1. Introduction
The therapeutic scenario for multiple myeloma (MM) has changed drastically in recent years with the introduction of new molecules, which lead to a more precise medicine, improving overall response rate (ORR) and progression-free survival (PFS). Nowadays, anti-CD38 monoclonal antibodies (mAb) represent the backbone of induction therapies in newly diagnosed MM patients (NDMM). In contrast, antibody-drug conjugates (ADCs) and chimeric antigen receptor T cell (CAR-T) therapy represent optimal therapeutic strategies in the setting of relapse refractory patients (RRMM). On 5 August , the FDA approved belantamab mafodotin-blmf (BLENREP; GlaxoSmithKline), an ADC directed against BCMA, for treating patients with RRMM who have received at least four prior therapies, including an anti-CD38 monoclonal antibody, a proteasome inhibitor, and an immunomodulatory drug (IMD). Other ADCs are currently under evaluation [Citation1].
2. BCMA role and belantamab mafodotin mechanism of action
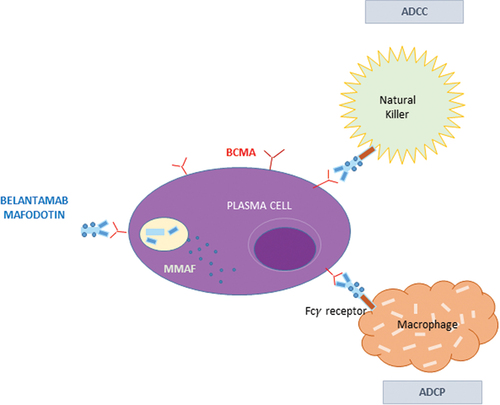
BCMA, also known as TNFRSF17 or CD269, belongs to the tumor necrosis factor superfamily, and it is mainly expressed in mature B lymphocytes and plasma cells (PCs). The interactions between BCMA and its natural ligands, such as B-cell activating factor (BAFF) and a proliferation-inducing ligand (APRIL), determine PCs survival and proliferation through signaling pathways including AKT, NF-kB, and MAPK, resulting in MM progression. Belantamab mafodotin is a BCMA-directed humanized afucosylated monoclonal IgG1 antibody (J6MO) conjugated via a non-cleavable linker to mono-methyl auristatin F (MMAF). The interaction between the drug and BCMA promotes the activation of caspase-3-dependent apoptosis after the intracellular release of MMAF. The afucosylation of the antibody permits the linkage with the Fc-bearing NK cells, inducing antibody-dependent cellular cytotoxicity (ADCC). Macrophages mediate another mechanism of cell death via antibody-dependent cellular phagocytosis (ADCP) following the release of antigens by apoptotic MM cells. Finally, the antibody blocks the interaction of BCMA with its natural ligands BAFF and APRIL () [Citation2].
Figure 1. Mechanisms of action of belantamab mafodotin. The interaction between the drug and BCMA promotes the activation of caspase-3 dependent apoptosis after the intracellular release of MMAF. The afucosylation of the antibody permits the linkage with the Fc-bearing NK cells, inducing antibody-dependent cellular cytotoxicity (ADCC). Another mechanism of cell death is mediated by macrophages via antibody-dependent cellular phagocytosis (ADCP) following the release of antigens by apoptotic MM cells.
3. Clinical studies
3.1. Belantamab mafodotin as single agent
The efficacy of belantamab mafodotin was first investigated in a phase I first-in-human study (BMA117159, DREAMM-1, NCT02064387). This clinical trial included 73 RRMM previously receiving an alkylator, a proteasome inhibitor (PI), and an IMiD. The majority of patients underwent ≥ 5 lines of therapy. In part 1, dose-finding, of the study, 38 patients were treated with drug dosages ranging from 0.03 to 4.6 mg/Kg every 3 weeks for 16 cycles. The recommended dose was finally established at 3.5 mg/Kg. In part 2, the expansion cohort included 35 patients, 37% of whom were refractory to novel mAb such as Daratumumab. The ORR was 60%, with 43% very good partial response (VGPR). The median PFS was 12 months, with a median duration of response (DoR) of 14.3 months. Most common adverse events (AEs) were represented by corneal events characterized by photophobia, blurred vision, and dry eyes. Such AEs were reported in 63% of patients. In 34% of patients, a dose reduction was necessary while in 40% of the population, AEs led to dose interruptions or delays () [Citation3,Citation4].
