?Mathematical formulae have been encoded as MathML and are displayed in this HTML version using MathJax in order to improve their display. Uncheck the box to turn MathJax off. This feature requires Javascript. Click on a formula to zoom.
?Mathematical formulae have been encoded as MathML and are displayed in this HTML version using MathJax in order to improve their display. Uncheck the box to turn MathJax off. This feature requires Javascript. Click on a formula to zoom.ABSTRACT
Background
This study investigated the ability of patients, naïve to adalimumab treatment and self-injection with an autoinjector (AI), to successfully self-administer AVT02, an adalimumab biosimilar, using a custom, ergonomic AI (Alvotech hf., Reykjavik, Iceland).
Research design and methods
This was a single-arm, open-label study, consisting of an 8-week active period and 48-week extension phase. Patients with moderate to severe rheumatoid arthritis (RA) self-administered 40 mg AVT02 subcutaneously via AI in the active period, followed by prefilled syringe in the extension phase. The primary endpoint was the percentage of successful self-injections up to Week 8. Usability and robustness of the AI were evaluated in the active period; safety, efficacy, pharmacokinetic and immunogenicity data were assessed throughout the study.
Results
The AI success rate was 100%. No handling events were noted up to Week 8. Both Ctrough measurements and immunogenicity profile were in line with expectations from previous studies, with no unexpected safety signals.
Conclusions
This study demonstrated that AVT02-AI can be successfully and reliably used for repeated self-injections of AVT02 by moderate to severe RA patients, despite no previous experience of adalimumab self-administration. The extension phase provides long-term efficacy and safety data for AVT02 in RA.
Study identifier
NCT04224194
1. Introduction
Rheumatoid arthritis (RA) is a common autoimmune disease which can lead to significant morbidity in affected patients due to the inflammation and destruction of joints [Citation1], and is also associated with a high economic burden [Citation2]. Treatment approaches for RA usually involve administration of disease-modifying anti-rheumatic drugs (DMARDs), such as methotrexate (MTX), which can be combined with biologics such as Humira® (reference adalimumab), a recombinant, fully humanized anti-tumor necrosis factor-alpha (TNF-α) monoclonal immunoglobulin G1 antibody [Citation3,Citation4]. Reference adalimumab is globally approved for a wide range of inflammatory indications, including RA [Citation3,Citation4]. Inhibitors of TNF-α have also been shown to be potent DMARDs, particularly when combined with MTX [Citation5,Citation6]. As an alternative to reference adalimumab, biosimilars such as AVT02 can be used to effectively treat RA.
AVT02 is a biosimilar to reference adalimumab and is available at a high concentration (100 mg/mL), in a citrate-free formulation. This matches the newest formulation of the reference product which is associated with reduced injection site pain [Citation7], which may also increase treatment adherence [Citation8].
AVT02 is currently approved in the EU, Canada, Switzerland, and the UK, and is under review in other jurisdictions. Reference adalimumab and biosimilars (including AVT02) are typically administered via an autoinjector (AI) or a prefilled syringe (PFS), either by a healthcare professional or they are self-administered. Subcutaneous (s.c.) self-injection of medication has benefits for the patient and healthcare system, but there are barriers including dexterity problems and injection anxiety that can prevent self-injection being used effectively [Citation9].
Usability assessments of injection devices performed in real-world clinical settings can provide the most relevant data regarding patients’ ability to use the product, and the potential clinical impact [Citation10]. Demonstration of usability in the appropriate population(s) is important to assess the potential impact of population-specific characteristics. Patients with RA often experience limitation in grip strength, hand dexterity and pain during precise movement [Citation9], often resulting in injection-related anxiety, making this population highly relevant for such investigation. The current study investigates the capability of patients, naïve to both adalimumab treatment and AI self-injection, to successfully self-administer the Alvotech proprietary AVT02-AI as per the instructions provided. This is a novel AI, utilizing the Ypsomate platform, created to be more convenient for self-administration by those with impaired dexterity. During an 8-week period (active phase), the usability and robustness of the proposed AI was evaluated in patients with moderate to severe RA who self-injected AVT02 s.c. Subsequently, the same cohort of patients was treated with AVT02-PFS over a 48-week period (extension phase); safety, efficacy, pharmacokinetic (PK) and immunogenicity data were assessed throughout the study.
