Abstract
Objectives. To describe current on- (isolated coronary arterty bypass grafting, iCABG) and off-label (non-iCABG) use of aprotinin and associated safety endpoints in adult patients undergoing high-risk cardiac surgery in Nordic countries. Design. Data come from 10 cardiac surgery centres in Finland, Norway and Sweden participating in the European Nordic aprotinin patient registry (NAPaR). Results. 486 patients were given aprotinin between 2016 and 2020. 59 patients (12.1%) underwent iCABG and 427 (87.9%) non-iCABG, including surgery for aortic dissection (16.7%) and endocarditis (36.0%). 89.9% were administered a full aprotinin dosage and 37.0% were re-sternotomies. Dual antiplatelet treatment affected 72.9% of iCABG and 7.0% of non-iCABG patients. 0.6% of patients had anaphylactic reactions associated with aprotinin. 6.4% (95 CI% 4.2%–8.6%) of patients were reoperated for bleeding. Rate of postoperative thromboembolic events, day 1 rise in creatinine >44μmol/L and new dialysis for any reason was 4.7% (95%CI 2.8%–6.6%), 16.7% (95%CI 13.4%–20.0%) and 14.0% (95%CI 10.9%–17.1%), respectively. In-hospital mortality and 30-day mortality was 4.9% (95%CI 2.8%–6.9%) and 6.3% (95%CI 3.7%–7.8%) in all patients versus mean EuroSCORE II 11.4% (95%CI 8.4%–14.0%, p < .01). 30-day mortality in patients undergoing surgery for aortic dissection and endocarditis was 6.2% (95%CI 0.9%–11.4%) and 6.3% (95%CI 2.7%–9.9%) versus mean EuroSCORE II 13.2% (95%CI 6.1%–21.0%, p = .11) and 14.5% (95%CI 12.1%–16.8%, p = .01), respectively. Conclusions. NAPaR data from Nordic countries suggest a favourable safety profile of aprotinin in adult cardiac surgery.
Introduction
According to the European Medicines Agency (EMA) the registered indication for aprotinin is prophylactic use to reduce blood loss and blood product transfusions in patients undergoing isolated coronary artery bypass graft surgery (iCABG), who are at high risk of major blood loss [Citation1,Citation2].
Aprotinin, which is a naturally appearing polypeptide, inhibits several proteolytic enzymes including plasmin, trypsin, and kallikrein. In contrast, the lysine analogues epsilon aminocaproic acid (EACA) and tranexamic acid (TXA) impede the alteration of plasminogen to plasmin. Aprotinin gained clinical interest when Royston and co-workers in 1987 first reported much less perioperative bleeding with aprotinin, and an almost 90% reduction in transfusion rate after repeat open-heart surgery [Citation3]. However, as several observational studies and one randomised clinical trial (BART) about 15 years ago hinted that use of aprotinin increased mortality [Citation4–7] as well as renal impairment [Citation4,Citation6] in cardiac surgery, the Marketing Authorisation Holder (MAH – Bayer) removed the drug from the market in 2007 [Citation8], followed by the European Medicines Agency (EMA) decision to repeal its European licence in 2008 [Citation9]. Nevertheless, after a re-examination in 2010 of the data that led to aprotinin’s withdrawal, the EMA recommended lifting its suspension in 2012. In 2013, the Committee for Medicinal Products for Human Use (CHMP) “concluded that evidence from randomised clinical trials and observational studies support the use of aprotinin in reducing the incidence of massive bleeding, the need for transfusion of blood products and the need for re-surgery for bleeding;” [Citation10]. CHMP also “concluded that the BART data and the signal on increased mortality associated with aprotinin compared to EACA and TXA were not considered reliable, based on the totality of evidence now available since the review of aprotinin undertaken in 2007, including more recent observational studies, the new analysis of the BART study data and they identified major study flaws, and taking the advice of the scientific advisory board (SAG) into account.” [Citation10]. Therefore, the CHMP determined that “the balance of risks and benefits for aprotinin is positive under normal conditions of use, subject to the revision of the indication as described” [Citation10] above. The background for the decision has also been discussed elsewhere [Citation11–13]. Finally, from February 2016, aprotinin was again introduced in Europe, although the EMA required the new MAH (Nordic Pharma) to implement a patient registry to monitor its use in real-life [Citation10].
