KEYWORDS:
1. Introduction
During the last decades, the scenario of the therapeutic approaches in multiple myeloma (MM) has dramatically improved. The introduction of small molecules such as proteasome inhibitors (PIs) and immunomodulatory drugs (IMiDs) in the early 2000s has changed the natural history of the disease and patients’ outcomes. Subsequently, identifying CD38 and SLAMF7 as appropriate targets led to immunotherapeutic approaches using monoclonal antibodies (mAbs) such as daratumumab and elotuzumab, respectively. Isatuximab (Isa) is a novel immunoglobulin G1 (IgG1) monoclonal antibody directed against a distinct epitope on CD38, which is highly expressed on malignant MM cells [Citation1].
2. CD38 role and isatuximab mechanism of action
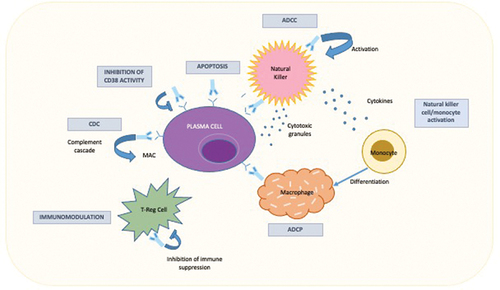
CD38 is a type II transmembrane protein expressed in immune cells and modulates leukocyte migration, activation process, and calcium signaling. In normal conditions, CD38 is expressed at low levels in immune cells [Citation1], while healthy individuals and MM patients show significantly higher CD38 expression in plasma cells [Citation2]. Isa determines MM cell death through complement-dependent cytotoxicity (CDC), antibody-dependent cellular phagocytosis (ADCP), and antibody-dependent cellular cytotoxicity (ADCC) through the binding of Fcγ receptors on immune effector cells with consequent release of toxic agents, such as perforin and granzyme. Isa can also directly induce MM cell death through caspase-dependent apoptosis, modulate the enzymatic activity of CD38, and enhance the natural killer cell- and T-cell-mediated immune response [Citation2]. Although Dara acts similarly to Isa, the mechanism of action of the two mAbs is slightly different. First, the two drugs target different CD38 epitopes. Furthermore, daratumumab must be combined with cross-linking agents to induce apoptosis, while Isa can directly induce cell death [Citation1,Citation2] ().
3. Clinical studies
3.1. Isatuximab in relapsed-refractory multiple myeloma
3.1.1. Isatuximab as a single agent
The first single-agent study evaluated Isa’s safety and tolerability in 84 heavily pretreated relapsed-refractory MM (RRMM) patients (median lines of therapy 5) (). The study demonstrated that Isa as monotherapy at doses ≥10 mg/kg is very effective against the disease, showing an overall response rate (ORR) of 23.8% and a median progression-free survival (PFS) of 3.7 months, with low toxicity profile (51% of the patients experienced grade 1 or 2 infusion-related reactions – IRR) [Citation3]. A dose-finding study, which included 97 RRMM patients, found that a dosage of ≥10 mg/kg determined an ORR of 24.3%. Elderly patients and those harboring high-risk cytogenetic lesions experienced a higher ORR [Citation4]. As a backbone, the addition of dexamethasone (Dex) in stage 2 of this study made obtaining a higher ORR with an acceptable safety profile possible [Citation5].
Table 1. Selected clinical studies of isatuximab in RRMM.
3.1.2. Isatuximab in combination with immunomodulatory drugs
Isa was tested with pomalidomide (Pom) and Dex in phase 1 and 3 studies [Citation7,Citation8]. Phase 1 trial compared Isa-Pom-Dex with Pom-Dex, and it included 45 RRMM, 82% of whom were refractory to lenalidomide (Lena). After a median follow-up of 9.2 months, the ORR was 62.2% versus 31% obtained with Pom-Dex alone [Citation7]. In the phase 3 trial (ICARIA-MM), 307 RRMM patients, who had received at least two previous lines of therapy, including Lena and PIs (patients refractory to previous anti-CD38 and Pom were excluded), were assigned to receive Isa-Pom-Dex (Isa group, n = 154) or Pom-Dex (control group, n = 153) (). Patients have also been stratified based on the number of previous lines of therapy (2–3 vs. >3) and age (<75 vs. ≥75 years). In the Isa group, Isa 10 mg/kg was administered weekly for the first 4-week cycle and then on days 1 and 15. Both groups received Pom 4 mg for 21 consecutive days and weekly Dex 40 mg (20 mg if aged ≥ 75 years) until disease progression. The primary end-point was PFS; the secondary end-points were ORR and OS. The median follow-up at data cutoff (1 October 2020) [Citation6] was 35.3 months. Adding Isa to Pom–Dex significantly improved PFS reducing by 40% the risk of disease progression or death. That benefit was proven in all the subgroups analyzed (age ≥75 years [Citation9], ISS stage III, renal impairment, high-risk cytogenetics [Citation10], including chromosome 1q21 abnormalities, and number of previous lines >3 double-refractoriness). Median overall survival (OS) was 24.6 months in the Isa group and 17.7 months in the control group. More patients in the experimental arm achieved a partial remission (PR) (60% vs. 35%), a very good partial remission (VGPR) (27% vs. 7%), and a complete response (CR) (5% vs. 1%). A study [Citation11] demonstrated that conventional disease assessments (IFE) used in the trial resulted in a 7.1% underestimation of the CR rate in the Isa-Pd arm because of the presence of the therapeutic antibody in the serum. Regarding grade 3 adverse events (AEs), only neutropenia appeared prevalent in the Isa group (76% vs. 52%) [Citation8].