2. Patients and methods
2.1. Study design
This was a single-arm, open-label, AI handling study (NCT04224194), consisting of an 8-week active period and a 48-week extension phase. The active period assessed the performance of the AI. During the optional extension phase, AVT02 was delivered via single-use PFS. Efficacy, safety, PK, and immunogenicity in RA were assessed throughout the study. The study was conducted at 9 sites in the countries of Georgia and Ukraine, in compliance with the ethical principles in the Declaration of Helsinki, International Council for Harmonization of Technical Requirements for Pharmaceuticals for Human Use guidelines, and all applicable local regulatory authorities’ requirements. The study protocol was approved by an Independent Ethics Committee. Written informed consent was obtained from each patient before any study-related procedures were performed.
2.2. Study population
The study population included adult patients (18–80 years) diagnosed with RA for at least four months, currently classified according to the 2010 American College of Rheumatology (ACR)/European League Against Rheumatism classification criteria for RA (score ≥6/10), and with moderate to severe active RA. Moderate to severe active RA was defined as ≥6 swollen (out of 66) and ≥6 tender (out of 68) joint counts, abnormal elevation of C-reactive protein (CRP) (≥6 mg/L) and fulfillment of one of the following two criteria at screening: a) positive rheumatoid factor or b) evidence of one joint erosion on radiological assessment of the hands or wrist of the dominant hand at screening. Patients were not eligible if they had previous experience of self-administering medications using an AI or pen (either marketed or investigational), had prior treatment with more than one biologic or one protein kinase inhibitor DMARD for RA or had previously received adalimumab or a biosimilar of adalimumab.
2.3. Trial procedures
During the active period, patients were trained on the s.c. injection of AVT02 via AI and the disposal of the used syringe. The injection was administered in the abdomen or thigh. The observer assessment tool (OAT) and participant assessment tool (PAT) questionnaires were used to document success or failure of AVT02 injection, the number of handling events, ease of use, and adequacy of the instructions for use of the AI. The OAT and PAT questionnaires are detailed in Supplementary Appendix I. The first (trained) AVT02 40 mg dose was injected on-site on Week 0 (Day 0) by qualified staff. The second dose (trained self-injection) was injected by the patient on-site at Week 2 and observed by qualified staff. On Week 4, Week 6, and Week 8, the patient self-injected the dose on-site, observed by qualified staff (observed self-injection). After Week 8, patients were offered participation in an optional 48-week extension phase (Weeks 9–56) during which AVT02 40 mg was administered via PFS starting at Week 10 until the last dose at Week 54. Patients were offered the option of self-injections at home during the extension phase, while at Weeks 14, 24 and 36, AVT02 administration occurred at the clinic. End of study (EOS) was at Week 56. Quantitation of serum AVT02 concentrations was performed using a validated sandwich assay on 96-well microtiter plates using Meso Scale Discovery electrochemiluminescence technology in a one assay setup as per current guidelines [Citation11,Citation12] and recommendations [Citation13]. The detection of anti-drug antibodies (ADAs) and neutralizing antibodies (NAbs) was performed as a one assay setup following current recommendations [Citation14]. A multi-tiered approach was used consisting of a screening assay, confirmation assay, titration assay and neutralizing assay to evaluate immunogenicity as per the FDA and EMA guidelines [Citation15,Citation16].
2.4. Outcome measures
The primary endpoint was the percentage of successful self-injections as reported in the OAT and PAT questionnaires, up to Week 8. All attempts to operate the AI were observed and documented by both the observer and patient. An attempt was classified as ‘Successful’ if, after performing all steps required to prepare and inject with the AI, the entire dose was expelled into the injection site. An attempt was deemed ‘Unsuccessful’ if the patient was unable to perform at least one of the key steps in the injection procedure, or the patient was unable to expel the dose without any unexpected interruption resulting in a visible stream of drug delivery outside of the injection site. If the OAT and PAT disagreed, then the attempt was considered ‘Unsuccessful.’
AI-related secondary endpoints were: (1) Frequency of any AI handling events during the self-injection process reported in the OAT and PAT up to Week 8. An AI handling event was any of the following that prevented the patient from successfully self-injecting the full contents of the AI: failure to remove the clear needle cap of the autoinjector, failure to pinch skin and position pen over injection site, or failure to push and keep pushing the pen down against the injection site; (2) Patient’s assessment of ease of use and adequacy of the instructions for use; (3) Assessment of AI robustness (defined by number of returned AIs with any signs of damage, malfunctioning or injection incompleteness identified by visual inspection); (4) Percentage of patients achieving ACR −20/-50/-70 response at Weeks 4, 8, 14, 24, 36, and 56; (5) Percentage of patients achieving Simple Disease Activity Index (SDAI) improvement at Weeks 4, 8, 14, 24, 36, and 56 in comparison to baseline; (6) Change from baseline in Disease Activity Score 28 C-reactive protein (DAS28 CRP); and (7) Health Assessment Questionnaire (HAQ) at Weeks 4, 8, 14, 24, 36, and 56. ACR-20/-50/-70 response, SDAI, DAS28 and HAQ are defined in Supplementary Appendix II.