A first report of the European Nordic aprotinin patient registry (NAPaR) outcome data was published recently to describe current on- and off-label use of aprotinin and associated safety endpoints, including in-hospital mortality, in over 5000 adult patients undergoing cardiac surgery from 83 sites in 9 European countries [Citation14]. The analysis showed that the incidence of adverse events subsequent the administration of aprotinin in patients at high risk of death or blood loss undergoing cardiac surgery, including complex cardiac surgeries other than iCABG, is comparable to data from recent literature where aprotinin was not used. However, in comparison with other European countries the participating Nordic countries (Sweden, Finland, and Norway) had a much high entry rate of non-compulsory data, including 30-day mortality and EuroSCORE II data, and mostly used the high dose regimen (also called full-Hammersmith).
Thus, the primary aim of this study was to compare 30-day mortality data with an estimation of 30-day mortality via EuroSCORE II. Secondary aims were to compare a selection of patients, aprotinin regimen and outcome data between NAPaR centres in Nordic and other European countries regarding other safety outcome parameters.
Material and methods
Data were analysed from 10 cardiac surgery centres in Finland, Norway and Sweden that were participating in the NAPaR, which is a non-interventional, post-authorization safety study (PASS) executed at EMA’s request. Data from the Nordic countries were compared with other European countries participating in the NAPaR registry, which was required for any European centre that wanted to use aprotinin in cardiac surgery.
The Pharmacovigilance Risk Assessment Committee (PRAC) of the EMA specifically asked for data on three aspects of adherence to the Summary of Product Characteristics (SmPC) regarding test dose, anaphylactic reactions, and activated coagulation time (ACT) during cardiopulmonary bypass (CPB) [Citation14]. Adult patients who agreed to take part in the study including entry of their anonymised data were included in the registry if they had received aprotinin during any type of cardiac surgery using CPB at the involved centres. The treating doctors determined if patients were to be given aprotinin based on their clinical judgement. The study started when commercial aprotinin became available at each centre, from February 2016 and the data lock point of the registry was November 2, 2020. Patients were treated according to the routine clinical practice of each centre. Aprotinin was given according to the guidelines of the SmPC [Citation1].
Ethics
The study was performed in agreement with the Independent Ethics Committees (IECs)/Institutional Review Boards (IRBs), the Declaration of Helsinki, and the Good Epidemiology and Good Pharmacoepidemiology Practices (GEP/GPP) guidelines. As the NAPaR registry was non-interventional, EMA did not find it necessary to declare it to the relevant IEC/IRB. Despite this, ethical approval of the study was given by relevant authorities for each participating hospital in the Nordic countries. The study code is NG-APRO-PASS-01.3 with the Swedish Ethical Committee Board Number 2015/1976-31.4. Each patient asked to participate was well informed about aprotinin before possible agreement to participate in the study and after signing a written agreement, patients could still refuse data collection to the registry. The study protocol is available on the European Network of Centres for Pharmacoepidemiology and Pharmacovigilance website (www.ENCePP.com, EU PAS Register number: EUPAS11384).
Statistical analysis
A power calculation was not performed as the NAPaR registry was primarily a descriptive study without a control group. The analysis corresponded to the procedure and their matching mean European System for Cardiac Operative Risk Evaluation (EurosSCORE II): iCABG versus non-iCABG, surgery in patients with active endocarditis, and surgery for aortic dissection.
Categorical variables are presented as numbers and percentages and quantitative variables are presented as mean, and standard deviation (SD) when normally distributed otherwise as median and interquartile range (IQR). Comparisons were made with Student-t, Chi2 or Fisher’s exact test when appropriate, and we computed the 95% confidence interval (CI) with the Wald test. Statistical significance was set by p < .05 (two-sided). Statistical analyses were performed with SPSS version 24 for Windows (IBM SPSS Statistics, NY, US).
