Abstract
Objectives: Hemodynamic gain index (HGI), a novel hemodynamic index obtained from cardiopulmonary exercise testing (CPX), is associated with adverse cardiovascular outcomes. However, its specific relationship with ventricular arrhythmias (VAs) is unknown. We aimed to assess the association of HGI with risk of VAs in a prospective study. Design: Hemodynamic gain index was estimated using heart rate and systolic blood pressure (SBP) responses ascertained in 1945 men aged 42–61 years during CPX from rest to maximum exercise, using the formula: [(Heart ratemax x SBPmax) – (Heart raterest x SBPrest)]/(Heart raterest x SBPrest). Cardiorespiratory fitness (CRF) was measured using respiratory gas exchange analysis. Hazard ratios (HRs) (95% confidence intervals, CIs) were estimated for VAs. Results: Over a median follow-up duration of 28.2 years, 75 cases of VA were recorded. In analysis adjusted for established risk factors, a unit (bpm/mmHg) higher HGI was associated with a decreased risk of VA (HR 0.72, 95% CI: 0.55–0.95). The results remained consistent on adjustment for lifestyle factors and comorbidities (HR 0.72, 95% CI: 0.55–0.93). Comparing the top versus bottom tertiles of HGI, the corresponding adjusted HRs (95% CIs) were 0.51 (0.27–0.96) and 0.52 (0.28–0.94), respectively. The associations were attenuated on addition of CRF to the model. HGI improved risk discrimination beyond established risk factors but not CRF. Conclusions: Higher HGI is associated with a reduced risk of VAs in middle-aged and older Caucasian men, but dependent on CRF levels. Furthermore, HGI improves the prediction of the long-term risk for VAs beyond established risk factors but not CRF.
Introduction
Cardiovascular diseases (CVDs) remain a leading cause of global morbidity and mortality, with ventricular arrhythmias (VAs) emerging as a significant contributor to this burden [Citation1]. Ventricular arrhythmias represent a complex group of cardiac disorders that encompass premature ventricular contractions, ventricular tachycardia (VT), and ventricular fibrillation (VF) [Citation1]. These ventricular arrhythmic events can lead to sudden cardiac death, a catastrophic event with profound societal and individual implications [Citation1]. Established risk factors for VAs include structural heart disease, previous myocardial infarction, hypertension, smoking, obesity, and diabetes [Citation2]. Despite advances in identifying these risk factors, the occurrence of VAs remains unpredictable in a substantial proportion of cases. Consequently, there exists an unmet need to discover additional risk factors that could enhance the precision of risk stratification and facilitate targeted interventions to mitigate the incidence of VAs.
Cardiopulmonary exercise testing (CPX) has emerged as a valuable tool in assessing cardiovascular function and involves the systematic evaluation of an individual’s physiological response to incremental exercise stress [Citation3]. Parameters such as heart rate and blood pressure responses, in conjunction with cardiorespiratory fitness (CRF) as measured using peak oxygen uptake (VO2peak), provide critical insights into cardiovascular health and prognosis [Citation4–6]. A growing body of evidence has demonstrated an inverse relationship between CRF and adverse cardiovascular outcomes, including VAs [Citation7–10]. Individuals with higher CRF levels tend to exhibit better cardiovascular health, reduced incidence of cardiovascular events, and improved overall survival.
The Hemodynamic Gain Index (HGI) has emerged as an innovative index derived from the combined assessment of exercise heart rate and systolic blood pressure (SBP) responses during CPX [Citation11]. It represents a novel approach that captures the dynamic interaction between cardiovascular responses to exercise stress, reflecting the efficiency of the cardiovascular system in adapting to increased demands. Recent research has underscored the predictive capabilities of HGI for adverse cardiovascular outcomes such as cardiovascular mortality, sudden cardiac death, heart failure and all-cause mortality [Citation11–16], offering a promising avenue for refining risk assessment strategies. While HGI has shown promise in predicting these major adverse cardiovascular outcomes, its relationship with the specific endpoint of VAs has not been explored. This prospective cohort study aims to fill this knowledge gap by investigating the potential link between HGI and the risk of VAs. We also assessed HGI's ability to predict and reclassify the long-term risk of VAs beyond established cardiovascular risk factors.