Clinical-related secondary endpoints were: (1) PK, assessed by monitoring serum trough levels of AVT02 at steady state at Week 0 (Day 0; pre-dose), and Weeks 2, 8, 24, 36, and 56 measured from one 4-mL serum sample; (2) Immunogenicity, assessed by monitoring the presence of ADAs and NAbs to AVT02 at Week 0 (Day 0; pre-dose), Weeks 2, 8, 24, 36, and 56, measured from two 4-mL serum samples; and (3) Routine safety parameters, including laboratory safety, vital sign measurements, 12-lead electrocardiogram results, chest X-ray and physical examination findings assessed throughout the study. The frequency, type, and severity of treatment-emergent adverse events (TEAEs) were also assessed.
2.5. Statistical analyses
Data are reported using summary statistics. No formal statistical testing was performed. The percentage of successful self-injections was calculated as follows:
The 95% Wilson score confidence intervals (CI) were displayed for the primary endpoint at Weeks 4, 6, 8 and overall for Weeks 4, 6, and 8 combined. The calculation for robustness is included in Supplementary Appendix III. Analysis populations are described in Supplementary Appendix IV. The primary and secondary endpoint data are presented for the full analysis set. Demographic, baseline characteristics, PK, immunogenicity, and safety data are presented for the safety set. All statistical analyses were performed using SAS v9.4 (SAS Institute, Inc., Cary, North Carolina). Graphics were prepared using the same version of SAS.
3. Results
3.1. Patient disposition and characteristics
Of the 137 patients screened, 107 were enrolled into the study, 106 completed the study, and 1 patient withdrew due to a positive Hepatitis B surface antigen at screening (). All patients who completed the 8-week active period (n = 106) took part in and completed the optional extension phase up to Week 56 (). Patient demographics and baseline characteristics are shown in . The mean ± SD age of the enrolled patients was 54.4 ± 11.1 years, with 85.0% under 65 years. Ninety-six patients (89.7%) were female, and 11 (10.3%) were male. All patients were White, and 105 patients (98.1%) were not Hispanic or Latino. At baseline, the mean ± SD time from RA diagnosis was 125.2 ± 89.7 months, the mean ± SD tender joint count was 19.7 ± 5.6, the mean ± SD swollen joint count was 16.8 ± 5.3, and the mean ± SD DAS28 CRP score was 6.7 ± 0.8. Prior and concomitant medications are shown in . The mean ± SD dose of MTX at baseline was 14.1 ± 1.7 mg/week.
Figure 1. Patient disposition. *One patient was initially enrolled and received a dose of AVT02, and subsequently withdrew due to a positive Hepatitis B surface antigen at screening.
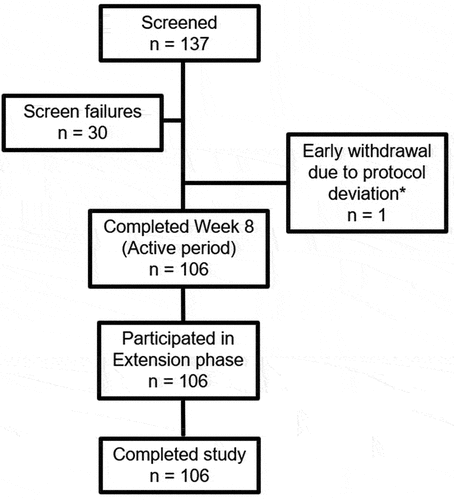
Table 1. Baseline characteristics (Safety population).
3.2. Primary endpoint
The AI success rate was 100% as reported by both the trial site personnel and by patients ().
Table 2. AI performance (Full analysis population).
3.3. Secondary endpoints – AI-related
No handling events were noted with the AI up to Week 8 (Supplementary Table 1). Over 90% of patients (97/107, 91.5%) felt the instructions provided with the AI were ‘very useful’ and most felt the instructions were ‘very clear’ (). Across all time points, approximately 80% of patients found the AI ‘very easy’ to use and, in general, less difficulty was reported as the study progressed (). Over 80.0% (81.1–86.8%) of patients reported ‘no steps were really difficult’ to follow; ‘getting ready’ was the step which caused the most difficulty (4.7–10.4%; ). The first 110 AIs used were inspected for robustness and none showed any sign of damage or malfunction (100.0% [95% CI: 96.6, 100.0]).
Table 3. Ease of use of the AI (Full analysis population).
