Abstract
Background. Sudden cardiac arrest (SCA), often also leading to sudden cardiac death (SCD), is a common complication in coronary artery disease. Despite the effort there is a lack of applicable prediction tools to identify those at high risk. We tested the association between the validated GRACE score and the incidence of SCA after myocardial infarction. Material and methods. A retrospective analysis of 1,985 patients treated for myocardial infarction (MI) between January 1st 2015 and December 31st 2018 and followed until the 31st of December of 2021. The main exposure variable was patients’ GRACE score at the point of admission and main outcome variable was incident SCA after hospitalization. Their association was analyzed by subdistribution hazard (SDH) model analysis. The secondary endpoints included SCA in patients with no indication to implantable cardioverter-defibrillator (ICD) device and incident SCD. Results. A total of 1985 patients were treated for MI. Mean GRACE score at baseline was 118.7 (SD 32.0). During a median follow-up time of 5.3 years (IQR 3.8–6.1 years) 78 SCA events and 52 SCDs occurred. In unadjusted analyses one SD increase in GRACE score associated with over 50% higher risk of SCA (SDH 1.55, 95% CI 1.29–1.85, p < 0.0001) and over 40% higher risk for SCD (1.42, 1.12–1.79, p = 0.0033). The associations between SCA and GRACE remained statistically significant even with patients without indication for ICD device (1.57, 1.30–1.90, p < 0.0001) as well as when adjusting with patients LVEF and omitting the age from the GRACE score to better represent the severity of the cardiac event. The association of GRACE and SCD turned statistically insignificant when adjusting with LVEF. Conclusions. GRACE score measured at admission for MI associates with long-term risk for SCA.
KEY MESSAGES
What is already known about this subject?
Nearly 50% of cardiac mortality is caused by sudden cardiac death, often due to sudden cardiac arrest.
Despite the effort, there is a lack of applicable prediction tools to identify those at high risk.
What does this study add?
This study shows that GRACE score measured at the point of admission for myocardial infarction can be used to evaluate patients’ risk for sudden cardiac arrest in a long-term follow-up.
How might this impact on clinical practice?
Based on our findings, the GRACE score at the point of admission could significantly affect the patients’ need for an ICD device after hospitalization for MI and should be considered as a contributing factor when evaluating the patients’ follow-up care.
Introduction
In western nations the incidence of Sudden Cardiac Death (SCD) is approximately 100/100 000 inhabitants and covers roughly 50% of the cardiac mortality and 15–20% of all-cause mortality [Citation1–3]. SCD is often a continuation of Sudden Cardiac Arrest (SCA) and in >30% of the cases a first clinical marker of coronary artery disease (CAD) [Citation4]. AHA/ESC guidelines describe sudden cardiac arrest (SCA) as a sudden cessation of heart activity resulting in loss of circulation and unresponsiveness [Citation2, Citation5]. A minority of SCA patients survive to hospital discharge [Citation6]. Various comorbidities like hypertension, atrial fibrillation, diabetes, and sleep apnea, as well as other traditional cardiovascular risk factors (high BMI, smoking, family history, dyslipidemia etc) and patients’ different medications are associated with a higher risk of SCA and SCD [Citation7–11]. Even though the similar risk profile has been seen to effect on the prognosis of both men and women the incidences of SCA and SCD are drastically higher among male patients regardless of the prevalence of the other risk factors [Citation3, Citation12–15]. This is despite women being at higher risk of both mortality and adverse outcomes after the primary infarction [Citation16, Citation17].
Consistently and accurately predicting the risk for SCA/SCD events has been established to be difficult [Citation4, Citation18, Citation19]. In 2016 Deo et al. developed one of the first validated risk prediction tools for SCD in patients without known heart disease [Citation20]. The results from the PRE-DETERMINE study has later defined the association between SCD and both left ventricular ejection fraction and New York Heart Association (NYHA) heart failure classification in patients with established CAD [Citation21]. However, previous risk calculations and association analyses rely on risk factors that can vary with time and there is still a need for replicated results before they can be used in clinical practice.