Materials and methods
We conducted this study in accordance with STROBE (STrengthening the Reporting of OBservational studies in Epidemiology) guidelines for reporting observational studies in epidemiology (Supplementary Material 1). The Kuopio Ischemic Heart Disease (KIHD) study, a general population-based prospective cohort study designed to evaluate potential risk factors for CVD outcomes and other related chronic diseases, was employed for this analysis. The study included a representative sample of 2682 men aged 42–61 yr who were recruited from the city of Kuopio and its surrounding rural communities in eastern Finland [Citation12, Citation13, Citation17]. A total of 1945 men with non-missing information on HGI, potential confounding variables, and VAs were included in the present analysis (Supplemental Material 2). All VAs that occurred from study enrollment to 12/31/2018 were included. Baseline assessments, questionnaire administration and physical examinations took place between March 1984 and December 1989. Ethical approval for the study protocol was sought from the institutional review board of the University of Eastern Finland (reference #:143/97) on December 1, 1983, all participants provided written informed consent and all study procedures were conducted according to the Declaration of Helsinki.
For the assessment of HGI and other CPX measures, a maximal symptom-limited cycle exercise test was conducted between 8:00 and 10:00 am using an electronic braked cycle ergometer as described in previous reports [Citation10, Citation18–20]. Cardiorespiratory fitness was measured using VO2peak, which was directly assessed using a computerized metabolic measurement system (Medical Graphics, USA). The standardized testing protocol included a 3-min warm-up at 50 watts (W; 1 W = 6.12 kgm/min), which was followed by 20 W/min increases in workload with direct analyses of expired respiratory gases. Respiratory gas exchange was measured by the breath-by-breath method, which involved breath sample collection via a face-mask. The respiratory gas analyzer expressed VO2peak as an average value recorded over 8 s. Peak oxygen uptake was defined as the highest or peak attained value for oxygen consumption, expressed as mL/kg/min [Citation10, Citation17]. Electrocardiographic indices, blood pressure, and heart rate were measured both at rest and during the exercise testing phase [Citation12, Citation13]. The HGI was derived from the following formula: ([Heart ratemax x SBPmax] – [Heart raterest x SBPrest])/(Heart raterest x SBPrest) [Citation11]. The diagnostic classification of VAs was coded according to ICD-9 codes (427.41) or ICD-10 codes (I47.2, I49.0) codes. The definition of non-sustained or sustained VT and/or VF was based on electrocardiography, which was ascertained from hospital documents [Citation21]. Documents were cross-checked in detail by two physicians. The Independent Events Committee, masked to clinical data, performed classification of outcomes.
Hazard ratios (HRs) with 95% CI for VA were estimated with multivariable Cox proportional hazards models after confirming no major departure from the assumptions of the proportionality of hazards using Schoenfeld residuals [Citation22]. Given no evidence of a non-linear relationship with various adverse cardiovascular outcomes [Citation12, Citation13], HGI was modelled as a continuous [per unit (bpm/mmHg) increase] variable, with subsidiary modelling using tertiles. Given the low event rate and to avoid over-adjustment, HRs were adjusted for in four models: (Model 1) age (years); (Model 2) model 1 plus total cholesterol, high-density lipoprotein cholesterol (HDL-C), body mass index (BMI), history of hypertension and alcohol consumption; (Model 3) age, smoking status, prevalent type 2 diabetes (T2D), prevalent coronary heart disease (CHD) and physical activity; and (Model 4) age, smoking status, prevalent T2D, hypertension and CHD, and CRF. The covariates were selected based on their previously established roles as risk factors for VAs, evidence from previous research [Citation12, Citation13], previously published associations with VAs in the KIHD study [Citation10], or their potential as confounders based on known associations with VAs and observed associations with HGI using the available [Citation23]. Given the high mortality rate in the KIHD cohort, we conducted additional analyses to estimate the baseline cumulative subhazard of VA considering mortality as a competing outcome to VAs. We used the competing-risks extension of the Cox proportional hazards models, as proposed by Fine and Gray [Citation24]. To evaluate whether adding information on HGI to established risk factors for VAs is associated with improvement in risk prediction, we calculated measures of discrimination (Harrell’s C-index [Citation25] and −2 log likelihood[Citation26, Citation27] and reclassification [Citation28, Citation29]. To investigate the change in C-index on the addition of HGI, two VA risk prediction models were fitted: one model based on traditional risk factors (i.e. age, smoking status, prevalent T2D, hypertension and CHD, and physical activity) and the second model with these risk factors plus HGI. We also tested for differences in the −2 log likelihood of prediction models with and without inclusion of HGI; it is a more sensitive measure when evaluating the added predictive value of a new measurement [Citation26, Citation27]. Reclassification was assessed using the category-free version of net-reclassification-improvement (NRI) [Citation28, Citation29] and the integrated-discrimination-improvement (IDI) [Citation28] by comparing the model containing traditional risk factors to the predicted risk from the model containing traditional risk factors plus HGI. We repeated the process for a model comprising age, smoking status, prevalent T2D, hypertension and CHD, plus CRF. A p value of ≤ 0.05 was deemed statistically significant. All statistical analyses were performed using Stata version MP 18 (Stata Corp).