3.4. Secondary endpoints – efficacy and health-related quality of life
At Week 8, 49.1%, 5.7% and 0.9% patients achieved ACR-20/-50/-70 responses, respectively, and at Week 56 these had increased to 70.8%, 47.2% and 13.2%, respectively (). The mean ± SD SDAI scores decreased consistently across the whole study from 55.0 ± 12.2 at baseline to 26.8 ± 11.4 and 10.2 ± 6.4 at Weeks 8 and 56, respectively (, Supplementary Table 2). Similarly, the mean ± SD DAS28 CRP scores decreased consistently across the whole study from 6.7 ± 0.8 at baseline to 4.6 ± 1.0 and 3.2 ± 0.9 at Weeks 8 and 56, respectively (, Supplementary Table 3). The HAQ scores for all domains consistently decreased across the whole study. The domains with the biggest mean ± SD change from baseline to Week 8 were Eating (−1.0 ± 0.8), Grip (−0.9 ± 0.8), Dressing and Grooming (−0.8 ± 0.7) and Reach (−0.8 ± 0.8), and from baseline to Week 56 were Eating (−1.5 ± 0.8), Grip (−1.3 ± 0.9) and Reach domains (−1.3 ± 0.9) (Supplementary Table 4).
Figure 2. Mean (± SE) of SDAI Score from baseline by visit (Full analysis set).
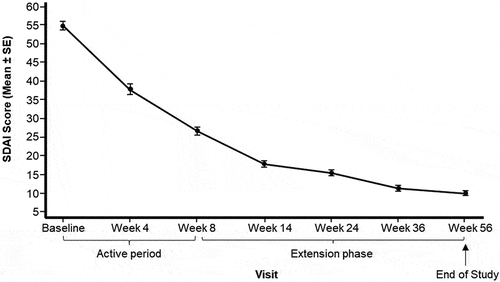
Figure 3. Mean (± SE) of DAS28 CRP from baseline by visit (Full analysis set).
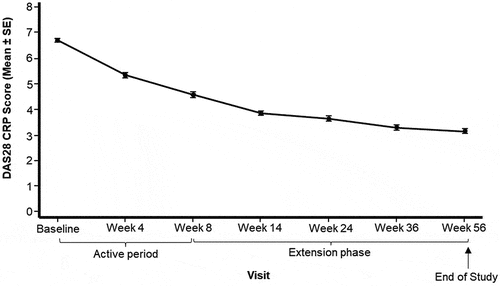
Table 4. ACR-20/-50/-70 response (Full analysis population).
3.5. Secondary endpoints – PK and Immunogenicity
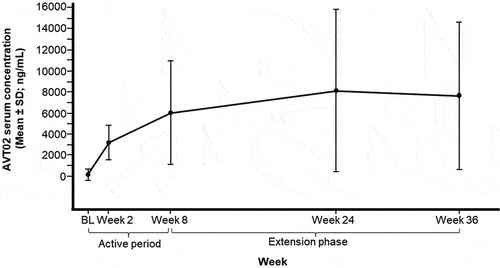
The mean serum concentration of AVT02 increased from 165.3 ng/mL at baseline to 7024.5 ng/mL at Week 56, reaching a peak of 8141.3 ng/mL at Week 24 (, Supplementary Table 5). There was no significant difference in serum concentrations of AVT02 based on injection site (data not shown). The mean ± SD ADA titers were variable at baseline (123.6 ± 481.2), at Week 8 (10,484.0 ± 64,820.2), and at Week 56 (90,813.6 ± 406,374.9) (Supplementary Table 6). The percentage of patients with ADAs increased from 16.8% at baseline to 65.1% at Week 8, and then decreased to 49.1% at Week 56 (Supplementary Table 7). Of ADA positive patients, most were also positive for NAbs at Week 8 (62.3%) increasing to 90.4% at Week 56. Patients who were ADA positive had lower serum concentrations of the study drug compared to those that were ADA negative at Week 8 (mean ± SD: 5345.6 ± 4748.3 vs 8958.5 ± 4642.4 ng/mL) and Week 56 (mean ± SD: 6232.6 ± 5396.0 vs 15,558.9 ± 7305.5 ng/mL). Similarly, patients who were NAb positive had lower serum concentrations of the study drug compared to those that were NAb negative at Week 8 (mean ± SD: 3142.8 ± 3808.0 vs 8602.7 ± 4347.9 ng/mL) and Week 56 (mean ± SD: 5194.8 ± 4998.2 vs 11,882.8 ± 6261.2 ng/mL).