Outcomes
The European System for Cardiac Operative Risk Evaluation (EuroSCORE) II was finalised before each procedure to estimate the risk of postoperative death [Citation15]. Aprotinin safety was assessed by in-hospital and 30-day mortality, as well as myocardial infarction, stroke, or other reported thromboembolic events (TEEs); renal impairment based on acute kidney injury (AKI) defined as pre- to postoperative rise in plasma creatinine level >44 µmol/l (0.5 mg/dL) or new renal replacement therapy (dialysis); any hypersensitivity or allergic response to aprotinin; and re-exploration for bleeding/tamponade. Change in plasma creatinine was used because it enabled comparisons with prior publications showing renal injury [Citation4].
Data collection
NAPaR data were collected and registered into an electronic web-based record form accessible worldwide. NAPaR followed the template of the reports of the European Association for Cardio-Thoracic Surgery [Citation16]. The design of the registry and comparisons to published data have been described in detail before [Citation14].
Role of the funding source
The study sponsor (Nordic Group B.V.) did not have any impact or influence on the selection of surgery centres that wished to participate, on the inclusion of patients, on the analysis of data and on the results and conclusions reported in this article.
Results
From 2016 to 2020, 486 adult patients undergoing cardiac surgery with cardio-pulmonary bypass were treated with aprotinin in the Nordic countries of Finland, Norway, and Sweden, whereby altogether 10 centres participated in the NAPaR registry. describes the pre-, intra- and postoperative variables of the 486 patients from the Nordic countries and a comparison with the 4823 NAPaR patients from other European countries [Citation14] after subtraction of the patients from the Nordic countries. The mean age of Nordic patients, 59.8 ± 14.6 years, was lower than in other European countries (62.6 ± 14.8, p < .01), with 9.9% of the patients >75 years vs. 19.8% in other European countries (p < .05). Only 12.1% of Nordic patients underwent iCABG and most of these, 72.9%, were on dual antiplatelet treatment (DAPT) compared with 27.1% (p < .01) and 18.9% (p < .01) in other European countries, respectively. On the other hand, the two most frequent procedures in the Nordic countries were surgery for endocarditis (36.0%) and aortic dissection (16.7%), compared with significantly lower incidences, 8.7% (p < .01) and 12.1% (p < .01), respectively, in the other European countries. Also, the incidence of the Full Hammersmith regimen of aprotinin was higher in the Nordic countries (89.9%, p < .01) compared with the other European countries (50.2%, p < .01). Operation urgency differed between the two groups with almost one quarter of procedures being elective (26.3%) in Nordic countries, compared with more than half of surgeries in other European countries (54.6%, p < .01). Incidence for urgent, emergent and salvage procedures were 51.9%, 17.0% and 4.9%, respectively, in Nordic countries compared with 27.3% (p < .01), 16.9% (n.s.), and 1.2% (p < .01), respectively, in other European countries. Prevalence of outcome variables including in hospital mortality, re-exploration for bleeding, anaphylactic reactions, postoperative thromboembolic events, permanent stroke, or myocardial infarction did not differ significantly between the two groups, except for new AKI and new dialysis for any reason, which were significantly higher in the Nordic countries.
Table 1. Pre-, intra- and postoperative variables in adult patients exposed to aprotinin during cardiac surgery with cardiopulmonary bypass.
The transfusion rate of packed red blood cells (PRBC) until 48 h after surgery for all patients in the Nordic countries was 61.1% (326/486 patients) with corresponding median PRBC volume of 500 ml (25th/75th percentile: 0 ml/1005 ml, range: 0–13,859 ml).
In the Nordic countries only 59 patients underwent iCABG with a mean EuroSCORE II of 7.9 ± 10.7, and 8.5% of the iCABG procedures were elective, 63.0% urgent, 28.8% emergent and 1.7% salvage. The in-hospital and 30-day mortality of iCABG were 5.1% (3/59) and 8.5% (5/59), respectively. Seven of the 59 (11.9%) iCABG patients were in cardiogenic shock and 3 of these patients died within the hospital stay. In comparison, the in-hospital mortality of iCABG was 1.1% (14/1259, p < .05) in other European countries [Citation14].
In , the variables of patients in the NAPaR registry not undergoing iCABG, the licenced indication for aprotinin by the European Medical Agency, are compared between the Nordic (N = 427) and other European countries (N = 3519). There were similar significant differences between the two groups as when all patients were compared () except for two outcome variables. The prevalence of in hospital mortality (4.9%) and postoperative thromboembolic events (4.9%) were significantly lower in the Nordic countries compared with 8.7% (p < .01) and 7.5% (p < .05), respectively, in the other European countries.