Results
Baseline characteristics
The overall mean ± standard deviation (SD) age at baseline for the 1945 men was 53 ± 5 years, while the mean ± SD HGI and CRF was 2.53 ± 1.05 bpm/mmHg and 30.5 ± 7.9 mL/kg/min, respectively. Hemodynamic measures such as resting heart rate, peak SBP, heart rate recovery, and diastolic blood pressure recovery were similar between those who developed or did not develop VAs at the end of follow-up duration. Those who developed VAs were older; more likely to have lower levels of HGI, CRF and peak heart rate; higher levels of resting SBP and SBP recovery; and have pre-existing hypertension ().
Table 1. Baseline characteristics of study participants overall and by ventricular arrhythmias.
Associations of HGI with VA
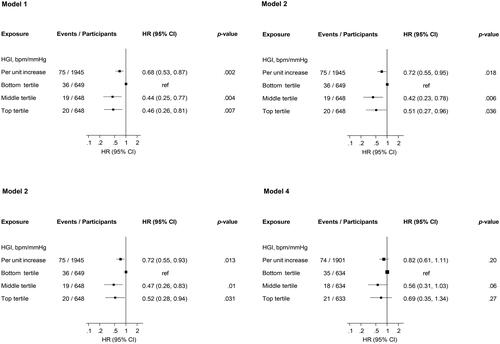
Over a median (IQR) follow-up duration of 28.2 (18.7, 31.3) years, 75 cases of VA occurred. In analysis adjusted for age, each 1 unit increase in HGI was associated with a lower risk of VA (HR 0.68, 95% CI: 0.53–0.87) (-Model 1), which was minimally attenuated to (HR 0.72, 95% CI: 0.55–0.95) on further adjustment for total cholesterol, HDL-C, BMI, history of hypertension and alcohol consumption (-Model 2). The results remained consistent in a third model that adjusted for age, smoking status, prevalent T2D and CHD, and physical activity (-Model 3). When physical activity was replaced with CRF in the third model, the HR (95% CI) was attenuated to 0.82 (0.61–1.11) (-Model 4). Alternatively, comparing the top versus bottom tertiles of HGI, the corresponding adjusted HRs (95% CIs) for VA were 0.46 (0.26–0.81), 0.51 (0.27–0.96), 0.52 (0.28–0.94) and 0.69 (0.35–1.34), respectively (). A total of 1191 deaths occurred during follow-up and 58 individuals died before experiencing VA. In analyses including mortality as a competing risk event, the subhazard of VA was (SHR 0.71, 95% CI: 0.41–1.23) per unit increase in HGI.
Figure 1. Association of hemodynamic gain index with ventricular arrhythmias.
HGI, hemodynamic gain index; ref, reference;
Model 1: Adjusted for age;
Model 2: Model 1 plus total cholesterol, high-density lipoprotein cholesterol, body mass index, history of hypertension and alcohol consumption;
Model 3: Age, smoking status, prevalent type 2 diabetes, hypertension and coronary heart disease and physical activity;
Model 4: Age, smoking status, prevalent type 2 diabetes, hypertension and coronary heart disease and cardiorespiratory fitness
HGI and VA risk prediction
A VA risk prediction model containing traditional risk factors (age, smoking status, prevalent T2D, hypertension and CHD, and physical activity) yielded a C-index of 0.6443 (95% CI: 0.5826 to 0.7061). After addition of information on HGI, the C-index was 0.6701 (95% CI: 0.6081 to 0.7322), representing a modest increase of 0.0258 (95% CI: −0.0095 to 0.0611; p=.15). The −2 log likelihood was significantly improved on addition of HGI to the risk model (p for comparison=.012). The continuous NRI and IDI were 49.17% (95% CI: −6.92 to 105.27; p=.086) and 0.0044 (0.0009 to 0.0079; p=.014), respectively.