3.6. Safety
During the 8-week active period, mean (range) exposure to AVT02 was 56.6 (55.6–57.7) days, with an average of five injections (one investigator-led and four self-injections). In the extension phase, mean (range) exposure to AVT02 was 308.8 (295.0–335.0) days, with an average of 23 injections, and the mean ± SD cumulative dose administered was 918.1 ± 27.3 mg. Over the whole study period, mean (range) exposure to AVT02 was 376.2 (1.0–404.0) days with an average of 27.7 injections (AI or PFS). The mean ± SD cumulative dose administered was 1107.7 ± 107.7 mg. No local injection site reactions were reported during the study (). TEAEs are shown in . A total of 67 TEAEs were reported by 40 patients (37.4%); five patients (4.7%) each reported one treatment-related TEAE. Overall, most TEAEs were mild in severity; two patients (one in the active phase and one in the extension phase) reported severe TEAEs (one patient reported headache and one patient reported increased hepatic enzyme). Three serious TEAEs were reported by two patients (1.9%); one patient reported cervical vertebral fracture and wound dehiscence, and one patient reported increased hepatic enzyme, which was considered to be treatment-related by the investigator. TEAEs of special interest were reported by five patients (4.7%; one patient reported leukopenia; two patients reported hepatic enzyme increased; and two patients reported mycobacterium tuberculosis complex test positive). There were no deaths.
Table 5. Safety (Safety population).
4. Discussion
Previous studies have demonstrated PK and clinical similarity between AVT02 and reference adalimumab [Citation17,Citation18]. These biological medicines are typically administered by either a PFS or AI device, and it is important to show that these devices have no impact on PK [Citation10,Citation19]. In addition, as RA patients may have limited dexterity due to their underlying condition, it is also important to ensure new devices are convenient and easy to use. In this study we investigated the capability of patients naïve to both adalimumab and self-administration with AI to successfully use the AVT02-AI to self-administer, after receiving appropriate training. While the sample size in the present study was not based on any statistical powering, so that comparison between AI and PFS was not possible, such a comparison has been assessed in another study [Citation20].
Patients with moderate to severe RA were enrolled in this study, which is reflected in the severity of tender joint count, swollen joint count, DAS28 CRP score and MTX dose reported at baseline. The study population was predominantly female (89.7%), as opposed to an approximately two- to three-fold increased frequency in females over males in the real-world situation [Citation21], but gender was not considered a factor in the primary endpoint of this study.
In our real-world study, all patients were able to successfully self-administer AVT02 s.c. using the AI without prior experience of self-injection with AI (100% success rate). RA patients typically found the AI easy to use, with ease of use persisting over time. There were no handling events, and the AI was found to be robust, supporting use in a patient population that exhibits limited dexterity [Citation22,Citation23].
The study population of participants with ‘active moderate to severe rheumatoid arthritis’ was selected as a robust challenge for the AI (mean ± SD tender joint count 19.7 ± 5.6; swollen joint count 16.8 ± 5.3; DAS28 CRP score 6.7 ± 0.8). Furthermore, patients were not eligible if they had previous experience of self-administering medications. Therefore, it was expected that a certain percentage of the study population would still experience difficulties with self-administration, even with the patient-centric design process of the AVT02 AI. While it is accepted that one self-injection device cannot support the needs of all patient groups, increasing family and HCP support can help reduce non-adherence that may be the result of difficulty with self-injection [Citation24]. A multi-factorial approach to avoiding noncompliance may therefore mitigate the difficulties faced by those patients with severely impaired mobility.
The PK parameter, Ctrough (an indicator of drug absorption and a predictor of efficacy), observed for AVT02 was in line with expectations from previous results [Citation17,Citation18]. Efficacy of AVT02 has previously been demonstrated in patients with moderate to severe chronic plaque psoriasis [Citation18], showing highly similar improvement in Psoriasis Area and Severity Index score at Week 16 (91.6%) compared to the reference adalimumab (89.6%) [Citation18]. Additionally, the similarity in efficacy between AVT02 and reference adalimumab was consistent in a subgroup analysis of a subset of these patients with psoriatic arthritis. While these results support the use of AVT02 to treat other indications through extrapolation [Citation25,Citation26], it is nevertheless of interest to assess efficacy in the RA population. The ACR-20, −50, and −70 results suggest a gradual response to treatment and improvement over time, and the SDAI and DAS28 CRP results indicate a reduction in disease severity. These observations are supportive of the use of AVT02 to treat adult patients with moderate to severe RA, with the treatment effect persisting beyond Week 8 and up to Week 56 (EOS). It should be noted however, that this study was not powered to demonstrate efficacy. In addition to the clinical benefits, the encouraging health-related quality of life results may lead to physical and social benefits for patients [Citation27,Citation28]. Immunogenicity analyses revealed the presence of drug-reactive, preexisting antibodies at baseline, a phenomenon observed in other clinical studies with reference product biosimilars [Citation18,Citation29–31]. Reasons for the presence of drug reactive endogenous preexisting antibodies vary and the exact cause is often unknown, but they may be an outcome of prior exposure to a protein or glycan with a similar epitope. We also observed that the proportion of patients who were ADA positive decreased from Week 8 to Week 56. This decrease may be due to concomitant administration of MTX, as it is known to increase drug levels of several TNF inhibitors, most likely via a decrease in ADA formation [Citation32].
