ABSTRACT
Chronic musculoskeletal (MSK) pain, specifically low back pain (LBP) and osteoarthritis (OA), are a major cause of global disability, reduced quality of life and high socioeconomic burden. Research in high income countries suggests MSK pain is often comorbid with other long-term conditions / non-communicable diseases (NCDs) including diabetes, hypertension, and cardiovascular disease. However, the epidemiology of comorbid NCDs and MSK pain in low- and middle-income countries (LMICs) is unclear. This systematic review aims to describe the prevalence and pattern of comorbid NCDs in adults with MSK pain in LMICs. Nine databases were searched for epidemiological studies in LMICs (World Bank categories). Paired researchers independently identified studies, extracted data, and completed critical appraisal using Hoy risk of bias tool. Random-effect meta-analysis was used to estimate prevalence of NCDs comorbid with MSK pain. From 2112 citations; 14 studies (n=6093 adults with MSK pain, mean age=46.9years) were included. Overall prevalence of MSK pain with comorbid NCDs was 46.1% (95%CI 32.3 - 59.9). Systemic hypertension had the highest comorbid prevalence with MSK pain (42.6%, 95%CI 25.6-59.6), followed by diabetes (26.7%, 95%CI 16.1-37.3) and mental health conditions (anxiety/depression; 24.9% 95%CI 11.5-38.4). A high proportion of patients with MSK pain in LMICs experience comorbid NCDs. Variable data/population samples, and under-reporting limit accurate capture of prevalence estimates. Understanding the true burden of MSK pain (specifically lower back pain and hip/knee osteoarthritis) and comorbid NCDs is critical to informing effective treatment strategies. The health systems implications of these findings are imperative towards person-centred care, organisation of care and efficient resource utilisation in LMICs.
Introduction
In recent years, global health has been faced with a double burden of deaths and disability from infectious diseases (including pandemics) and an increase in the impact of non-communicable diseases (NCDs) such as cardiovascular disease, chronic lung illnesses, diabetes and arthritis (Ferreira et al. Citation2023; Steinmetz et al. Citation2023; Vos et al. Citation2020). Chronic musculoskeletal (MSK) pain, a common symptom from conditions such as osteoarthritis and lower back pain (LBP), is a large contributor to the global disability burden. Specifically, osteoarthritis saw a 104.9% increase in Disability Adjusted Life Years (DALYs) between 1990 and 2016, while LBP remains the number one cause of disability worldwide (Briggs et al. Citation2018; Hay et al. Citation2017). The most significant increase has been seen in low- and middle-income countries (LMICs) (Ferreira et al. Citation2023; Hay et al. Citation2017; Steinmetz et al. Citation2023; Vos et al. Citation2020).
Many NCDs share common risk factors, such as obesity, socioeconomic inequality, unhealthy lifestyle, older age and low health literacy (Vos et al. Citation2020). They are also often found to be comorbid with one another; a pattern which has long been seen in high-income countries (HICs). Comorbidity between NCDS and musculoskeletal (MSK) pain conditions such as osteoarthritis and LBP is also likely increasing in LMICs because of lifestyle changes and population ageing (Blyth et al. Citation2019). Similar to HICs, emerging evidence increasingly suggests increased consumption of unhealthy fast food, less physical activity due to growing prevalence of sedentary activities as a result of increased use of computers (relating to employments), televisions and/smart-phones (for social relaxation) in LMICs (NCD Citation2022). Other common factors changing NCDs and comorbidity profile in LMICs include social desirability and increased use of transportation systems designed to minimise physical energy use, increased alcohol misuse and stress under prevailing harsh economic conditions. The projection is such that the economic burden of NCDS in LMICs may reach up to US$ 7 trillion by 2025 (Vos et al. Citation2020). Many LMICs are not well-equipped to deal with this increasing burden of NCDs, which is expected to continue to rise in the coming years. The potential disability, reduced quality of life, decreased ability to work and consequent socio-economic impact of living with chronic MSK pain and NCDs in these settings are huge and can impact achievement of Sustainable Development Goals (Blyth et al. Citation2019; Briggs et al. Citation2018).
Policy changes to drive healthcare progress are difficult to plan, initiate or implement without robust and contextual epidemiological data from LMICs. There is a knowledge gap on the pooled prevalence and pattern of multimorbidity in LMICs (Arokiasamy et al. Citation2015; Xu, Mishra, and Jones Citation2017). This is as most research into prevalence, management and health policy requirements for NCDs has taken place in HICs, and only 5% of global research into multimorbidity originates or take account of data from LMICs (Catalá-López et al. Citation2018; Xu, Mishra, and Jones Citation2017). Current projections about the burden of NCDS in LMICs are thus mostly extrapolated from countries with higher volumes of relevant research (Blyth et al. Citation2019; Briggs et al. Citation2018; Cieza et al. Citation2020; Hay et al. Citation2017). Not accounted for in these projections are factors such as the rapid epidemiological transition in many LMICs relative to more stable dynamics in HICs. Pooled data from studies conducted in LMICs could generate more precise estimates of prevalence of MSK pain, specific patterns, and effects of comorbid NCDs in LMICs. An understanding of a more precise estimate of the MSK pain-NCDs comorbidity profile in LMICs is therefore imperative in order to strengthen the evidence base for future policy reforms as well as drive better policy integration of MSK into NCDs plans (Blyth et al. Citation2019). To the best of our knowledge, no previous systematic review has investigated the prevalence and pattern of NCDS and comorbid MSK pain (specifically osteoarthritis and LBP) in LMICs. This study therefore aimed to summarise and appraise currently available evidence regarding the prevalence and pattern of NCDs comorbid with MSK pain due to osteoarthritis or LBP among adults in LMICs.
Methods
Design: Systematic review and meta-analysis
Research Question: In adults living with MSK pain due to osteoarthritis or LBP in LMICs, what is the current pattern and prevalence of comorbid NCDs such as diabetes, cardiovascular diseases, respiratory diseases and mental illness – anxiety/depression?