A VA risk prediction model containing traditional risk factors (age, smoking status, prevalent T2D, hypertension and CHD) plus CRF yielded a C-index of 0.6766 (95% CI: 0.6159 to 0.7374). After addition of information on HGI, the C-index was 0.6859 (95% CI: 0.6258 to 0.7460), representing a non-significant increase of 0.0092 (95% CI: −0.0097 to 0.0282; p=.34). The −2 log likelihood was not significantly improved on addition of HGI to the risk model (p for comparison=.20). The continuous NRI and IDI were −28.67% (95% CI: −85.35 to 28.01; p=.32) and 0.0007 (-0.0011 to 0.0026; p=.44), respectively.
Discussion
In this prospective evaluation of the association between HGI and risk of VAs in a cohort of middle-aged and older Finnish men aged 42–61 years, HGI was strongly and inversely associated with the future risk of VAs, which was independent of several established and emerging risk factors, but was partly dependent on CRF levels. Given the high mortality rate in our study cohort which might have hindered VA development (n = 17 VA events available for analysis), the association between HGI and VAs was less robust when mortality was adjusted for as a competing risk event. This was also not a surprising finding as HGI is independently associated with mortality in the cohort. Furthermore, risk prediction analyses suggested that HGI may improve the discrimination but not reclassification of long-term VAs in the general population beyond common established risk factors. However, HGI did not improve risk discrimination or reclassification beyond established risk factors plus CRF levels.
This is the first evaluation of the nature and magnitude of the prospective association between HGI derived from CPX and VA risk, as well as the potential utility of HGI in VA risk prediction. However, an emerging number of studies have evaluated associations between this novel index and other adverse cardiovascular outcomes [Citation11–15]. In the pioneering clinical and statistical validation studies by Vainshelboim and colleagues, it was shown that higher HGI was associated with a lower risk of all-cause mortality in men and women, which was independent of potential confounders and remained robust in several sensitivity analyses [Citation11, Citation14]. In another related study, Chaikijurajai and colleagues showed that lower HGI was independently associated with an increased risk of all-cause mortality, and this association persisted in subgroups of men, women, and patients with and without heart failure, CHD, and betablocker use [Citation15]. Vainshelboim and Myers have recently shown higher HGI to be associated with a lower risk of heart failure incidence [Citation16].
The robust association between the HGI and the risk of VAs can be attributed to the intricate interplay of cardiovascular responses that HGI encapsulates. HGI, derived from the dynamic interaction between exercise-induced heart rate and SBP responses, offers insights into the compliance of the vasculature and efficiency of the cardiovascular system’s adaptation to increased demands [Citation11]. This efficiency reflects not only the integrity of autonomic regulation but also the compensatory mechanisms that maintain hemodynamic stability during exercise stress. Dysfunction in cardiac autonomic nervous system control, as manifested by aberrant heart rate and blood pressure responses, may signify an underlying substrate of sympathetic overactivity or impaired vagal tone. Such dysregulation can lead to electrical instability within the ventricles, rendering them susceptible to arrhythmogenic triggers. Moreover, the combined assessment of heart rate and blood pressure responses captured by HGI reflects the complex interplay between preload, afterload, and contractility. Disruptions in these factors can influence ventricular repolarization and refractoriness, creating a conducive environment for reentrant circuits that underlie certain types of VAs.