There were no unexpected safety signals observed in this study. Most TEAEs were mild, and there were no deaths. The lack of injection site reactions observed in this study is consistent with the immunosuppressive effects of concomitant MTX use in the study population. Overall, AVT02 was well-tolerated by RA patients, and the observed safety profile was in line with other AVT02 studies [Citation17,Citation18], and adalimumab studies [Citation33].
The AVT02-AI has been developed using a patient-centric approach, accounting for and addressing obvious barriers to users [Citation34], who are initiating or switching to an adalimumab biosimilar, and thus easing the accompanying burden of illness. In addition, results from this study suggest that the AI device can be used for other biosimilars in similar patient populations.
This study has some known limitations. The single-arm, open-label design nature prevents effective comparison to other comparator groups. The sample size was not statistically determined, and analyses were descriptive.
5. Conclusions
This study demonstrated that the proposed AVT02-AI can be successfully and reliably used for repeated self-injections of AVT02 by patients with moderate to severe RA, despite dexterity limitations and no previous experience of adalimumab AI self-administration. The extension phase provides long-term efficacy and safety data on AVT02, supporting previous AVT02 studies [Citation17,Citation18].
Abbreviations
| AI | = | Autoinjector |
| ACR | = | American College of Rheumatology |
| ADAs | = | Anti-drug antibodies |
| CI | = | Confidence interval |
| CRP | = | C-reactive protein |
| DAS28 CRP | = | Disease Activity Score 28 C-reactive protein |
| DMARDs | = | Disease-Modifying Anti-Rheumatic Drugs |
| EOS | = | End of Study |
| HAQ | = | Health Assessment Questionnaire |
| MTX | = | Methotrexate |
| NAbs | = | Neutralizing antibodies |
| OAT | = | Observer assessment tool |
| PAT | = | Patient assessment tool |
| PK | = | Pharmacokinetic |
| PFS | = | Prefilled syringe |
| RA | = | Rheumatoid arthritis |
| SDAI | = | Simple Disease Activity Index |
| SD | = | Standard deviation |
| s.c. | = | Subcutaneous |
| TEAEs | = | Treatment-emergent adverse events |
| TNF-α | = | Anti-tumor necrosis factor alpha |
Author contributions
N Damjanov, H Stroissnig, M Steiger, J Sobierska, E Guenzi, H Otto, A Sattar, HN Haliduola, E Edwald and F Berti were involved in the conception and design of the study. N Kirvalidze, N Kurashvili, M Steiger and J Sobierska were involved in the provision of study materials and patients and acquisition of the data. H Stroissnig, J Sobierska, H Otto, A Sattar, HN Haliduola and F Berti did the analysis and/or the interpretation of the data. All authors revised the report critically. All authors approved the final version.
Declaration of interests
H Stroissnig, M Steiger, J Sobierska, H Otto, A Sattar, HN Haliduola and F Berti are employees of Alvotech. E Guenzi was an employee of Alvotech at the time of the study. The authors have no other relevant affiliations or financial involvement with any organization or entity with a financial interest in or financial conflict with the subject matter or materials discussed in the manuscript apart from those disclosed.
Reviewer disclosures
Peer reviewers on this manuscript have no relevant financial or other relationships to disclose.
Ethical approval
The study was conducted in compliance with the ethical principles in the Declaration of Helsinki, International Council for Harmonisation of Technical Requirements for Pharmaceuticals for Human Use guidelines, and all applicable local regulatory authorities’ requirements. The study protocol was approved by an Independent Ethics Committee. Written informed consent was obtained from each patient before any study-related procedures were performed.
