 4.4 cm diameter) ascending aneurysms followed for up to 4.3 years [Citation20] and Kim et al. in a large (n = 4654) patient sample followed by echocardiography, found only 14 cases of AD/rupture during 14431 patient-years follow-up [Citation21]. Remote aortic segments (arch and downstream) were either not reported [Citation17,Citation21] or did not display any events (dissection, rupture or death) during follow-up [Citation16,Citation17,Citation20].
Relative survival decreased gradually and was significantly reduced at 10-year follow-up. An ultimate goal of any management strategy for the dilated proximal aorta – surveillance or pre-emptive surgery – is the improvement of prognosis towards normalization of survival. This goal was not met; despite liberal use of prophylactic operations in 11/80 patients, another 15/69 suffered remote aortic events or aortic death, and hence the relative survival was significantly reduced.
Apart from increasing descending aortic diameter, no other clinical or radiological predictors were identified for any other adverse outcome studied (death of any cause, aortic death, local or remote event, all events combined). Apparently, in this group, improved prediction based on other clinical, radiological, or other (biochemical, genetic, functional) variables would be of the utmost importance and hopefully serve the purpose of proceeding to surgical repair in the properly selected patients.
The present study was based on radiological (CT) examinations of the entire aorta. Consequently, relatively few clinical variables were recorded, and echocardiographic findings were not systematically collected. Therefore, important cardiovascular features remain incompletely outlined, including aortic valve cuspidity, except in patients operated for progressive and/or symptomatic aortic valve dysfunction. Bicuspid aortic valve (BAV) is associated with dilation of the proximal aorta [Citation22,Citation23]. However, when followed prospectively BAV patients very rarely suffer acute aortic events [Citation22,Citation23]. In this study, all aortic events other than prophylactic operations, occurred in the remote (distal) aorta. This part has been reported as normal in BAV patients [Citation24,Citation25]. Studies of mixed populations (bicuspid and tricuspid valves) have indicated negligible differences in the aspects of aortic events and mortality, making this deficit less concerning [Citation10,Citation21]. We speculate that in the present patient cohort, the distribution of events suggests that very few, if any, were related to BAV.
Study limitations
The present study carries the drawbacks common to all limited single-center retrospective studies. In effect, no causal relationships could be established, per study design, and findings need to be interpreted cautiously. Part of the present patient cohort was recruited in an earlier era, which might modify the findings. There was a paucity of clinical variables, but the study group consisted of patients actively followed-up, as surgical candidates, should the indication arise. Therefore, severe forms of cerebrovascular and peripheral arterial disease were very uncommon, as was severe renal or heart failure.
Conclusion
This study following patients with proximal aortic diameter just below threshold for surgical repair found worse outcomes than previous studies [Citation11,Citation16,Citation20,Citation21]: more elective proximal aortic repairs, more remote aortic events (type B aortic dissection or repair), more aortic-related deaths and worse overall survival compared to a matched normal population. Accordingly, proximal aortic diameter, growth, or common radiological findings all appeared insufficient to properly identify patients at risk for adverse events. Even in the context of a “borderline” dilated ascending aorta at baseline, the behaviour of the descending aorta was the driver of adverse outcomes, both acute events (TBAD) and death. Long-lasting surveillance of the entire aorta, with a commitment to handle progression in a timely manner, appears strongly recommended but will not in itself identify all patients experiencing adverse aortic events Ideally, novel and stronger predictors should be developed and incorporated in the management.
Supplemental Material
Download MS Word (26.4 KB)Disclosure statement
The authors report there are no competing interests to declare.
Data availability statement
The data underlying this article will be shared on a reasonable request to the corresponding author.
Additional information
Funding
References
- Coady MA, Rizzo JA, Hammond GL, et al. What is the appropriate size criterion for resection of thoracic aortic aneurysms? J Thorac Cardiovasc Surg. 1997;113(3):476–491. doi: 10.1016/S0022-5223(97)70360-X.
- Elefteriades JA. Natural history of thoracic aortic aneurysms: indications for surgery, and surgical versus nonsurgical risks. Ann Thorac Surg. 2002;4(5):S1877–S1880. doi: 10.1016/s0003-4975(02)04147-4.
- Erbel R, Aboyans V, Boileau C, et al. 2014 ESC guidelines on the diagnosis and treatment of aortic diseases: document covering acute and chronic aortic diseases of the thoracic and abdominal aorta of the adult. The task force for the diagnosis and treatment of aortic diseases of the european society of cardiology (ESC). Eur Heart J. 2014;35(41):2873–2926.
- Hiratzka LF, Bakris GL, Beckman JA, et al. 2010 ACCF/AHA/AATS/ACR/ASA/SCA/SCAI/SIR/STS/SVM guidelines for the diagnosis and management of patients with thoracic aortic disease. J Am Coll Cardiol. 2010;55(14):e27–e129. doi: 10.1016/j.jacc.2010.02.015.
- Boodhwani M, Andelfinger G, Leipsic J, et al. Canadian cardiovascular society position statement on the management of thoracic aortic disease. Can J Cardiol. 2014;30(6):577–589. doi: 10.1016/j.cjca.2014.02.018.
- Parish LM, Gorman JH, 3rd, Kahn S, et al. Aortic size in acute type a dissection: implications for preventive ascending aortic replacement. Eur J Cardiothorac Surg. 2009;35(6):941–946. doi: 10.1016/j.ejcts.2008.12.047.