In approximately 19–25% of the SCA events the initial recorded rhythm is shockable ventricular arrythmia and the rate has been declining over the past decades [Citation22]. It is known that the severity of the suffered myocardial infarction (MI) increases the incidence of ventricular arrythmias in the follow-up [Citation23]. However, this is a property that is not much considered in the previously known risk factors of SCA/SCD. Various risk prediction tools, such as the Global Registry of Acute Coronary Events (GRACE) score, have been shown to accurately evaluate patients’ long-term mortality due to cardiac and non-cardiac reasons [Citation24–28]. GRACE is a clinically endorsed risk score that is easy to use in practice by using web-based GRACE ACS Risk and Mortality calculators and is highly repeatable [Citation27–29]. As GRACE primarily measures the severity of the MI based on patients’ symptoms and vital measurements our hypothesis was that the score measured at the point of admission for previous MI event would associate with the incidence of SCA and SCD in a long-term follow-up. The aim of our study was to investigate whether previously measured GRACE score (1.0) [Citation28] would associate with the incidence of SCA/SCD and could be used to identify individuals with increased risk of SCA and SCD among patients hospitalized for MI.
Material and methods
Study population
This study is a part of the retrospective MADDEC (Mass Data in Detection and Prevention of Serious Adverse Events in Cardiovascular Disease) registry study. In MADDEC conventional electronic health record (EHR) data is combined with the clinical cardiovascular phenotype data collected by treating physicians into a dedicated KARDIO registry. The register contains high-level risk prediction data complemented with detailed data on hospital EHSs. Data is collected from all patients treated at Tays Heart Hospital, located in Tampere, Finland, which is the sole provider of specialized cardiac care for approximately 0.5 million inhabitants in the region of Pirkanmaa [Citation30, Citation31].
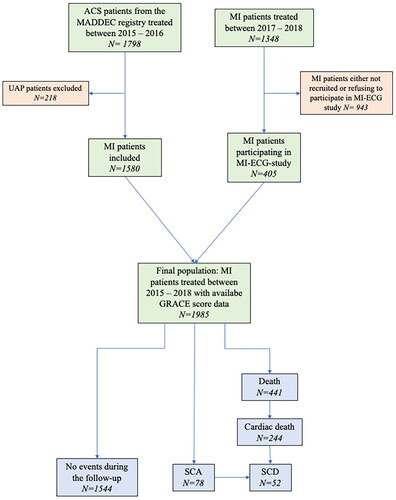
This study focuses on subset of MADDEC patients that were treated for MI between January 1st 2015 and December 31st 2018. In this patient population the GRACE score was collected retrospectively for all patients treated in 2015–2016 [Citation24, Citation32] and prospectively for all patients participating in the prospective MI-ECG study (NCT03231826) (enrollment of patients in 2017–2018). Between 2017–2018 the GRACE score was not calculated from MI patients not participating into MI-ECG study thus being unavailable to be included into the study population. Follow-up of the patients lasted until the December 31st 2021 ().
Main exposure and outcome variables
Main exposure variable of this study was the patients’ GRACE score (1.0) originally designed to estimate six-month mortality risk after admission for MI. The score was calculated based on the information collected at the point of admission as described by Granger et al. [Citation27] and by the formula later developed and validated by Fox et al. [Citation28]. This data was gathered retrospectively for patients treated in 2015–2016 and prospectively from the participants of the MI-ECG study. All data was available in electronical format recoded to written hospital medical records or electronic health records monitoring patients’ vitals (blood pressure, heart rate etc.).
The mortality data was provided by Causes of Death -register maintained by Statistics Finland that covers 100% of the mortality data of Finnish citizens deceased in Finland and nearly 100% of Finnish citizens deceased abroad [Citation33–35]. This data includes causes of death data in ICD format and copies of death certificates which comprise detailed written accounts of the circumstances leading to death. In Finland it is mandated by the legislature that death certificate should be written by the last treating physician based on the patients’ clinical information and post-mortem inspection, as well as medical autopsy information if the cause of death cannot be determined with abovementioned methods. If the death is sudden, unforeseen or within one month of medical procedure the cause of death is mandated to be determined by a forensic autopsy in which case the forensic pathologist is responsible for the death certificate. Thus, all sudden deaths are being registered by either forensic pathologist or treating physician if patient is diagnosed and hospitalized during or immediately after witnessed SCA. The autopsy rate among SCA deaths in MADDEC population was 49% (n = 226/535) between 2007–2018 [Citation2]. In this study, the death certificates with written descriptions of the circumstances leading to death (written by the last treating physician or forensic pathologist) were reviewed individually for possible SCD (defined below) in patients who passed away during the study period. Uncertain cases were discussed between researchers (M.J.K and J.H.). In addition to using written death certificates for endpoint adjudication, written patient records for all patients were also reviewed for incident out-of-hospital cardiac arrests successfully resuscitated and for adequate ICD therapies for hemodynamically compromising VT/VF in patients with ICD-devices.