Protocol and registration
An a priori protocol was developed and registered with PROSPERO, an international systematic review register (CRD42019134690). This systematic review has been reported according to the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) (Page et al. Citation2021).
Eligibility criteria
Epidemiological studies (cross-sectional, cohort and case-control) of adults ≥18 years of age with clinically confirmed LBP or osteoarthritis of the knee or hip were considered for inclusion. For the purpose of this review, focus was on chronic MSK pain due to low back pain, and/or osteoarthritis (at any peripheral joint) due to substantial evidence that these are the most prevalent MSK pain conditions globally (Hay et al. Citation2017). However, where available, evidence was reviewed for other MSK pain conditions including chronic widespread pain. This review was limited to populations residing in LMICs as classified by the world bank (2013–2017). LMICs were defined as countries with a gross national income per capita of <US$1006 (low income) or between US$1006 and US$3995 (lower-middle income) (World Bank Citation2020). Further criteria for inclusion consist of report of at least one comorbid NCDs specifically: diabetes, cardiovascular diseases, respiratory diseases, and mental illness – anxiety/depression alongside MSK pain. Studies focusing only on specific population subgroups (specifically, cancers) were excluded from this review, due to differences in management, and care pathways for cancer-related comorbidities in LMICs. Detailed eligibility criteria are presented in .
Table 1. Eligibility Criteria.
Information sources and search strategy
A comprehensive search strategy including terms related to musculoskeletal pain, comorbidities and developing/LMIC countries was developed with the help of an information specialist (see appendix 1). The search identified publications from the following databases from their inception until May 2021: MEDLINE, Embase and AMED, CINAHLPlus, PsychINFO, Web of Science, Global Health Database and Global Index Medicus. Hand searching through references of relevant studies was also conducted for grey literature, but no grey literature-specific search engines were used. No restrictions were put in place regarding language of publication or research date.
Study selection
Two researchers independently assessed the eligibility of the primary studies through title and abstract screening using Covidence (systematic review software:www.covidence.org) and pre-determined eligibility criteria, blinded to one another’s decisions. Full-text review was conducted following this, in accordance with the same criteria. Conflicts regarding eligibility were resolved through discussion between paired researchers, or by an adjudicating third reviewer.
Data collection process and data items
The publications fully satisfying pre-defined criteria were subjected to data extraction and quality appraisal. A data collection proforma designed and piloted prior to extraction included country and region of study, study setting, study design, sampling strategy, sample size, study population demographics (mean age, BMI, gender proportion, occupational status). Data extracted included raw participant estimates with and without NCDs comorbid with MSK and controls where available. Information on the prevalence (as a percentage) or risk (as an odds ratio – OR) of NCDs comorbid with MSK pain was also extracted and tabulated. Data extraction was performed independently by two researchers before cross-referencing and discussing disagreements where applicable.
Risk of bias within studies
Two independent reviewers assessed methodological quality of eligible full texts based on the risk of bias tool by Hoy et al. (Citation2012). Reviewers were blinded to each other’s assessment until independent reviewers were completed. Disagreements were resolved with discussion.
Summary measures and data analyses
A random effects meta-analysis was conducted due to anticipated variations in the study design, participants and methodologies (Egger, Davey Smith, and Phillips Citation1997). Where given, period prevalence was reported. Absolute numbers were also used to calculate proportion/percentages of people with MSK and comorbid NCD where required. Point prevalence (proportion of the study sample having co-morbid NCDs) were used in statistical pooling. Pooled prevalence estimates were reported with 95% confidence intervals (CI). Heterogeneity was assessed by inspection of forest plots and calculation of I2 with a score of > 60% indicating high heterogeneity. Meta-analysis was performed for data involving MSK pain overall and any comorbid NCDS. Subgroup analyses on prevalence of comorbid NCDS with hip/knee OA and LBP were presented including consideration for relative contributions of data from studies with low, moderate, and high risk of bias as a form of sensitivity analysis. Analyses were conducted using MetaXL using double arcsine transformation variant for meta-analysis of prevalence (Barendregt et al. Citation2013).
Results
Characteristics of included studies
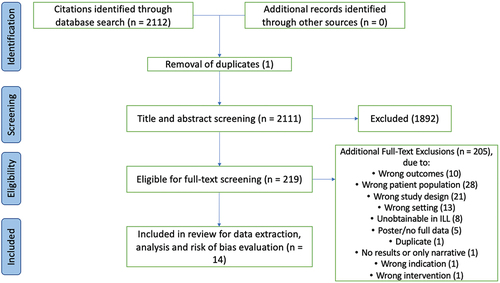
Comprehensive search yielded 2112 citations. Subsequent title and abstract screening resulted in 219 potentially eligible full texts (). Of these, 14 studies presenting data on comorbid NCDs across 6 United Nations geographical subregions (8 studies with participants in Africa and 6 in Asia) with MSK pain from 7 LMICs (i.e. Egypt, Ethiopia, India, Indonesia, India, Kenya, Nigeria and Pakistan) were included in this review. Primary studies data were collected between 2003 and 2018. On average, participants were aged 46.9 years (range: 20-69 years) (Aboderin and Nanyonjo Citation2017; Aggarwal et al. Citation2013). The majority (>50%) were female. Only eight studies recorded Body Mass Index (BMI). Where recorded, participants were typically overweight (mean BMI > 25kgm−2 in studies) or obese (BMI >30 in 3 studies). Employment status across most studies (8/14) was not reported, but of the 6 studies where information was provided, an average 63.9% of participants were employed. Detailed characteristics of included studies are presented in .
Table 2. Characteristics of included studies.