- Pape LA, Tsai TT, Isselbacher EM, et al. Aortic diameter > or = 5.5 cm is not a good predictor of type a aortic dissection: observations from the international registry of acute aortic dissection (IRAD). Circulation. 2007;116(10):1120–1127. doi: 10.1161/CIRCULATIONAHA.107.702720.
- Rylski B, Branchetti E, Bavaria JE, et al. Modeling of predissection aortic size in acute type a dissection: more than 90% fail to meet the guidelines for elective ascending replacement. J Thorac Cardiovasc Surg. 2014;148(3):944–948.e1. doi: 10.1016/j.jtcvs.2014.05.050.
- Heuts S, Adriaans BP, Rylski B, et al. Evaluating the diagnostic accuracy of maximal aortic diameter, length and volume for prediction of aortic dissection. Heart. 2020;106(12):892–897. doi: 10.1136/heartjnl-2019-316251.
- Guo MH, Appoo JJ, Saczkowski R, et al. Association of mortality and acute aortic events with ascending aortic aneurysm: a systematic review and meta-analysis. JAMA Netw Open. 2018;1(4):e181281. doi: 10.1001/jamanetworkopen.2018.1281.
- Solomon MD, Leong T, Sung SH, et al. Association of thoracic aortic aneurysm size with long-term patient outcomes: the KP-TAA study. JAMA Cardiol. 2022;7(11):1160–1169. doi: 10.1001/jamacardio.2022.3305.
- Oladokun D, Patterson BO, Sobocinski J, et al. Systematic review of the growth rates and influencing factors in thoracic aortic aneurysms. Eur J Vasc Endovasc Surg. 2016;51(5):674–681. doi: 10.1016/j.ejvs.2016.01.017.
- Dickman PW, Coviello E. Estimating and modeling relative survival. The Stata J. 2015;15(1):186–215. doi: 10.1177/1536867X1501500112.
- Kälsch H, Lehmann N, Möhlenkamp S, et al. Body-surface adjusted aortic reference diameters for improved identification of patients with thoracic aortic aneurysms: results from the population-based Heinz Nixdorf Recall study. Int J Cardiol. 2013;163(1):72–78. doi: 10.1016/j.ijcard.2011.05.039.
- Chang HW, Kim SH, Hakim AR, et al. Diameter and growth rate of the thoracic aorta-analysis based on serial computed tomography scans. J Thorac Dis. 2020;12(8):4002–4013. doi: 10.21037/jtd-20-1275.
- Gagné-Loranger M, Dumont É, Voisine P, et al. Natural history of 40-50 mm root/ascending aortic aneurysms in the current era of dedicated thoracic aortic clinics. Eur J Cardiothorac Surg. 2016;50(3):562–566. doi: 10.1093/ejcts/ezw123.
- McLarty AJ, Bishawi M, Yelika SB, et al. Surveillance of moderate-size aneurysms of the thoracic aorta. J Cardiothorac Surg. 2015;10(1):17. doi: 10.1186/s13019-015-0220-2.
- Wu J, Zafar MA, Liu Y, et al. Fate of the unoperated ascending thoracic aortic aneurysm: three-decade experience from the Aortic Institute at Yale University. Eur Heart J. 2023;44(43):4579–4588.
- Isselbacher EM, Preventza O, Hamilton Black J, 3rd, et al. 2022 ACC/AHA guideline for the diagnosis and management of aortic disease: a report of the American heart association/American college of cardiology joint committee on clinical practice guidelines. Circulation. 2022;146(24):e334–e482. doi: 10.1161/CIR.0000000000001106.
- Geisbüsch S, Stefanovic A, Schray D, et al. A prospective study of growth and rupture risk of small-to-moderate size ascending aortic aneurysms. J Thorac Cardiovasc Surg. 2014;147(1):68–74. doi: 10.1016/j.jtcvs.2013.06.030.
- Kim JB, Spotnitz M, Lindsay ME, et al. Risk of aortic dissection in the moderately dilated ascending Aorta. J Am Coll Cardiol. 2016;68(11):1209–1219. doi: 10.1016/j.jacc.2016.06.025.
- Michelena HI, Desjardins VA, Avierinos JF, et al. Natural history of asymptomatic patients with normally functioning or minimally dysfunctional bicuspid aortic valve in the community. Circulation. 2008;117(21):2776–2784. doi: 10.1161/CIRCULATIONAHA.107.740878.
- La Canna G, Ficarra E, Tsagalau E, et al. Progression rate of ascending aortic dilation in patients with normally functioning bicuspid and tricuspid aortic valves. Am J Cardiol. 2006;98(2):249–253. doi: 10.1016/j.amjcard.2006.01.096.
- Cecconi M, Manfrin M, Moraca A, et al. Aortic dimensions in patients with bicuspid aortic valve without significant valve dysfunction. Am J Cardiol. 2005;95(2):292–294. doi: 10.1016/j.amjcard.2004.08.098.
- Jackson V, Olsson C, Eriksson P, et al. Aortic dimensions in patients with bicuspid and tricuspid aortic valves. J Thorac Cardiovasc Surg. 2013;146(3):605–610. doi: 10.1016/j.jtcvs.2012.07.039.