Both SCA and SCD were defined based on AHA/ECS guidelines [Citation2, Citation5]. SCA was defined as an unforeseen stoppage of heart pulsation leading to hemodynamic catastrophe that was assumed to be of cardiac origin. On the other hand, SCD was defined as an unexpected death within 1 h of the start of the symptoms or within 24 h since the patient was last seen alive and without any symptoms. Patients who died later than 1 h after the symptoms due to successful resuscitation and suffered anoxic brain damage were classified as SCDs. In addition, if there was no mention or other suggestion of the death being sudden or unexpected in the death certificate or EHR the arrythmia was not considered as SCA nor the death as SCD. In other words, the expected arrythmias and deaths at the hospital ward, in patients with severe comorbidities, or with ambiguous details were not considered SCAs or SCDs.
We tested our hypothesis with three different endpoints. The primary endpoint was incident SCA (as defined above) detected during follow-up after discharge or transfer to a primary care unit. Then we analyzed the association between GRACE and SCAs in patients without an indication for implantable cardioverter-defibrillator (ICD) device which represent a true population in the need of primary prevention for SCA and SCD [Citation36, Citation37]. In this case the incidence of SCAs was thus observed only among those patients who did not have an ICD device implanted at baseline and if a patient received an ICD for any indication during follow-up they were no longer followed for SCA (censored from the analysis) regardless of whether they received therapy for SCA/SCD later. Finally, as a tertiary outcome variable, we used the incident SCDs occurring during the follow-up among all patients and similarly among patients with no ICDs (as described above).
Statistical analysis
The data from the retrospective registry of MI patients in 2015–2016 and from the MI-ECG registry were combined for analysis (the preliminary screening did not identify any interactions between GRACE score and study population associating with the incidence of SCA). A sub-distribution hazard (SDH) analysis was performed as described by Austin et al. [Citation38]. SDH analysis was used instead of traditional regression analysis to control the confounding by competing events, i.e. deaths to other causes. The continuous GRACE score was z-transformed and hazard ratios (HR) are presented per one standard deviation (SD) increase in the standardized score. The study population was further categorized into three equally sized groups based on GRACE score to better illustrate the possible linearity (or deviation from linearity) of the association between GRACE score and SCA. We also calculated a new GRACE score independent of age by excluding age from the score (reduced 0,0531*age from the original score [Citation28]) to test whether the association between GRACE score and SCA is independent on age. The analyses were also further adjusted for baseline left ventricular ejection fraction (LVEF, data missing in 138 patients). A p-value of < 0.05 was considered statistically significant. All analyses were performed with IBM SPSS Statistics version 28.0 (IBM Corporation, Armonk, NY, USA) and R software (packages survival and cmprsk).
Results
A total of 1985 patients were treated for MI during the study period 1341 (67.6%) of whom were male. The mean age of the study population was 68.5 years (SD 11.8 years) and the mean GRACE score was 118.7 (SD 32.0). Most of the patients had suffered a non-ST-elevation myocardial infarction (NSTEMI, 53%, n = 1052) and approximately one-third had suffered an ST-elevation myocardial infarction (STEMI, 37.6%, n = 747). The population characteristics and the distribution of GRACE score components are presented in more detail in .
Table 1. Baseline characteristics of the study population.
The median follow-up time was 5.3 years (IQR 3.8–6.1 years) during which 22.2% (n = 441) of the patients passed. The follow-up was ended when the patient died due to any cause, an event was censored, or patient reached the endpoint of their follow-up (31.12.2021). Two-thirds of the mortality were due to cardiovascular causes (55.3% of deaths, n = 244/441). Sixty-one patients died before discharge or transfer to the primary care unit (3.1% in-hospital mortality). A total of 78 SCA events occurred during the follow-up time. 87% (n = 68/78) of events were among patients without an ICD device. Of all SCAs, 52 were classifiable as SCDs (81% (n = 42/52) of which in patients without ICD device).