Table 2. Pre-, intra- and postoperative variables in adult patients exposed to aprotinin during cardiac surgery with cardio-pulmonary bypass other than isolated coronary artery bypass surgery.
Preoperative renal impairment (creatinine clearance <85 mL/min) affected 51.0% of patients in the Nordic countries. The prevalence of AKI and new dialysis for any reason was higher in the Nordic countries than other European countries for all NAPaR patients, 16.7% vs. 11.7% (p < .01) and 14.0% vs. 8.0% (p < .01), respectively, as well as when non-CABG patients were compared 16.9% vs. 5.3% (p < .01) and 15.9% vs. 10.3% (p < .01). In contrast, postoperative use of aminoglycosides was less frequent in the Nordic countries (13.1%) than in other European countries (31.3%, p < .01), whereas pre- and postoperative use of aminoglycosides or/and one preoperative dose of teicoplanin in Nordic countries (47.5%) was higher compared with postoperative aminoglycoside use in other European countries (31.3%, p < .01).
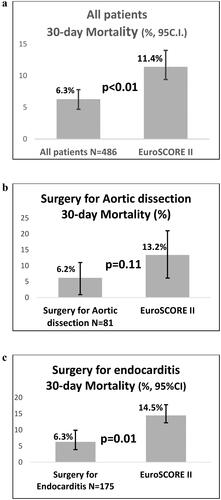
EuroSCORE II and 30-day mortality data were complete for the Nordic countries in contrast with other European countries where approximately half the population had missing data [Citation14]. According to , actual 30-day mortality rates in NAPaR patients from the Nordic countries are depicted for patients undergoing (a) all types of surgery, (b) aortic dissection surgery, and (c) surgery for endocarditis, and were 6.3% (CI 95% 3.7%–7.8%), 6.2% (CI 95% 0.9%–11.4%) and 6.3% (CI 95% 2.7%–9.9%), in comparison with 11.4% (CI 95% 8.4%–14.0%, p < .01), 13.2% (CI 95%: 6.1%–21.0%, p = .11), and 14.5% (CI 95% 12.1%–16.8%, p = .01), respectively, for the corresponding estimated 30-day mortality according to EuroSCORE II.
Discussion
The overall purpose of this study was to evaluate whether the comparison of outcome data in Nordic countries with those of other European countries participating in the NAPaR registry may provide further information on the safety of aprotinin in patients undergoing cardiac surgery with cardiopulmonary bypass. Interestingly, participating centres in the Nordic countries significantly more often used the full Hammersmith regimen of aprotinin than other European countries, 89.9% vs. 50.2% (p < .01), a relative difference of approximately 80%. Even after the exclusion of iCABG patients the difference between Nordic and European countries remained significant, with 89.0% vs. 69.4% (p < .01) of full-dose regimen, with a relative difference of 33%. As the effect of aprotinin on postoperative bleeding is dose dependent and as higher doses of aprotinin result in less blood loss per hour [Citation17], one may theoretically expect better efficacy outcomes with the high dose regimen or conversely a higher prevalence of complications, if aprotinin use in cardiac surgery indeed had an undesirable effect on outcome. Thus, the following safety signals are evaluated and discussed: mortality, reoperation rate, anaphylactic reactions, thromboembolic events including myocardial infarctions, and renal events.