Table 1. Selected clinical trials of belantamab mafodotin regimens.
Further dosage was investigated in phase 2 DREAMM-2, where belantamab mafodotin was tested at either 2.5 mg/kg or 3.4 mg/kg every 21 days until disease progression. The two groups were well-balanced in terms of ISS stage, cytogenetic, and previous therapies exposure. In particular, all participants were refractory to PI and IMiDs and were previously exposed to anti-CD38 mAb. More than 80% of patients received > 4 lines of therapy. The ORR, including VGPR or better, was slightly better in the group receiving 3.4 mg/Kg (34% vs. 31%). The median PFS was 4.9 months (95% CI (2·3–6·2) in the latter group, greater than in those receiving 2.5 mg/Kg (2.9 months – [95% CI 2·1–3·7]). The reason for the low ORR and limited PFS lies in the fact that the population treated in the trial was heavily pre-treated. Moreover, almost all patients had previously been treated with daratumumab, and they still represent unmet clinical needs. The benefit was seen across all subgroups of patients, including those harboring high-risk cytogenetic lesions and renal impairment. Regarding AEs, keratopathy (microcyst-like epithelial changes [MECs]) assessed by ophthalmic examination was the most common cause, and it was responsible for treatment discontinuation, mainly in the cohort utilizing the drug at 3.4 mg/Kg (10% of cases). Dose reductions were registered in 23% of the 2.5 mg/kg cohort and 27% in the 3.4 mg/kg cohort. Moreover, delays in treatment were observed in 47% of the 2.5 mg/kg group and 48% of the 3.4 mg/kg group. Other side effects included thrombocytopenia, anemia, transaminitis, and infusion-related reactions. All the events were predominant in the group treated with a higher dosage. Therefore, to reach the right balance between efficacy and safety, the 2.5 mg/kg dose was, at least, determined as the recommended dose [Citation5]. The final analysis of DREAMM-2 confirms earlier results regarding ORR and safety profile () [Citation6].
3.2. Belantamab mafodotin in combination with IMiDs
DREAMM-6 (NCT03544281) ARM A investigated the association of belantamab mafodotin with lenalidomide and dexamethasone. Part 1 of the study tested the dosage of 2.5 mg/Kg Q4W, while part 2 evaluated the dose of 1.9 mg/kg at Q8W or Q4W) and 2.5 mg/kg at Q4W or Q4W given as a divided dose. The trial included heavily pre-treated MM patients with a range of 1–11 prior therapies, 58% of whom were previously treated with lenalidomide. The best ORR was found in the cohort performing 1.9 mg/kg Q4W (75%) rather than the others. The high rate of ORR could be explained by a minor incidence of grade ≥ 3 and, by a consequent minor rate of drug interruption [Citation7].
The Algonquin study (NCT03715478) is an ongoing phase 1/2 clinical trial testing belantamab mafodotin in association with pomalidomide and dexamethasone in the setting of RRMM. The median number of prior treatments was 3 (range 2–5). More than half of the cohort received prior stem cell transplant (68.6%), and all patients were exposed to PI (80% refractory), lenalidomide (88.6% refractory), and daratumumab (45.7% exposed, 100% refractory). Seventy-one percent of patients were double refractory, and 37.1% were triple refractory. Sixty patients received belantamab mafodotin in four different drug cohorts, i.e. 1.92 mg/kg Q4W or 2.5 mg/kg Q4W or 2.5 mg/kg Q8W or 2.5 mg/kg Q12W with Pd. Twenty-eight patients were triple-refractory. Considering all drug cohorts, the ORR was 89%, with 72% achieving VGPR. At a median follow-up of 8.6 months (range 0.9–27.9), the median PFS was 24.2 months. The most common AE was keratopathy which occurred in quite all patients (96.9%), and 56.7% were declared as grade 3/4 [Citation8].
3.3. Belantamab mafodotin in combination with PIs
In the DREAMM-6 (NCT03544281) ARM B cohort, 18 patients received belantamab mafodotin 2.5 mg/kg Q3W plus bortezomib and dexamethasone. Remarkably, 89% of patients were previously exposed to bortezomib and half to daratumumab. The ORR was 78%, especially in the PIs exposed group (75%), including the rate of VGPR or better, and in less pre-treated patients. Keropathy and thrombocytopenia were the most frequent AEs [Citation9]. In particular, keratopathy occurs in 100% of patients which leads to dose reduction in 39% of patients. None of them discontinued the treatment.