GRACE score and SCA
The GRACE score was associated with a higher risk of SCA in unadjusted analysis (). One SD change in GRACE score associated with over 50% higher risk of SCA [SDH 1.55 (95% CI 1.29–1.85), p < 0.001]. As the analysis was further adjusted with potential confounding factors (Sex, hypertension, diabetes, peripheral artery disease, atrial fibrillation or flutter, previous MI, previous stroke) the results did not significantly change and the association between GRACE and SCA remained statistically significant [1.48 (1.21–1.81), p > 0.001]. Among the adjusted factors only previous MI (severity of which is measured by GRACE) was statistically significant [1.94 (1.18–3.21), p = 0.009]. In addition, female sex was borderline insignificant [0.61 (0.36–1.02), p = 0.057]. Other factors were clearly insignificant (p > 0.1). As the results were analyzed among sex-specific subpopulations the results remained fairly similar [1.55 (1.25–1.92), p > 0.001 among male and 1.71 (1.30–2.25), p > 0.001 among female patients]. As the unadjusted results were adjusted with LVEF measured at baseline the association remained significant [1.40 (1.13–1.72), p = 0.002]. The association was observed even if patients with ICD devices were excluded from the analysis [1.57 (1.30–1.90), p < 0.001]. In addition, the results remained fairly similar when omitting the age from the score yet the risk slightly decreased, as was expected [SDH 1.42 (1.19–1.66), p < 0.001 for age independent GRACE score], and remained significant even when adjusted with LVEF [1.28 (1.00–1.64), p = 0.048] (). The GRACE score was also associated with overall mortality [HR 2.78 (2.51–3.06), p < 0.001].
Table 2. Associations between GRACE score and age independent GRACE score with SCA and SCD.
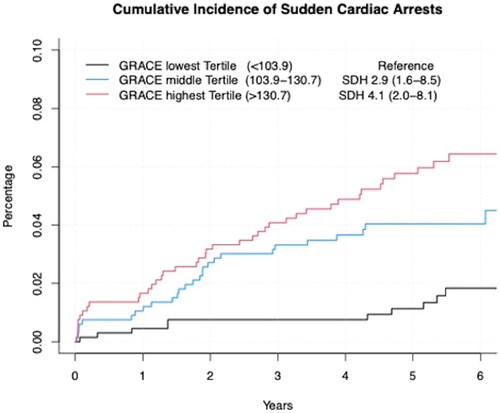
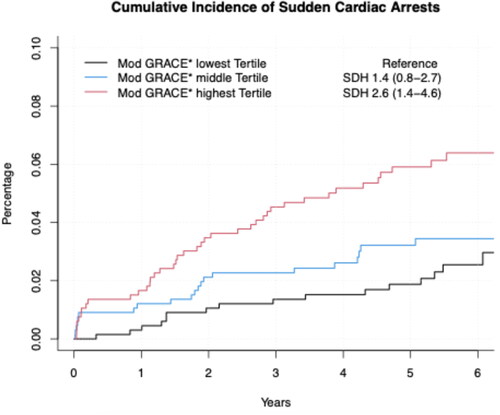
When categorizing the study population into three groups of equal size by GRACE score we observed that patients in the middle and top tertile had approximately three to four times higher risk for future SCA when compared to patients in the lowest tertile [SDH 2.88 (95% CI 1.59–8.50), p = 0.004 for middle tertile and 4.07 (2.04–8.13), p < 0.001 for top tertile, as well as p < 0.001 for the linear trend] (). Although somewhat attenuated, a similar association was observed when the study population was divided into three categories of equal size by age independent GRACE score ().
The association of GRACE score with SCD
In unadjusted analysis the patients’ GRACE score also associated with higher risk of future SCD, the risk being over 40% [1.42 (1.12–1.79), p = 0.003]. As the results were adjusted with abovementioned confounding factors the results remained statistically significant [1.35 (1.04–1.76), p = 0.027]. Only previous MI was borderline insignificant [1.83 (0.99–3.36), p = 0.051] and others were clearly insignificant (p > 0.1). However, when the unadjusted result was adjusted with baseline LVEF the association turned statistically insignificant [1.28 (0.96–1.69), p = 0.092]. There was also a statistically significant association between age independent GRACE and SCD [1.40 (1.15–1.67), p < 0.001], yet this association turned insignificant when adjusted with baseline LVEF [1.27 (0.98–1.64), p = 0.077]. In patients without ICD the unadjusted analyses showed a statistically significant association between GRACE score and SCD [1.40 (1.08–1.81), p = 0.012 for GRACE and 1.38 (1.10–1.73), p = 0.006 for age independent GRACE], yet the results turned statistically insignificant when adjusting with baseline LVEF [1.31 (0.96–1.80), p = 0.088 for GRACE and 1.30 (0.96–1.77), p = 0.095 for age independent GRACE] ().