Methodological quality appraisal
Detailed risk of bias assessment is summarised in . Of the 14 included studies, seven (Aboderin and Nanyonjo Citation2017; Adebusoye, Ogunbode, and Alonge Citation2013; Aggarwal et al. Citation2013; Akintayo et al. Citation2019; El-; Sagheer, Khan, and Sharif Citation2013; El-Sayed et al. Citation2010; Yerima and Adelowo Citation2017) were assessed as having low risk of bias (having satisfied > 50% of criteria based on the Hoy et al. (Citation2012) risk of bias tool). Although most studies reported adequate case definitions (85.7%), methods used to define and diagnose low back pain and hip/knee OA often lacked clear validity. Only four studies (Abdel-Nasser et al. Citation1998; Adebusoye, Ogunbode, and Alonge Citation2013; Aggarwal et al. Citation2013) used standardised criteria to diagnose/define comorbid NCDs as this data were largely collected via self-reporting. Seven studies (Abdel-Nasser et al. Citation1998; Iqbal et al. Citation2011; Kusumaningtyas, Tamtomo, and Murti Citation2018; Mahmoud et al. Citation2019; Narayan, Thabah, and Poduval Citation2017; Omoke and Amaraegbulam Citation2016; Shah, Kataria, and Joshi Citation2011) were assessed as at high risk of bias. Two most common reasons for classifying studies as at high risk of bias included an absence of random sampling techniques (n = 10) and samples not closely representing wider populations (: study population and eligibility criteria notes).
Table 3. Risk of bias assessment.
Prevalence of NCDs (hypertension, diabetes mellitus and anxiety/depression) with MSK pain
Among adults in LMICs, a wide range of NCDs were reported to be comorbid with MSK pain: diabetes (number of studies (n) = 8), hypertension (n = 7), mental health conditions (including anxiety and depression, n = 5), obesity (n = 8), peptic ulcer (n = 3), toxic goitre/thyroid disease (n = 2), coronary artery disease (n = 3), chronic obstructive pulmonary disease/bronchial asthma (n = 3), and metabolic syndrome (n = 1). For this review, analysis was focused on the three most common NCDs reported, i.e. hypertension, diabetes and mental health conditions including anxiety and depression. Studies predominantly reported point prevalence (i.e. in absolute numbers or percentages) of comorbid NCDs among adult patients with MSK pain. A summary of results and forest plots of meta-analysis is presented in .
Figure 2a. Forest plot: Overall MSK and NCD comorbidities with indications of overall risk of bias per study.
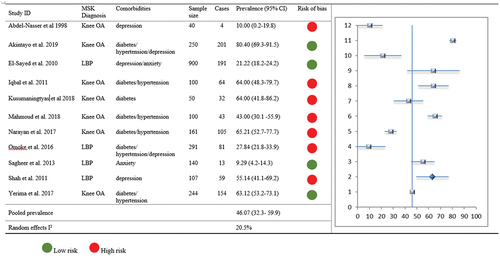
Figure 3. Forest Plot: MSK prevalence with comorbid Hypertension with indications of overall risk of bias per study.
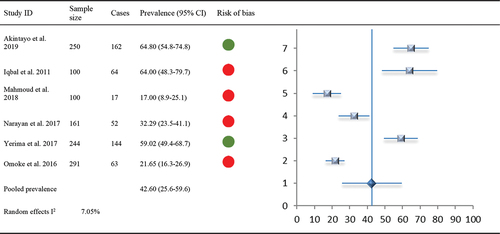
Figure 4. Forest Plot: MSK prevalence with comorbid Diabetes with indications of overall risk of bias per study.
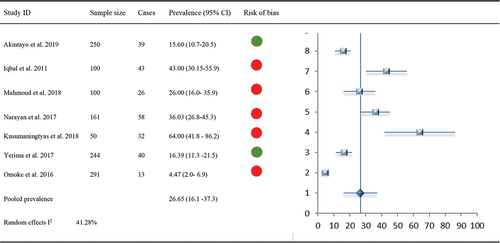
Figure 5. Forest Plot: MSK prevalence with comorbid Depression and Anxiety with indications of overall risk of bias per study.
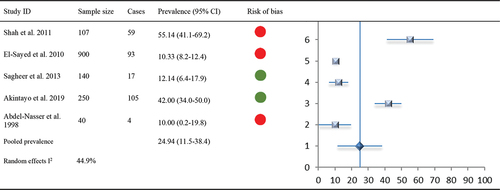
Figure 6. Forest Plot: Subgroup analysis of comorbid NCDs with specific MSK Pain (i.e. hip/knee OA and LBP) with indications of overall risk of bias per study.
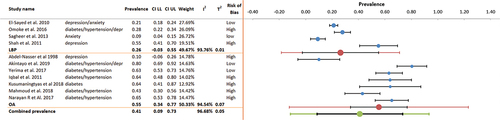
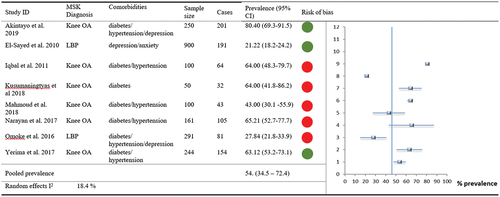
For any of the three most common NCDs (i.e. hypertension, diabetes and mental health conditions) comorbid with MSK pain, 11 of 14 included studies (n = 2383) provided suitable data for meta-analysis. Overall prevalence of any one NCD comorbid with MSK pain () was 46.07% (95% CI 32.3–59.9, I2 20.5%). Furthermore, eight studies reported MSK pain comorbid with at least two or more NCDs in over half of their participants - . Pooled prevalence was 53.5% (95% CI 34.5–72.4, I2 18.4%).
Comorbid hypertension with MSK pain was reported in seven studies (n = 2007), with prevalence ranging from 17.0%-64.8%. The range was 17.0–64.80% amongst African populations and 32.3–64.0% amongst Asian populations. Of the seven studies, two were low risk of bias and four were at high risk of bias. One study (Aboderin and Nanyonjo Citation2017) reported the risk of hypertension in this population as an unadjusted OR but was not included in meta-analysis. Pooled prevalence of comorbid hypertension with MSK pain (6 studies, n = 1146) was 42.6% (95% CI 25.6–59.6, I2 7.1%).