Though the current findings suggest that CRF remains a stronger risk and prognostic indicator for adverse cardiovascular outcomes than HGI as shown in previous studies [Citation12, Citation13], these findings may still offer a novel and refined approach to cardiovascular risk assessment in clinical practice. Incorporating HGI into risk stratification algorithms has the potential to identify individuals at increased risk of VAs who might otherwise be overlooked by conventional risk factors alone. This personalized risk assessment could enable healthcare professionals to allocate preventive interventions more effectively, thereby mitigating the occurrence of life-threatening arrhythmic events. Furthermore, the integration of HGI into routine CPX protocols may enhance the accuracy of risk prediction, offering clinicians a streamlined tool to comprehensively evaluate both cardiovascular fitness and arrhythmogenic potential. While it’s true that cardiac imaging modalities like echocardiography (echo) and magnetic resonance imaging (MRI) are valuable tools for risk stratification in VAs, the potential role of a non-specific marker like HGI should not be overlooked for the following reasons: HGI offers a simple and practical approach to assessing hemodynamic function by integrating heart rate and blood pressure responses into a single metric. Its ease of estimation and low cost make it accessible in both clinical and research settings. HGI provides complementary information to cardiac imaging; while echo and MRI offer anatomical and structural insights. HGI offers functional hemodynamic data, enhancing the comprehensive evaluation of cardiovascular health and VA risk. Numerous studies have demonstrated the prognostic value of HGI in predicting adverse cardiovascular outcomes [Citation11–16] and now VA. The emerging evidence supports its potential role as an additional risk stratification tool alongside cardiac imaging markers. Given the low event rate and being the first study to report these findings, further large-scale studies are warranted to replicate these findings and investigate the potential clinical value of HGI in risk assessment.
This study offers notable strengths and acknowledges certain limitations. The prospective cohort study design and long follow-up duration enhances the study’s credibility by capturing real-time data and facilitating the establishment of temporal relationships. The relatively large sample size and adjustment for established risk factors contribute to the robustness of the findings. Additionally, the utilization of CPX for HGI assessment enhances the study’s relevance. The limitations included the low VA event rate and the use of single baseline values of HGI, which could potentially introduce regression dilution bias. We were unable to account for relevant variables such as use of medications (e.g. beta-blockers, which affect heart rate) and structural heart disease, because of unavailability of data; furthermore, because of the low event rate, comprehensive adjustments for confounders could not be done because of the likelihood of over-fitting models. We acknowledge that our sample only included males, which restricts the generalizability of our findings to females. Future studies should aim for more diverse participant demographics to ensure broader applicability. The data were collected in the 1980s, which may limit the generalizability of our findings to contemporary populations, especially concerning advancements in therapy. While this is a valid concern, the longitudinal nature of the study allows for valuable insights into the long-term effects of various factors on VAs. We acknowledge the controversy surrounding the use of Harrel’s C-index, NRI and IDI and recognize their limitations. For instance, Harrel’s C-index is based on ranks rather than on continuous data and it can be insensitive in detecting differences [Citation30]; the categorical NRI only accounts for the direction of risk reclassification, and not the magnitude [Citation31]. We employed the category-free NRI, which has the advantage of not requiring pre-specified categories and does not lose information due to categorization [Citation30]. We also used the −2 log likelihood test, which is a sensitive risk discrimination method and well recommended [Citation26, Citation27]. Ascertainment of VAs was based on ICD-codes. We acknowledge that ICD-codes can have limitations due to potential misclassification or underreporting of events. However, using ICD-codes for outcome definition is a common practice in epidemiological studies [Citation32]. While this approach may not capture all VAs accurately, it provides a standardized method for identifying events across large datasets. Additionally, validation studies have demonstrated the utility of ICD-codes for identifying clinical outcomes, although some degree of misclassification may still exist [Citation33]. Therefore, while acknowledging its limitations, the use of ICD-codes remains a practical and widely accepted method for defining VAs in research settings. Finally, although the study establishes a strong association between HGI and VA risk, causation cannot be proved and the mechanistic underpinnings are speculative and necessitate further exploration.
Conclusions
Higher HGI is associated with a reduced risk of VAs in middle-aged and older Caucasian men, but dependent on CRF levels. Furthermore, HGI improves the prediction of the long-term risk for VAs beyond established risk factors but not CRF.
Authors’ contributions
S.K.K.: study design, data analysis and interpretation, drafting manuscript, and revising manuscript content and approving final version of manuscript; S.Y.J.: study design, interpretation, revising manuscript content and approving final version of manuscript; S.K.: study design, interpretation, revising manuscript content and approving final version of manuscript; J.A.L.: study design and conduct, responsibility for the patients and data collection, and revising manuscript content and approving final version of manuscript.
Supplemental Material
Download MS Word (63.1 KB)Acknowledgements
We thank the staff of the Kuopio Research Institute of Exercise Medicine and the Research Institute of Public Health and University of Eastern Finland, Kuopio, Finland for the data collection in the study.