Mortality
The validated EuroSCORE II risk calculation of 30-day mortality data were compared with the actual 30-day mortality rate of NAPaR patients in Nordic countries as these variables were complete for Nordic countries. The actual 30-day mortality rate for all types of surgery was 6.3% (CI 95% 3.7%–7.8%), in comparison with 11.4% (CI 95% 8.4%–14.0%, p < .01) for the corresponding estimated 30-day mortality according to EuroSCORE II, a reduction of the expected 30-day mortality by approximately 50%. However, a similar comparison with EuroSCORE II data and the actual 30-day mortality rate of all European countries is difficult to perform as it may be subjected to selection bias with almost half this population having missing EuroSCORE II data (mean ± SD 10.7% ± 12.7%) and 30-day mortality rates have not been presented [Citation14]. Moreover, the 30-day mortality of NAPaR patients undergoing surgery for aortic dissection in Nordic countries (6.2% (5/81), CI 95% 0.9% to11.4%) was less than half the expected rate according to EuroSCORE II (13.2%, CI 95% 6.1%–21.0%, p = .11), with the lack of significance being probably due to a type II error (N = 81). In comparison, the in-hospital mortality for surgery of the unambiguous diagnosis of aortic dissection in other European countries was 16.9% (100-5/642-81 = 95/561, CI 95% 13.1%–19.0%) [Citation14], which was more than twice as high in comparison with the 30-day mortality of Nordic countries (p < .01), although still comparable with current literature [Citation14]. Furthermore, the 30-day mortality rate for NAPaR patients undergoing surgery for endocarditis in the Nordic countries showed a similar outcome with approximately halved percentages when compared with the expected 30-day mortality according to EuroSCORE II (6.3% vs. 14.5%, p < .01, ), Also, the in-hospital mortality rate of NAPaR patients not undergoing iCABG was significantly lower in the Nordic countries compared with other European countries (4.9% vs. 8.7%, p < .01, ).
In the Nordic countries significantly fewer patients of the NAPaR underwent iCABG compared with other European countries, 12.1% vs. 27.1%, but they had a higher risk of death with a higher EuroSCORE II of 7.9% ± 10.7 vs. 4.6% ± 6.3 (p < .01). Furthermore, the Nordic countries had a much lower rate of iCABG procedures that were elective, 8.5% vs. 60.8% (p < .01), and consequently 91.5% vs. 39.2% (p < .01) non-elective. Moreover, DAPT was much more prevalent in NAPaR iCABG patients in the Nordic countries (72.9%) than in other European countries (18.9%, p < .01). These data indicate that NAPaR patients undergoing iCABG in the Nordic countries were highly selected and had a much higher risk than in the other European countries. This selection bias may fully explain why the in-hospital (5.1%) and 30-day mortality (8.5%) of iCABG were significantly higher in the Nordic countries compared with the in-hospital mortality of 1.1% in other European countries [Citation14], thus making comparisons virtually futile.
All in all, actual 30-day mortality rates in comparison with individually calculated 30-day mortality risk according to EuroSCORE II in Nordic countries, as well as comparisons with in-hospital mortality rates between Nordic and other European countries indicate that the use of higher dosage of aprotinin in the Nordic versus other European countries may possibly be favourable in terms of mortality, and most importantly not the other way around.
Reoperation rate
Reoperation rates did not differ significantly between NAPaR patients in the Nordic and other European countries ( and ). One may have expected lower reoperation rates in the Nordic countries as the full Hammersmith regimen was used significantly more frequently in the Nordic countries. On the other hand, several other factors that promote bleeding and re-operation rates were often significantly more frequent or differed in the Nordic countries in comparison with other European countries, including higher use of DAPT, higher EuroSCORE II values, longer mean duration of CPB, a higher rate of non-elective procedures, as well as a higher rate of surgery for aortic dissection and endocarditis. Hence, one could speculate that all these listed factors promoting bleeding and re-operation may have been counteracted by the more frequent use of the full Hammersmith dose regime for aprotinin in the Nordic countries.
Anaphylactic/hypersensitivity reaction reactions
Prevalence of anaphylactic/hypersensitivity reactions associated with aprotinin were low and did not differ significantly between NAPaR patients in the Nordic countries (0.6%) compared to other European countries (0.2%). All patients recovered without sequelae and more details including timing have been described earlier [Citation14].
Thromboembolic events (TEEs)
The prevalence of postoperative TEEs (4.7%), permanent stroke (3.1%), and myocardial infarction (1.0%) did not differ significantly between NAPaR patients in Nordic and other European countries before and after the exclusion of iCABG patients except for postoperative TEEs, which was lower in the Nordic countries (4.9%) compared with in the other European countries (7.5%, p < .05). Thus, the effect of a higher use of full Hammersmith dosage of aprotinin in the Nordic countries had if anything a positive effect on this safety signal.