Discussion
There are multiple studies made on GRACEs components and their association with mortality, as well as on how to improve the score in its capacity to evaluate the risk of mortality [Citation24, Citation32, Citation39–41]. However, to our understanding there is no previous evidence of the association between GRACE score and SCA or SCD. Our hypothesis was that the severity of the patients’ primary MI assessed by GRACE score would associate with and help to predict the future risk of SCA and SCD as the more severe event makes the heart more vulnerable for deadly ventricular arrythmias after the MI [Citation23].
According to our analyses based on a retrospective registry data of patients treated for MI there is a strong association between the patients’ GRACE score upon admission and incidence of SCA later after discharge. With the increase of GRACE score from approximately 100 to 130 points (comparison of lowest and highest population tertiles) the risk of incident SCA increases over fourfold. Even when we omitted age from GRACE score the risk between lowest and highest population tertile remains well over twofold. The results also remained significant despite adjusting with patients’ LVEF and traditional confounding factors. There was also a significant association in the unadjusted analyses between the GRACE and SCD. However, these results turned statistically insignificant after adjusting with patients LVEF, most probably due to limited number of SCD events among the study population. When the results were investigated among sex-specific subpopulations the results among women were as good and even slightly better compared to men despite risk prediction of cardiac events and SCD has traditionally been established to be significantly harder among women [Citation3, Citation13, Citation14]. Based on our findings the GRACE score at the point of admission is a more reliable tool in estimating the risk of SCA than the traditional cardiovascular risk factors and performs well even among female population. Thus, it could be significant factor in analyzing patients’ need for an ICD device after hospitalization for MI.
In 2015 Deyell et al. suggested in their review that the most promising risk prediction tools would be imaging and measurements of both cardiac function and repolarization [Citation19]. Later the significance of repolarization measurements in risk evaluation have also been shown in multiple studies [Citation42–44]. In 2016 Deo et al. published a risk prediction model that takes into account 12 significant risk factors of SCD a majority of which are similar to GRACE score. The tool is based on ARIC (Atherosclerosis Risk in Communities) and CHS (Cardiovascular Health Study) studies [Citation20]. However, the study was conducted on patients with no previous history of heart diseases whereas ours included patients treated for MI, thus the results are not completely comparable.
Our study has several limitations that should be considered. First, the study population included only patients that underwent a coronary angiography and were treated due to MI, thus it cannot be generalized and applied to patients with no history of heart diseases. As the GRACE score is developed to evaluate the risk of ACS patient at the acute situation it is not suitable for evaluating a patient with no history of heart diseases in non-acute setting. The score is calculated based on patients’ acute findings at the point of admission due to the MI and if calculated from symptomless patient with no earlier MI, the score would always be low.
Second, as a single center study the population of our study is strongly emphasizing on a certain region of Finland. As a result, the results of the study may not be widely generalizable. This also resulted as limitations to further examine the different subpopulations of the study group. As a single center study we could only gather a limited amount of data and events in these subpopulations. Therefore, the power of the analyses remained too low to reliably evaluate the association of SCA or SCD and the different individual GRACE components. As we did not analyze the individual components, despite the omission of age, we cannot be certain whether the significant result is due to one or multiple significant factors of the index. However, as the presence, or absence, of one component only affects the total score by a certain amount the linearity between the total score and risk of SCA/SCD would suffer. Thus, we can assume that there are multiple significant components affecting the long-term risk of SCA.
Third, as the overall incidence of SCD and SCA even among patients who have suffered an MI is not high our analyses may be somewhat underpowered to detect the residual association between the GRACE score and SCD after adjusting for LVEF (which is also associated with Killip score for heart failure status, which is a major component of the GRACE score). As the association between GRACE and SCA clearly remained significant despite the LVEF adjustment it is suspected that with more endpoints the association between GRACE and SCD would have remained significant as well. However, LVEF measured at hospital is usually the result of the index event (i.e. MI) severity of which is depicted by patient status at admission which in turn is depicted by the GRACE score. Our results show clearly that the GRACE score is associated with SCA among patients who never end up receiving an ICD device. Implantation of an ICD is usually guided by high observed risk of arrhythmia or development of ischemic cardiomyopathy measured by decreasing LVEF. Therefore, we can assume that GRACE score is most probably a useful risk stratification tool for SCA and presumably for SCD as well even despite other more established risk factors.