Comorbid diabetes with any MSK pain was reported in eight studies (n = 2057). Of these, two were low risk of bias and five were high risk of bias. Prevalence ranged from 4.5%-64%; 36.0–64.0% (mixed point and period prevalence) amongst Asian populations, and 15.6–26.0% (period prevalence only) amongst African populations. One study reporting data as an unadjusted OR could not be included in the meta-analysis. Overall pooled prevalence of comorbid diabetes with MSK pain (7 studies, n = 1196) was 26.65% (95% CI 16.1–37.3, I2 41.3%).
Five studies (n = 1437) reported prevalence of comorbid anxiety/depression amongst patients with MSK pain (range: 10%-55.1%). Prevalence of comorbid anxiety/depression was higher amongst African populations. Of these, three had low risk of bias and two had high risk of bias. Overall prevalence of anxiety/depression amongst patients with MSK pain was 24.94% (95% CI 11.5–38.4, I2 44.9%).
Overall, compared to LBP at 26% prevalence (CI −0.03 to 0.55, I2 0.94%), studies consistently report a pattern of higher prevalence of comorbid NCDs 55% (CI 0.34–0.77, I2 95%) with knee OA (). Further subgroup analysis of comorbid NCDs based on specific MSK pain (hip/knee OA and LBP) is subsequently presented:
Comorbid NCDs with hip/knee OA
The prevalence of hypertension among patients with OA was reported in six studies (n = 1258). Two of these studies had a low risk of bias, and three had a high risk of bias. Five of the studies reported outcomes as percentage prevalence (range: 7.0% to 64.8%), with one reporting an unadjusted OR 2.8 (95% CI 1.7–4.6). Overall pooled prevalence of comorbid hypertension with hip/knee OA (5 studies, n = 855) was 46.9% (95% CI 29.1–64.2).
A total of seven studies (n = 1308) reported the prevalence of comorbid diabetes among patients with hip/knee OA. One study (Abdel-Nasser et al. Citation1998) reported unadjusted OR 1.4 (95% CI 0.7–2.7). For a total of 905 patients, six studies reported prevalence estimates in percentages (range: 15.60% to 64%) and were included in the meta-analysis. Pooled prevalence of comorbid diabetes with OA was 31.6% (95% CI 19.6–45.0).
Comorbidity of hip/knee OA with anxiety/depression was reported in two studies (n = 290) (Abdel-Nasser et al. Citation1998; Akintayo et al. Citation2019), with data presented as percentage prevalence (10%, 42%). A meta-analysis was not conducted due to the limited number of studies.
Comorbid NCDs with back pain
Only two studies (n = 649) reported prevalence of hypertension among patients with LBP: one as a percentage prevalence of 21.7% (28) and the other as OR 2 (95% CI 1.2–2.3) (Aboderin and Nanyonjo Citation2017). Similar to hypertension, comorbid prevalence of diabetes with LBP was reported by only two studies (n = 649) as a percentage prevalence of 4.5% and OR 1.2 (95% CI 0.6–2.5). These two studies (Aboderin and Nanyonjo Citation2017; Omoke and Amaraegbulam Citation2016) were accessed as at low and high risk of bias, respectively. Pooled prevalence of comorbid hypertension/diabetes with LBP was not estimated due to limited data.
Comorbid LBP with anxiety/depression was reported by four studies (n = 1307). Three of these were assessed as having low risk of bias (Aboderin and Nanyonjo Citation2017; El-Sayed et al. Citation2010; Sagheer, Khan, and Sharif Citation2013) and one as high risk of bias (Shah, Kataria, and Joshi Citation2011). Three studies reported prevalence estimates as a percentage (range: 10.3%-55.1%), while the fourth (Aboderin and Nanyonjo Citation2017) reported the risk of depression in patients with LBP OR 1.1 (95% CI 0.89–1.25). Based on three studies (n = 1147), pooled prevalence of anxiety/depression among patients with LBP in LMIC was estimated at 21.4% (95% CI 0.02–49.8).
Discussion
This systematic review aimed to appraise and synthesise current evidence on the prevalence of comorbid NCDs with MSK pain among adults in LMICs. Findings demonstrates a high prevalence of comorbid NCDs in over half of patients living with MSK pain in LMICs. Hypertension was found to have the highest comorbid prevalence (42.6%, 95% CI 25.6–59.6) with MSK conditions followed by diabetes (26.65%, 95% CI 16.1–37.3) and common mental health condition including anxiety and depression (24.94%, 95% CI 11.5–38.4).
Our results echo previously published findings of high prevalence of comorbid NCDs in LMICs (Abebe et al. Citation2020). According to our findings, up to two-thirds of adult patients consulting with MSK pain specifically LBP and hip/knee OA will also present with one or two other NCDS in LMIC settings. The pooled prevalence of hypertension amongst patients with hip/knee OA in this study at 46.9% is notably higher than the global prevalence estimated as 31.1% in 2010 (Mills, Stefanescu, and He Citation2020). This is an important finding and suggests that patients with OA in LMICs bear a greater burden of cardiovascular disease than the general population. Similarly, the pooled prevalence of diabetes mellitus (31.6%) among patients with hip/knee osteoarthritis found in this systematic review is notably higher than the global prevalence of diabetes mellitus, which was estimated at 9.3% in 2019 (Saeedi et al. Citation2019). The increased prevalence of cardiovascular health conditions and diabetes amongst patients with chronic MSK pain could be explained by shared risk factors amongst chronic MSK and cardiovascular conditions, such as obesity, nutritional (diet)/lifestyle and sociocultural factors germane to LMICs as well as possible indirect causative relationship between chronic MSK pain and cardiovascular disease. In addition, an interplay of polypharmacy from multiple help seeking (e.g. traditional and orthodox care) and self-medications could also affect comorbidity patterns, but these would all be subject to further research.