Device_303_study_manuscript_supplemental.docx
Download MS Word (84.7 KB)Acknowledgments
The authors thank the subjects who participated in the study and all the investigators who contributed, with particular thanks to Dr Viktoriia Yarosh of City Clinical Hospital 8, Kharkiv, Ukraine. The authors additionally thank Hildur Zoega for valuable discussions at the design stage of AI development. Medical writing support was provided by Sola Lawal and Anna Chapman-Barnes (eluSCIdate ltd, Meggen, Switzerland) and funded by Alvotech. This work was presented at EULAR 2022 in Copenhagen as Damjanov N, Stroissnig H, Steiger M, et al. AB0350 Assessment of Real-Life Patient Handling Experience of AVT02 Administered Subcutaneously Via Autoinjector in Patients with Moderate to Severe Active Rheumatoid Arthritis: An Open-Label, Single, Single-Arm Clinical Trial, Then an Extension Phase of AVT02 Administered with a Pre-Filled Syringe (ALVOPAD-PEN). Annals of the Rheumatic Diseases 2022;81:1301.
Data availability statement
The datasets generated and/or analyzed during the current study are available from the sponsor on reasonable request.
Supplementary material
Supplemental data for this article can be accessed online at https://doi.org/10.1080/14712598.2022.2131392
Additional information
Funding
References
- Aletaha D, Neogi T, Silman AJ, et al. 2010 rheumatoid arthritisclassification criteria: an American college of rheumatology/European league against rheumatism collaborative initiative. Arthritis Rheum . 2010;62(6):2569–2581.
- Hsieh P-H, Wu O, Geue C, et al. Economic burden of rheumatoid arthritis: a systematic review of literature in biologic era. Ann Rheum Dis. 2020;79(6):771–777.
- US Food and Drug Administration. Humira prescribing information 2021.
- European Medicines Agency. Humira summary of product characteristics 2021.
- van der Heijde D, Klareskog L, Rodriguez-Valverde V, et al. Comparison of etanercept and methotrexate, alone and combined, in the treatment of rheumatoid arthritis: two-year clinical and radiographic results from the TEMPO study, a double-blind, randomized trial. Arthritis Rheum. 2006;54(4):1063–1074.
- Breedveld FC, Weisman MH, Kavanaugh AF, et al. The PREMIER study: a multicenter, randomized, double-blind clinical trial of combination therapy with Adalimumab plus methotrexate versus methotrexate alone or Adalimumab alone in patients with early, aggressive rheumatoid arthritis who had not had previous methotrexate treatment. Arthritis Rheum. 2006;54(1):26–37.
- Nash P, Vanhoof J, Hall S, et al. Randomized crossover comparison of injection site pain with 40 mg/0.4 or 0.8 mL Formulations of adalimumab in patients with rheumatoid arthritis. Rheumatol Ther. 2016;3(2): 257–270.
- Bergman M, Patel P, Chen N, et al. Evaluation of adherence and persistence differences between adalimumab citrate-free and citrate formulations for patients with immune-mediated diseases in the United States. Rheumatol Ther. 2021;8(1): 109–118.
- Rekaya N, Vicik SM, Hulesch BT, et al. Enhancement of an auto-injector device for self-administration of etanercept in patients with rheumatoid arthritis confers emotional and functional benefits. Rheumatol Ther. 2020;7(3):537–552.
- US Food and Drug Administration. Guidance for industry rheumatoid arthritis: developing drug products for treatment -Draft guidance 2013 [Internet] [ cited 2022 May 06]. Available from: https://www.fdanews.com/ext/resources/files/archives/62813-01/06-07-13-RA.pdf.
- US Food and Drug Administration. Bioanalytical method validation. guidance for industry [Internet] [ cited 2022 June 01]. Available from: https://www.fda.gov/files/drugs/published/Bioanalytical-Method-Validation-Guidance-for-Industry.pdf.
- European Medicines Agency. Guideline on bioanalytical method validation [Internet] [ cited 2022 June 01]. Available from: https://www.ema.europa.eu/en/documents/scientific-guideline/guideline-bioanalytical-method-validation_en.pdf.
- Marini JC, Anderson M, Cai X-Y, et al. Systematic verification of bioanalytical similarity between a biosimilar and a reference biotherapeutic: committee recommendations for the development and validation of a single ligand-binding assay to support pharmacokinetic assessments. AAPS J. 2014;16(6):1149–1158.
- Civoli F, Kasinath A, Cai X-Y, et al. Recommendations for the development and validation of immunogenicity assays in support of biosimilar programs. AAPS J. 2019;22(1):7.
- US Food and Drug Administration. Immunogenicity testing of therapeutic protein products - developing and validating assays for anti-drug antibody detection. Guidance for industry [Internet] [ cited 2022 June 1]. Available from: https://www.fda.gov/media/119788/download.