Finally, in contrast to all consecutive patients treated in 2015–2016, our study sample from years 2017 and 2018 only included patients participating into the prospective MI-ECG study and therefore the results may be confounded by election bias as patients participating in a prospective observational trial do not completely represent all MI patients. In prospective studies even with no strict exclusion criteria, participants tend to be in better physical condition and may have lower in-hospital mortality when compared to those who do not participate. In our study, participants of the MI-ECG trial (from years 2017–2018) were significantly younger (mean age 4–5 years lower), had less decompensated heart failure (Killip ≥2) and lower in-hospital mortality rate when compared to other MI patients in 2017–2018. As a limitation, not having follow-up data from patients with worse clinical condition during hospitalization probably leads to reduced statistical power due to lower event rate for SCAs and SCDs.
This study opens many possibilities for future studies. As there is not a risk prediction tool that combines both more detailed repolarization measurements and patients’ other risk factors it could be possible to drastically improve the risk stratification by merging the components of both GRACE and prediction model made by Deo et al. as well as more detailed data from ECG results. This would also create a need to investigate the significance individual components of GRACE score even further. An external validation of these results, especially for evaluating the possibility of using GRACE score to estimate SCA/SCD risk in patients with no indication for primary preventive ICD device, is also suggested in the future.
Conclusions
There is a significant association between the patients’ clinical status measured by the GRACE score at admission due to MI and the incidence of SCA on a long-term follow-up. Based on our findings, the GRACE should be considered when evaluating the patients’ need for follow-up care.
Auhors’ contributions
All authors significantly and equally contributed to the planning, conducting, and reporting of this study. MH and JH are the authors responsible for the overall content of this study.
Ethical approval
Formal ethical approval was not required for the retrospective data of patients treated in 2015–2016 but the institutional research review board of Heart Hospital approved the study. For MI-ECG (NCT03231826) study, the institutional research review board, as well as the regional medical research ethics committee of Wellbeing Service County of Pirkanmaa (R17023) approved the study protocol, and all participants gave their written informed consent. This study was conducted according to the ethical principles of the Declaration of Helsinki on the use of human data.
Acknowledgements
The writers would like to thank Mr. Peitsa Leinonen for language check of this article.
Disclosure statement
Other than funding, the writers declare they have no competing interests.
Data availability statement
The anonymized data from this study are available upon request and for justified reasons.
Additional information
Funding
References
- Wong CX, Brown A, Lau DH, et al. Epidemiology of sudden cardiac death: global and regional perspectives. Heart Lung Circ. 2019;28(1):6–14. doi: 10.1016/j.hlc.2018.08.026.
- Koivunen M, Tynkkynen J, Oksala N, et al. Incidence of sudden cardiac arrest and sudden cardiac death after unstable angina pectoris and myocardial infarction. Am Heart J. 2023;257:9–19. doi: 10.1016/J.AHJ.2022.11.009.
- Albert CM, Chae CU, Grodstein F, et al. Prospective study of sudden cardiac death among women in the United States. Circulation. 2003;107(16):2096–2101. doi: 10.1161/01.CIR.0000065223.21530.11.
- Myerburg RJ, Junttila MJ. Sudden cardiac death caused by coronary heart disease. Circulation. 2012;125(8):1043–1052. doi: 10.1161/CIRCULATIONAHA.111.023846.
- Al-Khatib SM, Stevenson WG, Ackerman MJ, et al. 2017 AHA/ACC/HRS guideline for management of patients With ventricular arrhythmias and the prevention of sudden cardiac death: a report of the American college of cardiology/American heart association task force on clinical practice guidelines and the heart rhythm society. J Am Coll Cardiol. 2018;72(14):e91–e220. doi: 10.1016/J.JACC.2017.10.054.
- Johnson NJ, Salhi RA, Abella BS, et al. Emergency department factors associated with survival after sudden cardiac arrest. Resuscitation. 2013;84(3):292–297. doi: 10.1016/J.RESUSCITATION.2012.10.013.
- Bardai A, Blom MT, van Noord C, et al. Sudden cardiac death is associated both with epilepsy and with use of antiepileptic medications. Heart. 2015;101(1):17–22. doi: 10.1136/heartjnl-2014-305664.
- Huikuri HV. Psychotropic medications and the risk of sudden cardiac death. J Am Heart Assoc. 2022;4(2):e001894. doi: 10.1161/JAHA.115.001894.
- Adabag AS, Luepker RV, Roger VL, et al. Sudden cardiac death: epidemiology and risk factors. Nat Rev Cardiol. 2010;7(4):216–225. doi: 10.1038/nrcardio.2010.3.