We observed a general dearth of data regarding comorbid anxiety/depression with MSK pain among adults in LMICs, but our current finding of a 24.94% prevalence of anxiety/depression among adults with MSK pain from five studies involving 1437 participants is of high clinical relevance. This is as research in HICs has shown that anxiety and depression strongly predict outcome of care for MSK patients (ARMA Citation2018; Ogbeivor and Elsabbagh Citation2021). Reporting of mental health issues for patients with MSK pain in LMICs may be susceptible to several interrelated factors including the way health services are organised, awareness and cultural belief systems (Patel Citation2007). It is likely on an individual level that such conditions are underdiagnosed and therefore underreported due to reasons such as ongoing stigma and cultural awareness around mental health. Health professionals have an important role to play given that awareness and understanding of mental health problems co-morbid with physical health conditions are variable. Cultural/clinical minimisation of the impact of MSK pain on mental health of patients is a challenging issue that can easily prevent mental health diagnosis. Therefore, it would appear that further identification and treatment of common mental disorders such as anxiety and depression as part of overall care for MSK pain is policy imperative in LMICs.
This, as far as we are aware of, is the first systematic review and meta-analysis to specifically assess the prevalence and pattern of comorbid NCDs among patients with MSK pain in LMICs. In this study, we have made effort to conduct a robust review of available evidence ensuring alignment with established guidelines including a comprehensive search strategy in bibliographic databases, with additional hand searching of reference lists, and independent study selection by paired reviewers, which enhances the robustness of this review. However, we acknowledge that there are many other sources of MSK pain, including inflammatory arthritis, connective tissue disorders and chronic neck pain which were not specifically searched for in order to make the present review manageable. Our citation tracking of included studies and seminal articles in this field was designed to mitigate this, and we therefore think it is unlikely that important papers have been missed.
A major limitation is the small number of studies contributing to the evidence base in this systematic review and meta-analyses. Identified studies were also found to be limited in methodological rigor () and number of LMICs (n = 7) represented. The evidence base for this review of comorbid NCDs with MSK pain in LMICs was limited to three United Nations geographical regions (South East Asia, North Africa and Middle East, and Sub-Saharan Africa) but from seven countries only. Findings indicate high prevalence across these regions but studies from Africa reported higher prevalence estimates. Future studies need to explore further the reasons associated with this high prevalence as it has implications for health systems planning and socio-economic output. Limitations in methodological rigor and highlighted gaps in evidence are a direct reflection of research activities, priorities of global health research funders and research investment in NCDs in LMICs. On the other hand, a quick search of the Medline database on these subject yields potentially eligible epidemiological studies in hundreds from HICS. There is therefore an urgent need for capacity building regarding epidemiology of chronic MSK pain as well as comorbid NCDs in LMICs.
Furthermore, all included studies were cross-sectional in design and reported on point prevalence outcomes and often a narrow selection of population samples. We acknowledge the potential effects of these limitations on the pooled prevalence estimates of MSK pain and comorbid NCDs presented in this review. Statistically, moderate heterogeneity (I2 up to 44.9%, ) which was found across our analyses may be due largely to small sample sizes, variable population groups, definitions and diagnosis of NCDs. Furthermore, since many of the included studies were conducted in hospital settings or used unstandardised health care records data, it is highly plausible that accurate prevalence of NCDs comorbid with MSK pain in community settings is yet to be captured within the current evidence. It is therefore likely that real-world prevalence of comorbid NCDs and MSK pain in LMICs is yet unknown.
A similarly important question is the prevalence of comorbid NCDs and MSK conditions amongst younger populations living in LMICS. This is, however, outside the scope of this present review), population fell outside of the scope of this review due to significant differences in pathology, prognosis and management of children with MSK conditions and NCDs in LMICs. As such, any synthesis of younger populations would require separate analysis and interpretation in view of multiple other factors. It is essential that a further independent systematic review is undertaken to better understand the prevalence of comorbid NCDs amongst young patients with MSK conditions.
We do not draw conclusions regarding causal relationship between MSK pain and comorbid NCDs based on this review. However, the impact of living with chronic MSK pain on a patient’s lifestyle (e.g. reduction in exercise/physical activity avoidance because of pain, sedentary lifestyle and consequent weight gain – all contributing factors to the development of cardiovascular disease and other NCDs) has been suggested as possible mechanism for increasing prevalence of MSK pain co-morbidity with NCDs (Nuesch et al. Citation2011; Van der Zee‐Neuen et al. Citation2016; Williams et al. Citation2018). Clinician and patient awareness of this association may benefit from increased monitoring of risk factors, health education/promotion and prevention of worsened outcomes as part of routine MSK care in LMICs. Similarly, patients undergoing investigation and management of NCDs in LMICs would likely benefit from assessment and optimisation of any MSK problems which may be co-morbid or contributing to their presentation. Integrating MSK assessment into routine care of NCDs would likely pick up a significant number of MSK diagnoses which may otherwise be missed. This would allow for targeted management of any such MSK diagnoses, optimizing functional ability to facilitate lifestyle changes which are beneficial for a wide range of NCDs.