Isa was also tested in association with Lena-Dex. The combination showed an ORR of 48% in heavily pretreated patients and a rate even higher (52%) in those refractory to Lena with acceptable side effects () [Citation12].
3.1.3. Isatuximab in combination with proteasome inhibitors
In the IKEMA trial, 302 RRMM patients were randomized to receive Carfilzomib (K) and Dex (Kd) versus Isa-Kd (). Patients in the Isa-Kd arm received Isa 10 mg/kg weekly for 4 weeks and then every 2 weeks; patients in both arms received K twice a week (20 mg/m2 on days 1 and 2 of cycle 1; 56 mg/m2 on days 8, 9, 15, and 16 of cycle 1 and thereafter) and Dex 20 mg twice weekly. The triplet demonstrated promising results in terms of ORR and PFS. Indeed, an ORR of 87% has been registered in the experimental arm, with a PFS not reached at 20.7 months of follow-up. A PFS benefit was also demonstrated in patients with renal impairment [Citation13] and high-risk cytogenetics [Citation14], representing an efficient treatment option for hard-to-treat patients. Those data conflicted with those of the control arm, where ORR and PFS were 83% and 19.2 months, respectively. Moreover, Isa-Kd reduced by 47% the risk of disease progression or all-cause death. Of note, 41.4% of the Isa-Kd cohort reached minimal residual disease (MRD) negativity with very good partial response (VGPR) or better versus only 22.9% in the Kd population [Citation15]. Regarding safety, grade 3 AEs in the Isa group were slightly higher than in the Kd cohort (77% vs. 67%) [Citation13].
3.2. Isatuximab in newly diagnosed multiple myeloma
Several clinical studies have been conducted to investigate the activity of Isa in newly diagnosed MM (NDMM) patients. Relevant results have been shown in trials testing Isa in combination with Bortezomib (Bor), Lena, and Dex (VRD) or Bor, cyclophosphamide (C), and Dex (VCD) in transplant-ineligible NDMM patients. The trials reported an ORR of 100% and 93.3% for these two quadruplets, respectively [Citation16–18]. Moreover, the addition of Isa to VRD within the GMMG-HD7 clinical trial improved rates of MRD negativity (<105) than the control arm with VRD (50.1% vs. 35.6%) prior to autologous stem cell transplantation, resulting in an efficient induction therapy [Citation19]. Finally, an interim analysis of the GMMG-CONCEPT trial, published in 2022, reported the responses of high-risk NDMM patients who underwent a combination Isa-KRd. Quite the total achieved a VGPR. The median 24-month PFS was 75.5% [Citation20]. Based on those results, Isa appears to be a promising therapy for NDMM.
4. Recent updates
Recent updates regarding Isa have been shown during ASH 2022. A sub-analysis of the IKEMA trial showed as IsaKd improved outcome (PFS and depth of response), with a good safety profile in both early and late relapse MM patients [Citation21]. Moreover, updates from the phase 1b expansion study testing subcutaneous Isa administration in combination with Pom-Dex showed a safety profile consistent with intravenously (IV) administration, with no infusion reactions and efficacy similar to that observed in the phase 3 ICARIA study with IV Isa, in combination with Pd [Citation22]. Early results from the SKylaRk trial (Isa-KRd in all risk TE NDMM) suggested that Isa-KRd allowed of achieving deep responses in this setting of patients with a good safety profile [Citation23].
5. Expert opinion
Combinations of drugs, including Isa as a backbone, effectively treat MM. Based on these phase III pivotal trials, Isa has been approved by the FDA since 2021 in combination with Pom-Dex for the treatment of MM patients who have received at least two prior therapies, including Lena-Dex and a PI [Citation8] or in combination with Kd, for the treatment of MM patients who have received 1 to 3 prior lines of therapy [Citation13].