- European Medicines Agency. Guideline on Immunogenicity assessment of therapeutic proteins [Internet] [ cited 2022 June 01]. Available from: https://www.ema.europa.eu/en/documents/scientific-guideline/guideline-immunogenicity-assessment-therapeutic-proteins-revision-1_en.pdf.
- Wynne C, Schwabe C, Lemech C, et al. A randomized, adaptive design, double-blind, 3-arm, parallel study assessing the pharmacokinetics and safety of AVT02, a high-concentration (100 mg/mL) adalimumab biosimilar, in healthy adult subjects (ALVOPAD FIRST). Expert Opin Investig Drugs. 2022;31(9):965–976.
- Feldman SR, Reznichenko N, Pulka G, et al. Efficacy, safety and immunogenicity of avt02 versus originator adalimumab in subjects with moderate to severe chronic plaque psoriasis: a multicentre, double-blind, randomised, parallel group, active control, Phase III study. BioDrugs. 2021;35(6): 735–748.
- Wynne C, Stroig H, Dias R, et al. Multi-center, randomized, open-label, 2-arm parallel study to compare the pharmacokinetics, safety and tolerability of AVT02 administered subcutaneously via prefilled syringe or autoinjector in healthy adult volunteers. Ann Rheum Dis. 2022;81(Suppl 1):1891–1892.
- ClinicalTrials.gov. PK, safety and tolerability study of AVT02 (Adalimumab) pre-filled syringe (PFS) vs, AVT02 autoinjector (AI) [Internet]. Available from: https://clinicaltrials.gov/ct2/show/NCT03983876.
- Gerosa M, De Angelis V, Riboldi P, et al. Rheumatoid Arthritis: a Female Challenge. Women’s Health. 2008;4(2):195–201.
- Erol AM, Ceceli E, Uysal Ramadan S, et al. Effect of rheumatoid arthritis on strength, dexterity, coordination and functional status of the hand: the relationship with magnetic resonance imaging findings [Effect of rheumatoid arthritis on strength, dexterity, coordination and functional status of the hand: the relationship with magnetic resonance imaging findings]. Acta Reumatol Port. 2016;41(4):328–337.
- Da Sferra Silva G. De Almeida Lourenço M, De Assis MR. Hand strength in patients with RA correlates strongly with function but not with activity of disease. Adv Rheumatol. 2018;58:20.
- van den Bemt BJF, Gettings L, Domańska B, et al. A portfolio of biologic self-injection devices in rheumatology: how patient involvement in device design can improve treatment experience. Drug Deliv. 2019 Dec;26(1):384–392.
- US Food and Drug Administration. Biosimilar product regulatory review and approval.
- European Medicines Agency. Biosimilars in the EU.
- Martinec R, Pinjatela R, Balen D. Quality of life in patients with rheumatoid arthritis-a preliminary study. Acta Clin Croat. 2019;58(1):157–166.
- Katchamart W, Narongroeknawin P, Chanapai W, et al. Health-related quality of life in patients with rheumatoid arthritis. BMC Rheumatol. 2019;3:34.
- Cohen S, Genovese MC, Choy E, et al. Efficacy and safety of the biosimilar ABP 501 compared with adalimumab in patients with moderate to severe rheumatoid arthritis: a randomised, double-blind, phase III equivalence study. Ann Rheum Dis. 2017;76(10):1679–1687.
- Cohen SB, Alonso-Ruiz A, Klimiuk PA, et al. Similar efficacy, safety and immunogenicity of adalimumab biosimilar BI 695501 and humira reference product in patients with moderately to severely active rheumatoid arthritis: results from the phase III randomised VOLTAIRE-RA equivalence study. Ann Rheum Dis. 2018;77:914–921.
- Papp K, Bachelez H, Costanzo A, et al. Clinical similarity of the biosimilar ABP 501 compared with adalimumab after single transition: long-term results from a randomized controlled, double-blind, 52-week, phase III trial in patients with moderate-to-severe plaque psoriasis. Br J Dermatol. 2017;177(6):1562–1574.
- Burmester G-R, Kivitz AJ, Kupper H, et al. Efficacy and safety of ascending methotrexate dose in combination with adalimumab: the randomised CONCERTO trial. Ann Rheum Dis. 2015;74(6):1037–1044.
- Ghil J, Zielińska A, Lee Y. Usability and safety of SB5 (an adalimumab biosimilar) prefilled syringe and autoinjector in patients with rheumatoid arthritis. Curr Med Res Opin. 2019;35(3):497–502.
- Karlsdottir K, Gunnarsdottir AI, Grondal G, et al. A patients’ perspective towards the injection devices for humira® and imraldi® in a nationwide switching program. Front Med (Lausanne). 2022;9:799494.