Across many healthcare settings in LMICs, care may often be siloed in professional groups, orientated toward acute problems and the urgent needs of patients. However, this systematic review highlights a high prevalence of comorbid NCDs which means that adult MSK patients in LMICs are now presenting with complex health care needs. Not addressing complex needs of MSK patients with chronic NCDs may perpetuate a vicious cycle of increase healthcare utilisation, poor socio-economic outcomes and mortality. Given limited/scarce resources, effective care for this population group therefore requires a paradigm shift towards a dynamic MSK service that can manage diverse patient needs using a whole person-centred approach rather than in siloed patterns. This may help to alleviate the burden associated with MSK pain and improve health outcomes for patients in LMICs. This also raises questions regarding unmet research and training needs for MSK in LMICs (Arokiasamy et al. Citation2015; Blyth et al. Citation2019; Briggs et al. Citation2018). On the other hand, health systems strengthening efforts in LMICs can also be targeted towards NCDs, with consideration for MSK health in patients with hypertension, diabetes and mental health problems (Briggs et al. Citation2019, Citation2023). On this wise, extending service models for NCDs to better integrate MSK health will be sustainable and more person centred. We call on government, research institutions, funding bodies and professional organisations to prioritise efforts to help to improve quality of musculoskeletal care and research in LMICs. This has socio-economic impact and is key to the achievement of the sustainable development goals.
Conclusions
Findings from this systematic review evidence a high prevalence of comorbid NCDs including hypertension, diabetes, anxiety and depression among adult patients with MSK pain in LMICs. It highlights the co-existence and complex interaction between MSK pain and other comorbid NCDs previously documented in HICs in LMICs at a higher scale. To improve outcomes of care for MSK patients in LMICs and prevent a vicious cycle of increasing mortality and worsened socioeconomic outcomes, identification, assessment and addressing comorbid NCDS in MSK patients is an urgent policy imperative for health systems in LMICs. Further research and infrastructures such as integrated primary healthcare database including NCDs and increased awareness among clinicians could aid accurate assessment of the scale of this problem, adequate healthcare plan, person-centred care and improved health outcomes of MSK patients with comorbid NCDs in LMICs.
Availability of data and materials
All data generated or analysed during this study are included in this published article [and its supplementary information files].
Author contributions
OOB conceived the study concepts and design, OOB, ST, and JC drafted the study protocol with contributions from OOO,AO,BS, and FF. OOB, ST, JC led data accrual, and initial analysis. All authors contributed to data synthesis and initial interpretation of findings. OOB, ST, and JC, drafted initial manuscript, with significant contributions from MA, AO, BS, and FF.
Acknowledgements
Thanks to Dr xx for helping with the search strategy.
Disclosure statement
No potential conflict of interest was reported by the author(s).
References
- Abdel-Nasser, A. M., S. Abd El-Azim, E. Taal, S. A. El-Badawy, J. J. Rasker, and H. A. Valkenburg. 1998. “Depression and Depressive Symptoms in Rheumatoid Arthritis Patients: An Analysis of Their Occurrence and Determinants.” British Journal of Rheumatology 37 (4): 391–397. https://doi.org/10.1093/rheumatology/37.4.391.
- Abebe, F., M. Schneider, B. Asrat, and F. Ambaw. 2020. “Multimorbidity of Chronic Non-Communicable Diseases in Low- and Middle-Income Countries: A Scoping Review.” Journal of Comorbidity 10:2235042X2096191. https://doi.org/10.1177/2235042X20961919.
- Aboderin, I., and A. Nanyonjo. 2017. “Musculoskeletal Health Conditions Among Older Populations in Urban Slums in Sub-Saharan Africa.” Best Practice & Research Clinical Rheumatology 31 (2): 115–128. https://doi.org/10.1016/j.berh.2017.11.001.
- Adebusoye, L. A., A. M. Ogunbode, and T. O. Alonge. 2013. “Magnitude of Knee Osteoarthritis and Associated Risk Factors Among Adult Patients Presenting in a Family Practice Clinic in Nigeria.” Journal of Medicine in the Tropics 15 (2): 144. https://doi.org/10.4103/2276-7096.123607.
- Aggarwal, N., T. Anand, J. Kishore, and G. K. Ingle. 2013. “Low Back Pain and Associated Risk Factors Among Undergraduate Students of a Medical College in Delhi.” Education for Health 26 (2): 103. https://doi.org/10.4103/1357-6283.120702.
- Akintayo, R. O., A. Yerima, H. B. Olaosebikan, C. Uhunmwangho, and A. A. Akpabio. 2019. “How Much Gloom Is in Groans? Depression and Its Determinants in Nigerian Patients with Knee Osteoarthritis: A Multi-Center Cross-Sectional Study.” Clinical rheumatology 38 (7): 1971–1978. https://doi.org/10.1007/s10067-019-04497-2.
- Arokiasamy, P., U. Uttamacharya, K. Jain, R. B. Biritwum, A. E. Yawson, F. Wu, Y. Guo, et al. 2015. “The Impact of Multimorbidity on Adult Physical and Mental Health in Low-And Middle-Income Countries: What Does the Study on Global Ageing and Adult Health (SAGE) Reveal?” BMC Medicine 13 (1): 1–6. https://doi.org/10.1186/s12916-015-0402-8.
- Arthritis and Musculoskeletal Alliance (ARMA): Musculoskeletal and Mental Health. 2018. “Policy Position Paper.” http://arma.uk.net/wp-content/uploads/2018/11/MSK-and-Mental-Health-Nov2018.pdf.
- Barendregt, J. J., S. A. Doi, Y. Y. Lee, R. E. Norman, and T. Vos. 2013. “Meta-Analysis of Prevalence.” Journal of Epidemiology & Community Health 67 (11): 974–978. https://doi.org/10.1136/jech-2013-203104.
- Blyth, F. M., A. M. Briggs, C. H. Schneider, D. G. Hoy, and L. M. March. 2019. “The Global Burden of Musculoskeletal Pain—Where to from Here?” American Journal of Public Health 109 (1): 35–40. https://doi.org/10.2105/AJPH.2018.304747.
- Briggs, A. M., J. E. Jordan, S. Sharma, J. J. Young, J. Chua, H. E. Foster, S. A. Haq, et al. 2023. “Context and Priorities for Health Systems Strengthening for Pain and Disability in Low- and Middle-Income Countries: A Secondary Qualitative Study and Content Analysis of Health Policies.” Health Policy and Planning 38 (2): 129–149. https://doi.org/10.1093/heapol/czac061.