The high rates of ORR and the PFS and OS benefits can be achieved with a favorable AEs profile. Adding Isa to Pom increased anti-MM activity due to the direct toxicity and lysis of neoplastic plasma cells by effector cells. On the other hand, the Isa-PIs combination (i.e. carfilzomib) affects both MM and the microenvironment cells [Citation2]. Based on the excellent results mentioned above in the setting of RRMM, novel clinical trials (i.e. ClinicalTrials.gov Identifier NCT02513186) are currently evaluating the use of triplets containing Isa in NDMM, showing high ORR. A recent interim analysis of the GMMG-CONCEPT trial, including high-risk MM patients receiving induction therapy with Isa-KRd, demonstrated a VGPR or better in 45 out of the 50 enrolled patients and a median 24-month PFS of 75.5% [Citation20]. Nevertheless, there is still no indication of Isa in the same settings where daratumumab is recommended in association with bortezomib and melphalan or with Lena as upfront therapy in NDMM transplant-ineligible patients, despite the fact that several studies highlighted Isa’s safety. Nasal congestion, dry cough, and dyspnea are the most common nonhematologic adverse reactions that mainly occur during the first infusion. Thus, a proper premedication with diphenhydramine, methylprednisolone, ranitidine, and acetaminophen, 15–30 min before infusion, is mandatory. Regarding hematological AEs, neutropenia is the most common and easily manageable with the use of granulocyte colony-stimulating.
The MM therapeutic landscape constantly changes and aims to set anti-CD38+ monoclonal-based treatment as the frontline. Since it consists of continuous therapies, acquired resistance to those drugs is emerging. Several studies investigated such mechanisms. For example, the reduction of the CD38 expression phenomenon may be antagonized by adding all-trans retinoic acid (ATRA) or the histone deacetylase inhibitor ricolinostat [Citation2]. The MM microenvironment and decreasing immune cell functions are essential in acquired resistance. Thus, the combination with programmed anti-cell death-1 (PD-1) or anti-programmed death ligand-1 (PD-L1) could improve the efficacy of anti-CD38 antibodies [Citation24].
In conclusion, Isa represents an excellent tool in the treatment of MM. More studies are warranted to assess the best associations with other drugs and their appropriate application in clinical practice.
Declaration of interest
The authors have no relevant affiliations or financial involvement with any organization or entity with a financial interest in or financial conflict with the subject matter or materials discussed in the manuscript. This includes employment, consultancies, honoraria, stock ownership or options, expert testimony, grants or patents received or pending, or royalties.
Reviewer disclosures
Peer reviewers on this manuscript have no relevant financial or other relationships to disclose.
Additional information
Funding
References
- Abramson HN. Immunotherapy of multiple myeloma: promise and challenges. 2021;Volume 10(10):343–371.
- Zhu C, Song Z, Wang A, et al. Isatuximab acts through Fc-dependent, independent, and direct pathways to kill multiple myeloma cells. Front Immunol. 2020 14;11: 1771. . 10.3389/fimmu.2020.01771
- Martin T, Strickland S, Glenn M, et al. Phase I trial of isatuximab monotherapy in the treatment of refractory multiple myeloma. Blood Cancer J. 2019 29; 9(4): 41. 10.1038/s41408-019-0198-4.
- Mikhael J, Richter J, Vij R, et al. A dose-finding phase 2 study of single agent isatuximab (anti-CD38 mAb) in relapsed/refractory multiple myeloma. Leukemia. 2020;34(12):3298–3309. DOI:10.1038/s41375-020-0857-2
- Dimopoulos MA, Bringhen S, Anttila P, et al. Results from a phase II study of isatuximab as a single agent and in combination with dexamethasone in patients with relapsed/refractory multiple myeloma. Blood. 2018;132(suppl 1):155. DOI:10.1182/blood-2018-155
- Richardson PG, Perrot A, San-Miguel J, et al. Isatuximab plus pomalidomide and low-dose dexamethasone versus pomalidomide and low-dose dexamethasone in patients with relapsed and refractory multiple myeloma (ICARIA-MM): follow-up analysis of a randomised, phase 3 study. Lancet Oncol. 2022 Mar;23(3):416–427.
- Mikhael J, Richardson P, Usmani SZ, et al. A phase 1b study of isatuximab plus pomalidomide/dexamethasone in relapsed/refractory multiple myeloma. Blood. 2019;134(2):123–133. DOI:10.1182/blood-2019-02-895193
- Attal M, Richardson PG, Rajkumar SV, et al. Isatuximab plus pomalidomide and low-dose dexamethasone versus pomalidomide and low-dose dexamethasone in patients with relapsed and refractory multiple myeloma (ICARIA-MM): a randomised, multicentre, open-label, phase 3 study. Lancet. 2019;394(10214):2096–2107. DOI:10.1016/S0140-6736(19)32556-5
- Schjesvold F, Richardson PG, Facon T, et al. Isatuximab plus pomalidomide and dexamethasone in elderly patients with relapsed/refractory multiple myeloma: iCARIA-MM subgroup analysis. Haematologica. 2022 Mar 1;107(3):774–775. DOI:10.3324/haematol.2021.279160
- Harrison SJ, Perrot A, Alegre A, et al. Subgroup analysis of ICARIA-MM study in relapsed/refractory multiple myeloma patients with high-risk cytogenetics. Br J Haematol. 2021 Jul;194(1):120–131.