Renal events
In the literature there is a lack of consensus regarding the most appropriate definition of acute renal injury (AKI) following cardiac surgery, making comparisons between reports using different criteria challenging. The authors followed the definition used in the recently published first NAPaR publication [Citation14]. The same AKI criterium was used by two observational studies that noticed an increased AKI rate after aprotinin use in cardiac surgery [Citation4,Citation5]. A later study [Citation18] by the former group using the same data bank [Citation4] did, however, not notice an impaired renal function when evaluating risk factors for AKI. Also, the controversial largest randomized clinical trial (BART) comparing aprotinin with lysine analogues in cardiac surgery did not register an adverse renal effect in the aprotinin arm [Citation7]. This was corroborated by the authors [Citation19] in a following observational study of patients undergoing CABG comparing the use of aprotinin and tranexamic acid. Thus, the possible negative effect of aprotinin in cardiac surgery remains debatable although one should keep in mind that the renal elimination of aprotinin, normally within 4–5 h, is impaired in patients with renal insufficiency undergoing CPB [Citation20]. In the present study the prevalence of AKI and new renal replacement therapy for any reason was significantly higher in the Nordic countries. It is possible that this finding may be due to an adverse effect of aprotinin as the full Hammersmith regime was used significantly more often in the Nordic countries. On the other hand, as described above, NAPaR patients in the Nordic countries had a higher rate of factors associated with a worse renal outcome including the use of DAPT, higher EuroSCORE II values, longer mean duration of CPB, a higher rate of non-elective procedures, a higher rate of surgery for aortic dissection and for endocarditis. Such cases will likewise have a higher rate of AKI and renal replacement therapy due to severe fluid overload independent of their renal function. Also, pre- and postoperative use of aminoglycosides or/and one preoperative dose of teicoplanin in Nordic countries was significantly higher when compared with postoperative aminoglycoside use in other European countries.
Limitations
This study was an observational prospective study lacking direct comparisons with a placebo group or groups treated with lysine analogues. Although 30-day mortality rates in Nordic countries could be compared with validated EuroSCORE II rates, other comparisons were restrained to NAPaR results from other European countries or other similar publications of outcome and safety signals. Conversely, NAPaR patients and especially those in the Nordic countries had a higher rate of patients with a greater risk of adverse events than those studies having reservations about the safety of aprotinin [Citation4–7]. Moreover, EuroSCORE II generally performs well in risk estimation, but in some cardiac surgical populations such as iCABG and surgeries with the lowest and highest risks its calibration is weaker. Other possible limitations include that patients selected for aprotinin use differed between countries, and different procedures in similar countries may have varying clinical outcomes unrelated to aprotinin use.
Conclusions
In Nordic countries, the 30-day mortality rate of patients who underwent cardiac surgery and received aprotinin was approximately 50% lower than the calculated 30-day mortality risk according to EuroSCORE II. Comparisons between NAPaR patients in Nordic and other European countries indicated that the use of the higher dosage of aprotinin in the Nordic countries may favourably affect mortality and other safety signals, and most importantly not the other way around.
Supplemental Material
Download MS Word (13.9 KB)Acknowledgements
Presentation: preliminary data for this study were presented as a poster presentation at the Euroanaesthesia meeting, 4–6 June 2022, Milan and as a poster presentation at the European Association of Cardiothoracic Anaesthesiology and Intensive Care meeting 14–16 December 2022, Naples.
Disclosure statement
Jan van der Linden was a member of the Data Safety Monitoring Committee (DSMC) of the Nordic Aprotinin Patient Registry (NAPaR) for which Nordic Group B.V. (Hoofddorp, the Netherlands) was the sponsor and he has given honorary lectures financially supported by Nordic Pharma. The authors report there are no other competing interests to declare.
Additional information
Funding
References
- Summary of Product Characteristics: trasylol® (aprotinin). 2013. [cited 2023 April 5]. https://www.medicines.org.uk/emc/product/2472
- EMA. Antifibrinolytics containing aprotinin, aminocaproic acid and tranexamic acid. Aprotinin. Assessment report. 2013. [cited 2023 April 5]. https://www.ema.europa.eu/en/documents/referral/assessment-report-antifibrinolytic-medicines-aprotinin_en.pdf
- Royston D, Bidstrup BP, Taylor KM, et al. Effect of aprotinin on need for blood transfusion after repeat open-heart surgery. Lancet. 1987;2(8571):1289–1291. doi: 10.1016/s0140-6736(87)91190-1.