- Chen LY, Sotoodehnia N, Bůžková P, et al. Atrial fibrillation and the risk of sudden cardiac death: the atherosclerosis risk in communities study and cardiovascular health study. JAMA Intern Med. 2013;173(1):29–35. doi: 10.1001/2013.jamainternmed.744.
- Gami AS, Olson EJ, Shen WK, et al. Obstructive sleep apnea and the risk of sudden cardiac death: a longitudinal study of 10,701 adults. J Am Coll Cardiol. 2013;62(7):610–616. doi: 10.1016/j.jacc.2013.04.080.
- Deo R, Albert CM. Epidemiology and genetics of sudden cardiac death. Circulation. 2012;125(4):620–637. doi: 10.1161/CIRCULATIONAHA.111.023838.
- Ågesen FN, Lynge TH, Blanche P, et al. Temporal trends and sex differences in sudden cardiac death in the copenhagen city heart study. Heart. 2021;107(16):1303–1309. doi: 10.1136/HEARTJNL-2020-318881.
- Skjelbred T, Rajan D, Svane J, et al. Sex differences in sudden cardiac death in a nationwide study of 54 028 deaths. Heart. 2022;108(13):1012–1018. doi: 10.1136/HEARTJNL-2021-320300.
- Wittwer MR, Aldridge E, Hein C, et al. Sex differences in incidence and outcome of out-of-hospital cardiac arrest within a local health network. Front Cardiovasc Med. 2022;9:870696. doi: 10.3389/FCVM.2022.870696/FULL.
- Asleh R, Manemann SM, Weston SA, et al. Sex differences in outcomes after myocardial infarction in the community. Am J Med. 2021;134(1):114–121. doi: 10.1016/J.AMJMED.2020.05.040.
- Cenko E, Yoon J, Kedev S, et al. Sex differences in outcomes After STEMI: effect modification by treatment strategy and age. JAMA Intern Med. 2018;178(5):632–639. doi: 10.1001/JAMAINTERNMED.2018.0514.
- Goldberger JJ, Basu A, Boineau R, et al. Risk stratification for sudden cardiac death. Circulation. 2014;129(4):516–526. doi: 10.1161/CIRCULATIONAHA.113.007149.
- Deyell MW, Krahn AD, Goldberger JJ. Sudden cardiac death risk stratification. Circ Res. 2015;116(12):1907–1918. doi: 10.1161/CIRCRESAHA.116.304493.
- Deo R, Norby FL, Katz R, et al. Development and validation of a sudden cardiac death prediction model for the general population. Circulation. 2016;134(11):806–816. doi: 10.1161/CIRCULATIONAHA.116.023042.
- Chatterjee NA, Moorthy MV, Pester J, et al. Sudden death in patients with coronary heart disease without severe systolic dysfunction. JAMA Cardiol. 2018;3(7):591–600. doi: 10.1001/jamacardio.2018.1049.
- Jerkeman M, Sultanian P, Lundgren P, et al. Trends in survival after cardiac arrest: a swedish nationwide study over 30 years. Eur Heart J. 2022;43(46):4817–4829. doi: 10.1093/EURHEARTJ/EHAC414.
- Henkel DM, Witt BJ, Gersh BJ, et al. Ventricular arrhythmias after acute myocardial infarction: a 20-year community study. Am Heart J. 2006;151(4):806–812. doi: 10.1016/J.AHJ.2005.05.015.
- Hautamäki M, Lyytikäinen LP, Mahdiani S, et al. The association between charlson comorbidity index and mortality in acute coronary syndrome – the MADDEC study. Scand Cardiovasc J. 2020;54(3):146–152. doi: 10.1080/14017431.2019.1693615.
- Méndez-Eirín E, Flores-Ríos X, García-López F, et al. Comparación del valor predictivo pronóstico de los scores TIMI, PAMI, CADILLAC y GRACE en el SCACEST sometido a ICP primario o de rescate. Rev Esp Cardiol (Engl Ed). 2012;65(3):227–233. doi: 10.1016/j.recesp.2011.10.019.
- Raposeiras-Roubín S, Abu-Assi E, Cambeiro-González C, et al. Mortality and cardiovascular morbidity within 30 days of discharge following acute coronary syndrome in a contemporary european cohort of patients: how can early risk prediction be improved? The six-month GRACE risk score. Rev Port Cardiol. 2015;34(6):383–391. doi: 10.1016/j.repc.2014.11.020.
- Granger CB, Goldberg RJ, Dabbous O, et al. Predictors of hospital mortality in the global registry of acute coronary events. Arch Intern Med. 2003;163(19):2345–2353. doi: 10.1001/ARCHINTE.163.19.2345.