- Hulin C, Beksac M, Goodman HJ, et al. Antibody interference and response kinetics of isatuximab plus pomalidomide and dexamethasone in multiple myeloma. Blood Cancer J. 11(10). 169. 2021 Oct 20. 10.1038/s41408-021-00562-9.
- Martin T, Baz R, Benson DM, et al. A phase 1b study of isatuximab plus lenalidomide and dexamethasone for relapsed/refractory multiple myeloma. Blood. 2017;129(25):3294–3303. DOI:10.1182/blood-2016-09-740787
- Moreau P, Dimopoulos MA, Mikhael J, et al. Isatuximab, carfilzomib, and dexamethasone in relapsed multiple myeloma (IKEMA): a multicentre, open-label, randomised phase 3 trial. Lancet. 2021;397(10292):2361–2371. DOI:10.1016/S0140-6736(21)00592-4
- Spicka I, Moreau P, Martin TG, et al. Isatuximab plus carfilzomib and dexamethasone in relapsed multiple myeloma patients with high-risk cytogenetics: iKEMA subgroup analysis. Eur J Haematol. 2022;109(5):504–512.
- Martin T, Mikhael J, Hajek R, et al. Depth of response and response kinetics of isatuximab plus carfilzomib and dexamethasone in relapsed multiple myeloma: ikema interim analysis. Blood. 2020;136(suppl 1):7–8. DOI:10.1182/blood-2020-137681
- Ocio E, Otero PR, Bringhen S, et al. Preliminary results from a phase I study of isatuximab (ISA) in combination with bortezomib, lenalidomide, dexamethasone (VRd), and in patients with newly diagnosed multiple myeloma (NDMM) non-eligible for transplant. Blood. 2018;132(suppl 1):3160. DOI:10.1182/blood-2018-99-111244
- Ocio EM, Bringhen S, Oliva S, et al. A phase Ib study of isatuximab in combination with bortezomib, cyclophosphamide, and dexamethasone (VCDI) in patients with newly diagnosed multiple myeloma non-eligible for transplantation. Blood. 2017;130(suppl 1):3160.
- Ocio EM, Rodríguez Otero P, Bringhen S, et al. Updates from a phase Ib study of isatuximab (Isa), bortezomib (V) and dexamethasone (D) plus cyclophosphamide (C) or lenalidomide (R) in transplant-ineligible, newly diagnosed multiple myeloma (NDMM). J Clin Oncol. 2020;38(suppl 15):8529. DOI:10.1200/JCO.2020.38.15_suppl.8529
- Goldschmidt H, Mai EK, Nievergali E, et al. Addition of isatuximab to lenalidomide, bortezomib and dexamethasone as induction therapy for newly-diagnosed, transplant-eligible multiple myeloma patients: the phase III GMMG-HD7 trial. Blood. 2021;138(suppl 1):463.
- Leypoldt LB, Besemer B, Asemissen AM, et al. Isatuximab, carfilzomib, lenalidomide, and dexamethasone (Isa-KRd) in frontline treatment of high-risk multiple myeloma: interim analysis of the GMMG-CONCEPT trial. Leukemia. 2022;36(3):885–888.
- Facon T, Moreau P, Baker R, et al. Isatuximab plus carfilzomib and dexamethasone in patients with early versus late relapsed multiple Myeloma: ikema subgroup analysis. Blood. 2022;140(Supplement 1):1820–1822. DOI:10.1182/blood-2022-159105
- Quach H, Parmar G, Ocio EM, et al. Subcutaneous Isatuximab administration by an on-body delivery system (OBDS) in combination with Pomalidomide and Dexamethasone in patients with relapsed/refractory multiple Myeloma: phase 1b expansion study results blood. 2022;140(Supplement 1):4412–4414. DOI:10.1182/blood-2022-166840.
- O’donnell EK, Mo CC, Nadeem O, et al. A Phase II study of once weekly Carfilzomib, Lenalidomide, Dexamethasone, and Isatuximab in newly diagnosed, transplant-eligible multiple Myeloma (The SKylaRk Trial). Blood. 2022;140(Supplement 1):7282–7283. DOI:10.1182/blood-2022-156328
- Verkleij CPM, Jhatakia A, Broekmans MEC, et al. Preclinical rationale for targeting the PD-1/PD-L1 axis in combination with a CD38 antibody in multiple myeloma and other CD38-positive malignancies. Cancers (Basel). 2020;12(12):3713. DOI:10.3390/cancers12123713