- Briggs, A. M., J. G. Persaud, M. L. Deverell, S. Bunzli, B. Tampin, Y. Sumi, O. Amundsen, et al. 2019. “Integrated Prevention and Management of Non-Communicable Diseases, Including Musculoskeletal Health: A Systematic Policy Analysis Among OECD Countries.” BMJ Global Health 4 (5): e001806. https://doi.org/10.1136/bmjgh-2019-001806.
- Briggs, A. M., A. D. Woolf, K. Dreinhöfer, N. Homb, D. G. Hoy, D. Kopansky-Giles, K. Akesson, and L. March. 2018. “Reducing the Global Burden of Musculoskeletal Conditions.” Bulletin of the World Health Organization 96 (5): 366–368. https://doi.org/10.2471/BLT.17.204891.
- Catalá-López, F., A. Alonso-Arroyo, M. J. Page, B. Hutton, R. Tabarés-Seisdedos, and R. Aleixandre-Benavent. 2018. “Mapping of Global Scientific Research in Comorbidity and Multimorbidity: A Cross-Sectional Analysis.” PLOS 13 (1): e0189091. https://doi.org/10.1371/journal.pone.0189091.
- Cieza, A., K. Causey, K. Kamenov, S. W. Hanson, S. Chatterji, and T. Vos. 2020. “Global Estimates of the Need for Rehabilitation Based on the Global Burden of Disease Study 2019: A Systematic Analysis for the Global Burden of Disease Study 2019.” Lancet 396 (10267): 2006–2017. https://doi.org/10.1016/s0140-6736(20)32340-0.
- Egger, M., G. Davey Smith, and A. N. Phillips. 1997. “Meta-Analysis: Principles and Procedures.” BMJ 315 (7121): 1533–1537. https://doi.org/10.1136/bmj.315.7121.1533.
- El-Sayed, A. M., C. Hadley, F. Tessema, A. Tegegn, J. A. Cowan Jr, and S. Galea. 2010. “Back and Neck Pain and Psychopathology in Rural Sub-Saharan Africa: Evidence from the Gilgel Gibe Growth and Development Study, Ethiopia.” Spine 35 (6): 684. https://doi.org/10.1097/brs.0b013e3181b4926e.
- Ferreira, M. L., K. de Luca, L. M. Haile, J. D. Steinmetz, G. T. Culbreth, M. Cross, J. A. Kopec, et al. 2023. “Global, Regional, and National Burden of Low Back Pain, 1990–2020, Its Attributable Risk Factors, and Projections to 2050: A Systematic Analysis of the Global Burden of Disease Study 2021.” The Lancet Rheumatology 5 (6): e316–e329. https://doi.org/10.1016/S2665-9913(23)00098-X.
- Hay, S. I., A. A. Abajobir, K. H. Abate, C. Abbafati, K. M. Abbas, F. Abd-Allah, R. S. Abdulkader, A. M. Abdulle, T. A. Abebo, S. F. Abera, and V. Aboyans. 2017. “Global, Regional, and National Disability-Adjusted Life-Years (DALYs) for 333 Diseases and Injuries and Healthy Life Expectancy (HALE) for 195 Countries and Territories, 1990–2016: A Systematic Analysis for the Global Burden of Disease Study 2016.” Lancet 390 (10100): 1260–1344. https://doi.org/10.1016/S0140-6736(17)32130-X.
- Hay, S. I., A. A. Abajobir, K. H. Abate, C. Abbafati, K. M. Abbas, F. Abd-Allah, R. S. Abdulkader, et al. 2017. “GBD 2016 DALYs and HALE Collaborators. Global, Regional, and National Disability-Adjusted Life-Years (DALYs) for 333 Diseases and Injuries and Healthy Life Expectancy (HALE) for 195 Countries and Territories, 1990-2016: A Systematic Analysis for the Global Burden of Disease Study 2016.” Lancet 390 (10100): 1260–1344. https://doi.org/10.1016/S0140-6736(17)32130-X.
- Hoy, D. G., P. Brooks, A. Woolf, F. Blyth, L. March, C. Bain, P. Baker, E. Smith, and R. Buchbinder. 2012. “Assessing Risk of Bias in Prevalence Studies: Modification of an Existing Tool and Demonstration of Inter-Rater Agreement.” Journal of Clinical Epidemiology 65 (9): 934–939. https://doi.org/10.1016/j.jclinepi.2011.11.014.
- Iqbal, M. N., F. R. Haidri, B. Motiani, and A. Mannan. 2011. “Frequency of Factors Associated with Knee Osteoarthritis.” JPMA-Journal of the Pakistan Medical Association 61 (8): 786.
- Kusumaningtyas, M., D. Tamtomo, and B. Murti. 2018. “Factors Associated with the Occurrence of Osteoarthritis: A Path Analysis Evidence from Surakarta, Central Java.” Journal of Epidemiology & Public Health 4 (1): 9–19. https://doi.org/10.26911/jepublichealth.2019.04.01.02.
- Mahmoud, G. A., A. Moghazy, S. Fathy, and M. H. Niazy. 2019. “Osteoarthritis Knee Hip Quality of Life Questionnaire Assessment in Egyptian Primary Knee Osteoarthritis Patients: Relation to Clinical and Radiographic Parameters.” The Egyptian Rheumatologist 41 (1): 65–69. https://doi.org/10.1016/j.ejr.2018.05.001.
- Mahmoud, G. A., A. Moghazy, S. Fathy, and M. H. Niazy. 2019. “Osteoarthritis Knee Hip Quality of Life Questionnaire Assessment in Egyptian Primary Knee Osteoarthritis Patients: Relation to Clinical and Radiographic Parameters.” The Egyptian Rheumatologist 41 (1): 65–69.