Disclosure statement
No potential conflict of interest was reported by the authors.
Additional information
Funding
References
- Al-Khatib SM, Stevenson WG, Ackerman MJ, et al. 2017 AHA/ACC/HRS guideline for management of patients with ventricular arrhythmias and the prevention of sudden cardiac death: a report of the American college of cardiology/American heart association task force on clinical practice guidelines and the heart rhythm society. J Am Coll Cardiol. 2018;72(14):e91–e220. doi: 10.1016/j.jacc.2017.10.054.
- Harris P, Lysitsas D. Ventricular arrhythmias and sudden cardiac death. BJA Educ. 2016;16(7):221–229. doi: 10.1093/bjaed/mkv056.
- Ross R, Blair SN, Arena R, et al. Importance of assessing cardiorespiratory fitness in clinical practice: a case for fitness as a clinical vital sign: a scientific statement from the American heart association. Circulation. 2016;134(24):e653–e699. doi: 10.1161/CIR.0000000000000461.
- Balady GJ, Arena R, Sietsema K, et al. Clinician’s guide to cardiopulmonary exercise testing in adults: a scientific statement from the American heart association. Circulation. 2010;122(2):191–225. doi: 10.1161/CIR.0b013e3181e52e69.
- Guazzi M, Arena R, Halle M, et al. 2016 focused update: clinical recommendations for cardiopulmonary exercise testing data assessment in specific patient populations. Eur Heart J. 2016;39(14):1144–1161. doi: 10.1093/eurheartj/ehw180.
- Guazzi M, Adams V, Conraads V, et al. EACPR/AHA scientific statement. Clinical recommendations for cardiopulmonary exercise testing data assessment in specific patient populations. Circulation. 2012;126(18):2261–2274. doi: 10.1161/CIR.0b013e31826fb946.
- Laukkanen JA, Isiozor NM, Kunutsor SK. Objectively assessed cardiorespiratory fitness and all-cause mortality risk: an updated meta-analysis of 37 cohort studies involving 2,258,029 participants. Mayo Clin Proc. 2022;97(6):1054–1073. doi: 10.1016/j.mayocp.2022.02.029.
- Kunutsor SK, Jae SY, Kurl S, et al. The interplay between systolic blood pressure, cardiorespiratory fitness, and mortality risk: a prospective cohort study. J Cardiopulm Rehabil Prev. 2023;43(3):222–224.
- Laukkanen JA, Kunutsor SK, Yates T, et al. Prognostic relevance of cardiorespiratory fitness as assessed by submaximal exercise testing for all-cause mortality: a UK biobank prospective study. Mayo Clin Proc. 2020;95(5):867–878. doi: 10.1016/j.mayocp.2019.12.030.
- Laukkanen JA, Lavie CJ, Khan H, et al. Cardiorespiratory fitness and the risk of serious ventricular arrhythmias: a prospective cohort study. Mayo Clin Proc. 2019;94(5):833–841. doi: 10.1016/j.mayocp.2018.11.027.
- Vainshelboim B, Kokkinos P, Myers J. Prognostic value and clinical usefulness of the hemodynamic gain index in men. Am J Cardiol. 2019;124(4):644–649. doi: 10.1016/j.amjcard.2019.05.015.
- Laukkanen JA, Isiozor NM, Willeit P, et al. Hemodynamic gain index is associated with cardiovascular mortality and improves risk prediction: a prospective cohort study. J Cardiopulm Rehabil Prev. 2023;43(5):368–376. doi: 10.1097/HCR.0000000000000777.
- Laukkanen JA, Isiozor NM, Willeit P, et al. Haemodynamic gain index is associated with risk of sudden cardiac death and improves risk prediction: a cohort study. Cardiology. 2023;148(3):246–256. doi: 10.1159/000530637.
- Vainshelboim B, Kokkinos P, Myers J. Hemodynamic gain index in women: a validation study. Int J Cardiol. 2020;308:15–19. doi: 10.1016/j.ijcard.2020.03.066.
- Chaikijurajai T, Wu Y, Grodin JL, et al. Validation of prognostic value of the hemodynamic gain index in different groups of patients undergoing exercise stress testing. Am Heart J Plus. 2022;18. doi: 10.1016/j.ahjo.2022.100174.