- Mangano DT, Tudor IC, Dietzel C. The risk associated with aprotinin in cardiac surgery. N Engl J Med. 2006;354(4):353–365. doi: 10.1056/NEJMoa051379.
- Schneeweiss S, Seeger JD, Landon J, et al. Aprotinin during coronary-artery bypass grafting and risk of death. N Engl J Med. 2008;358(8):771–783. doi: 10.1056/NEJMoa0707571.
- Shaw AD, Stafford-Smith M, White WD, et al. The effect of aprotinin on outcome after coronary-artery bypass grafting. N Engl J Med. 2008;358(8):784–793. doi: 10.1056/NEJMoa0707768.
- Fergusson DA, Hébert PC, Mazer CD, et al. A comparison of aprotinin and lysine analogues in high-risk cardiac surgery. N Engl J Med. 2008;358(22):2319–2331. doi: 10.1056/NEJMoa0802395.
- Bayer Inc. Important safety information and availability of trasylol (aprotinin) - Notice to Hospitals. 2007. [cited 2023 April 5]. https://healthycanadians.gc.ca/recall-alert-rappel-avis/hc-sc/2007/14481a-eng.php
- EMA. European medicines agency recommends suspension of marketing authorisation aprotinin-containing medicines for systemic use. European Medicines Agency. 2007. [cited 2023 April 5]. https://www.ema.europa.eu/en/news/european-medicines-agency-recommends-suspension-marketing-authorisation-aprotinin-containing
- EMA. Antifibrinolytics containing aprotinin, aminocaproic acid and tranexamic acid. Aprotinin. Annex IV. 2013. [cited 2023 April 5]. https://www.ema.europa.eu/en/documents/referral/antifibrinolytic-medicines-article-31-referral-annex-iv-aprotinin_en.pdf
- EMA. European medicines agency recommends lifting suspension of aprotinin. 2012. [cited 2023 April 5]. https://www.ema.europa.eu/en/news/european-medicines-agency-recommends-lifting-suspension-aprotinin
- McMullan V, Alston RP. III aprotinin and cardiac surgery: a sorry tale of evidence misused. Br J Anaesth. 2013;110(5):675–678. doi: 10.1093/bja/aet008.
- Royston D, De Hert S, van der Linden J, et al. A special article following the relicence of aprotinin injection in Europe. Anaesth Crit Care Pain Med. 2017;36(2):97–102. doi: 10.1016/j.accpm.2017.02.001.
- De Hert S, Ouattara A, Royston D, et al. Use and safety of aprotinin in routine clinical practice: a european postauthorisation safety study conducted in patients undergoing cardiac surgery. Eur J Anaesthesiol. 2022;39(8):685–694. doi: 10.1097/EJA.0000000000001710.
- Nashef SA, Roques F, Sharples LD, et al. EuroSCORE II. Eur J Cardiothorac Surg. 2012;41(4):734–745. doi: 10.1093/ejcts/ezs043.
- Bridgewater B, Kinsman R, Walton P, et al. The 4th european association for cardio-Thoracic surgery adult cardiac surgery database report. Interact Cardiovasc Thorac Surg. 2011;12(1):4–5. doi: 10.1510/icvts.2010.251744.
- Royston D. Aprotinin versus lysine analogues: the debate continues. Ann Thorac Surg. 1998;65(4 Suppl):S9–S19. doi: 10.1016/s0003-4975(98)00071-x.
- Aronson S, Fontes ML, Miao Y, et al. Risk index for perioperative renal dysfunction/failure; critical dependence on pulse pressure hypertension. Circulation. 2007;115(6):733–742. doi: 10.1161/CIRCULATIONAHA.106.623538.
- Lindvall G, Sartipy U, Ivert T, et al. Aprotinin is not associated with postoperative renal impairment after primary coronary surgery. Ann Thorac Surg. 2008;86(1):13–19. doi: 10.1016/j.athoracsur.2008.03.033.
- O'Connor CJ, Brown DV, Avramov M, et al. The impact of renal dysfunction on aprotinin pharmacokinetics during cardiopulmonary bypass. Anesth Analg. 1999;89(5):1101–1107. doi: 10.1213/00000539-199911000-00006.