- Fox KAA, Dabbous OH, Goldberg RJ, et al. Prediction of risk of death and myocardial infarction in the six months after presentation with acute coronary syndrome: prospective multinational observational study (GRACE). BMJ. 2006;333(7578):1091–1094. doi: 10.1136/BMJ.38985.646481.55.
- GRACE ACS Risk and Mortality Calculator - MDCalc. Accessed May 22, 2023. https://www.mdcalc.com/calc/1099/grace-acs-risk-mortality-calculator.
- Hernesniemi JA, Mahdiani S, Tynkkynen JA, et al. Extensive phenotype data and machine learning in prediction of mortality in acute coronary syndrome – the MADDEC study. Ann Med. 2019;51(2):156–163. doi: 10.1080/07853890.2019.1596302.
- Hernesniemi JA, Mahdiani S, Lyytikäinen LP, et al. Cohort description for MADDEC – mass data in detection and prevention of serious adverse events in cardiovascular disease. In: Eskola H, Väisänen O, Viik J, Hyttinen J, eds. EMBEC NBC 2017 2017. IFMBE Proceedings, vol 65. Springer, Singapore. doi: 10.1007/978-981-10-5122-7_278
- Syyli N, Hautamäki M, Antila K, et al. Left ventricular ejection fraction adds value over the GRACE score in prediction of 6-month mortality after ACS: the MADDEC study. Open Heart. 2019;6(1):e001007. doi: 10.1136/openhrt-2019-001007.
- Salomaa V, Pääkkönen R, Pietilä A. Suomalaisen kuoleman trendit. Suom Laakaril. 2009;9:780–781.
- Statistics Finland - Quality Description:Causes of death 2015. Accessed November 16. 2023. https://www.stat.fi/til/ksyyt/2015/ksyyt_2015_2016-12-30_laa_001_en.html.
- Kuolemansyyt - Tilastokeskus. Accessed September 11, 2023. https://stat.fi/tilasto/ksyyt.
- Sydäntä synkronoivan tahdistinhoidon hoitovasteen ennustaminen sydämen vajaatoimintaa sairastavilla. Accessed November 6, 2022. https://www.kaypahoito.fi/nak08798#R1.
- Cleland JG, Abraham WT, Linde C, et al. An individual patient meta-analysis of five randomized trials assessing the effects of cardiac resynchronization therapy on morbidity and mortality in patients with symptomatic heart failure. Eur Heart J. 2013;34(46):3547–3556. doi: 10.1093/EURHEARTJ/EHT290.
- Austin PC, Lee DS, Fine JP. Introduction to the analysis of survival data in the presence of competing risks. Circulation. 2016;133(6):601–609. doi: 10.1161/CIRCULATIONAHA.115.017719/-/DC1.
- Zalewska-Adamiec M, Kuzma L, Dobrzycki S, et al. The GRACE scale in the prognosis of patients with takotsubo syndrome. J Interv Cardiol. 2020;2020:4340930–4340937. Wöhrle J, ed. doi: 10.1155/2020/4340930.
- Tonet E, Campo G, Maietti E, et al. Nutritional status and all-cause mortality in older adults with acute coronary syndrome. Clin Nutr. 2020;39(5):1572–1579. doi: 10.1016/j.clnu.2019.06.025.
- Zorzi A, Turri R, Zilio F, et al. At-admission risk stratification for in-hospital life-threatening ventricular arrhythmias and death in non-ST elevation myocardial infarction patients. Eur Heart J Acute Cardiovasc Care. 2014;3(4):304–312. doi: 10.1177/2048872614528796.
- Holkeri A, Eranti A, Haukilahti MAE, et al. Predicting sudden cardiac death in a general population using an electrocardiographic risk score. Heart. 2020;106(6):427–433. doi: 10.1136/HEARTJNL-2019-315437.
- Chatterjee NA, Tikkanen JT, Panicker GK, et al. Simple electrocardiographic measures improve sudden arrhythmic death prediction in coronary disease. Eur Heart J. 2020;41(21):1988–1999. doi: 10.1093/EURHEARTJ/EHAA177.
- Aro AL, Reinier K, Rusinaru C, et al. Electrical risk score beyond the left ventricular ejection fraction: prediction of sudden cardiac death in the Oregon sudden unexpected death study and the atherosclerosis risk in communities study. Eur Heart J. 2017;38(40):3017–3025. doi: 10.1093/EURHEARTJ/EHX331.