- Mills, K. T., A. Stefanescu, and J. He. 2020. “The Global Epidemiology of Hypertension.” Nature Reviews: Nephrology 16 (4): 223–237. https://doi.org/10.1038/s41581-019-0244-2.
- Narayan, R. V., M. M. Thabah, and M. Poduval. 2017. “Neuropathic Pain Among Patients with Primary Knee Osteoarthritis: Results of a Cross-Sectional Study from a Tertiary Care Center in Southern India.” Indian Journal of Rheumatology 12 (3): 132. https://doi.org/10.4103/injr.injr_90_16.
- NCD Countdown 2030 Collaborators. 2022. “NCD Countdown 2030: Efficient Pathways and Strategic Investments to Accelerate Progress Towards the Sustainable Development Goal Target 3.4 in Low-Income and Middle-Income Countries.” Lancet 399 (10331): 1266–1278. https://doi.org/10.1016/S0140-6736(21)02347-3.
- Nuesch, E., P. Dieppe, S. Reichenbach, S. Williams, S. Iff, and P. Juni. 2011. “All Cause and Disease Specific Mortality in Patients with Knee or Hip Osteoarthritis: Population-Based Cohort Study.” BMJ 342 (mar08 2): d1165. https://doi.org/10.1136/bmj.d1165.
- Ogbeivor, C., and L. Elsabbagh. 2021. “Management Approach Combining Prognostic Screening and Targeted Treatment for Patients with Low Back Pain Compared with Standard Physiotherapy: A Systematic Review & Meta-Analysis.” Musculoskeletal Care 14. https://doi.org/10.18502/jsp.v1i1.9767.
- Omoke, N. I., and P. I. Amaraegbulam. 2016. “Low Back Pain As Seen in Orthopedic Clinics of a Nigerian Teaching Hospital.” Nigerian Journal of Clinical Practice 19 (2): 212–217. https://doi.org/10.4103/1119-3077.175964.
- Page, M. J., J. E. McKenzie, P. M. Bossuyt, I. Boutron, T. C. Hoffmann, C. D. Mulrow, L. Shamseer, et al. 2021. “The PRISMA 2020 Statement: An Updated Guideline for Reporting Systematic Reviews.” PloS Medicine 18 (3): e1003583. https://doi.org/10.1371/journal.pmed.1003583.
- Patel, V. 2007. “Mental Health in Low-And Middle-Income Countries.” British Medical Bulletin 81-82 (1): 81–96. https://doi.org/10.1093/bmb/ldm010.
- Saeedi, P., I. Petersohn, P. Salpea, B. Malanda, S. Karuranga, N. Unwin, S. Colagiuri, et al. 2019. “Global and Regional Diabetes Prevalence Estimates for 2019 and Projections for 2030 and 2045: Results from the International Diabetes Federation Diabetes Atlas.” Diabetes Research & Clinical Practice 157:107843. https://doi.org/10.1016/j.diabres.2019.107843.
- Sagheer, M. A., M. F. Khan, and S. Sharif. 2013. “Association Between Chronic Low Back Pain, Anxiety and Depression in Patients at a Tertiary Care Centre.” JPMA: The Journal of the Pakistan Medical Association 63 (6): 688–690.
- Shah, S. H., L. R. Kataria, and D. Joshi. 2011. “Incidence of Depression in Chronic Low-Back Pain–A Hospital Based Study.” Healthline 2 (2): 35–40. https://doi.org/10.5555/20113327920.
- Steinmetz, J. D., G. T. Culbreth, L. M. Haile, Q. Rafferty, J. Lo, K. G. Fukutaki, J. A. Cruz, et al. 2023. “Global, Regional, and National Burden of Osteoarthritis, 1990–2020 and Projections to 2050: A Systematic Analysis for the Global Burden of Disease Study 2021.” The Lancet Rheumatology 5 (9): e508–e522. https://doi.org/10.1016/s2665-9913(23)00163-7.
- Van der Zee‐Neuen, A., P. Putrik, S. Ramiro, A. Keszei, R. de Bie, A. Chorus, and A. Boonen. 2016. “Impact of Chronic Diseases and Multimorbidity on Health and Health Care Costs: The Additional Role of Musculoskeletal Disorders.” Arthritis Care & Research 68 (12): 1823–1831. https://doi.org/10.1002/acr.22913.
- Vos, T., S. S. Lim, C. Abbafati, K. M. Abbas, M. Abbasi, M. Abbasifard, M. Abbasi-Kangecari, et al. 2020. “Global Burden of 369 Diseases and Injuries in 204 Countries and Territories, 1990–2019: A Systematic Analysis for the Global Burden of Disease Study 2019.” Lancet 396 (10258): 1204–1222. https://doi.org/10.1016/S0140-6736(20)30925-9.
- Williams, A., S. J. Kamper, J. H. Wiggers, K. M. O’Brien, H. Lee, L. Wolfenden, S. L. Yoong, et al. 2018. “Musculoskeletal Conditions May Increase the Risk of Chronic Disease: A Systematic Review and Meta-Analysis of Cohort Studies.” BMC Medicine 16 (1): 1–9. https://doi.org/10.1186/s12916-018-1151-2.
- World Bank. 2020. “World Bank Country and Lending Groups.” Accessed October 19, 2020. https://datahelpdesk.worldbank.org/knowledgebase/articles/906519-world-bank-country-and-lending-groups.
- Xu, X., G. D. Mishra, and M. Jones. 2017. “Mapping the Global Research Landscape and Knowledge Gaps on Multimorbidity: A Bibliometric Study.” Journal of Global Health 7 (1): 010414. https://doi.org/10.7189/jogh.07.010414.
- Yerima, A., and O. Adelowo. 2017. “Knee Osteoarthritis and Associated Cardio-Metabolic Clusters in a Tertiary Hospital in Nigeria.” Clinical rheumatology 36 (11): 2541–2548. https://doi.org/10.1007/s10067-017-3816-1.