- Vainshelboim B, Myers J. Haemodynamic gain index and heart failure incidence – prognostic and preventive value. Eur J Prev Cardiol. 2023;30(13):1404–1411. doi: 10.1093/eurjpc/zwad236.
- Kunutsor SK, Isiozor NM, Myers J, et al. Baseline and usual cardiorespiratory fitness and the risk of chronic kidney disease: a prospective study and meta-analysis of published observational cohort studies. Geroscience. 2023;45(3):1761–1774. doi: 10.1007/s11357-023-00727-3.
- Lakka TA, Venäläinen JM, Rauramaa R, et al. Relation of leisure-time physical activity and cardiorespiratory fitness to the risk of acute myocardial infarction. N Engl J Med. 1994;330(22):1549–1554. doi: 10.1056/NEJM199406023302201.
- Khan H, Kunutsor SK, Rauramaa R, et al. Long-term change in cardiorespiratory fitness in relation to atrial fibrillation and heart failure (from the kuopio ischemic heart disease risk factor study). Am J Cardiol. 2018;121(8):956–960. doi: 10.1016/j.amjcard.2018.01.003.
- Khan H, Kunutsor S, Rauramaa R, et al. Cardiorespiratory fitness and risk of heart failure: a population-based follow-up study. Eur J Heart Fail. 2014;16(2):180–188. doi: 10.1111/ejhf.37.
- Zaccardi F, Webb DR, Kurl S, et al. Inverse association between fasting plasma glucose and risk of ventricular arrhythmias. Diabetologia. 2015;58(8):1797–1802. doi: 10.1007/s00125-015-3646-0.
- Therneau TM, Grambsch PM. Modeling survival data: extending the cox model. New York, NY: Springer; 2000.
- Groenwold RH, Klungel OH, Grobbee DE, et al. Selection of confounding variables should not be based on observed associations with exposure. Eur J Epidemiol. 2011;26(8):589–593. doi: 10.1007/s10654-011-9606-1.
- Fine JP, Gray RJ. A proportional hazards model for the subdistribution of a competing risk. J Am Stat Assoc. 1999;94(446):496–509. doi: 10.1080/01621459.1999.10474144.
- Harrell FE, Jr., Lee KL, Mark DB. Multivariable prognostic models: issues in developing models, evaluating assumptions and adequacy, and measuring and reducing errors. Statist Med. 1996;15(4):361–387. doi: 10.1002/(SICI)1097-0258(19960229)15:4<361::AID-SIM168>3.0.CO;2-4.
- Cook NR. Use and misuse of the receiver operating characteristic curve in risk prediction. Circulation. 2007;115(7):928–935. doi: 10.1161/CIRCULATIONAHA.106.672402.
- Harrell FEJ. Regression modeling strategies. New York: Springer; 2001.
- Pencina MJ, D'Agostino RB, Sr., D'Agostino RB, Jr., et al. Evaluating the added predictive ability of a new marker: from area under the ROC curve to reclassification and beyond. Stat Med. 2008;27(2):157–172. doi: 10.1002/sim.2929.
- Pencina MJ, D'Agostino RB, Sr., Steyerberg EW. Extensions of net reclassification improvement calculations to measure usefulness of new biomarkers. Stat Med. 2011;30(1):11–21. doi: 10.1002/sim.4085.
- Cook NR. Quantifying the added value of new biomarkers: how and how not. Diagn Progn Res. 2018;2(1):14. doi: 10.1186/s41512-018-0037-2.
- Kerr KF, Wang Z, Janes H, et al. Net reclassification indices for evaluating risk prediction instruments: a critical review. Epidemiology. 2014;25(1):114–121. doi: 10.1097/EDE.0000000000000018.
- Ye Y, Larrat EP, Caffrey AR. Algorithms used to identify ventricular arrhythmias and sudden cardiac death in retrospective studies: a systematic literature review. Ther Adv Cardiovasc Dis. 2018;12(2):39–51. doi: 10.1177/1753944717745493.
- Tsai MJ, Tsai CH, Pan RC, et al. Validation of ICD-9-CM and ICD-10-CM diagnostic codes for identifying patients with out-of-Hospital cardiac arrest in a national health insurance claims database. Clin Epidemiol. 2022;14:721–730. doi: 10.2147/CLEP.S366874.