3.4. Belantamab mafodotin in combination with novel agents
DREAMM-4 (NCT03848845), a phase I/II trial, assessed the efficacy and safety of belantamab mafodotin with pembrolizumab in RRMM receiving ≥ 3 lines of therapy. Part 1 of the trial established the dose of belantamab mafodotin 2.5 mg/kg with Pembrolizumab 200 mg, both IV Q3W up to 35 cycles, followed by part 2 dose expansion. The patients’ cohort was characterized for 29% of high-risk cytogenetics and 26% of extramedullary disease. The ORR was 47%, most of which exceeded VGPR. At a follow-up of 14.7 months, the median PFS was 3.4 months. The most common AEs were keratopathy and blurred vision occurring in 35%, followed by thrombocytopenia, assessing no substantial differences in new AEs and efficacy compared with belantamab mafodotin monotherapy [Citation10].
DREAMM-5 (NCT04126200) is an ongoing Phase I/II study evaluating the safety and efficacy of belantamab mafodotin in combination with novel agents such as GSK3174998 (OX40 agonist), feladilimab (ICOS agonist), nirogacestat (GSI; PF-03084014), and dostarlimab (PD-1 inhibitor) in the setting of RRMM patients with ≥ 3 prior lines of therapy. The rationale of the combination resides in the ability of these agents, in particular nirogacestat, to increase the cellular expression of BCMA, allowing the use of lower doses of belantamab mafodotin and, therefore, determining lower rates of AEs [Citation11]
3.5. Ongoing randomized studies
In DREAMM-3 (NCT04162210), 35 heavily pre-treated RRMM patients, 57% of whom previously underwent ≥ 5 lines of therapies are stratified by International Staging System, prior exposure to anti-CD38, and the number of previous therapies. They are randomized (2:1) to receive either single-agent belantamab mafodotin or pomalidomide/dexamethasone until disease progression or unacceptable toxicity. The primary endpoint is progression-free survival, while the secondary endpoint is overall survival and ORR [Citation12]. On 22 November 2022, GSK announced the market withdrawal of belantamab mafodotin-blmf, as requested by the FDA. The reason consisted of the fact that the DREAMM-3 phase III trial did not fulfil meet its primary endpoint of PFS ().
In DREAMM-7 (NCT04246047) RRMM trial, cases are stratified by the Revised International Staging System, prior exposure to bortezomib, and the number of prior lines of therapy. Patients are randomized (1:1) to arm A (belantamab mafoditin-bortezomib-dexamethasone) or arm B (daratumumab-bortezomib-dexamethasone). The primary endpoint is PFS. Minimal residual disease negativity rate is, among others, a remarkable key secondary endpoint [Citation13]
DREAMM-9 (NCT04091126) is an ongoing phase I, dose and schedule evaluation trial including newly diagnosed MM patients considered ineligible to transplant, performing bortezomib-lenalidomide-dexamethasone or belantamab mafodotin, at different dosages in association with bortezomib-lenalidomide-dexamethasone, in both cases followed by maintenance with lenalidomide-dexamethasone. The primary endpoint is safety. Secondary endpoints include efficacy. Preliminary results suggest that the quadruplet does not reveal new AEs and shows high response rates, albeit with short follow-up [Citation14].
4. Expert opinion
MM remains an incurable disease with a high rate of relapse and mortality. Triple-exposed patients’ prognosis is still dismal, with an OS of 9.2 months [Citation15], and they still represent an unmet need. Nonetheless, new treatment options are improving patients’ outcomes due to their efficacy and safety. In particular, monoclonal antibodies, bispecific T cell, and CAR-T cell therapies allow high and durable response rates. Bispecific mAbs first recruit CD3+ immune effector T cells, then plasma cells expressing BCMA, leading to TCR-independent T cell activation and tumor killing through granzyme and perforin secretion. Higher levels of BCMA expression generally characterize neoplastic cells of RRMM patients, explaining the expected good results in this setting of patients. Beyond belantamab, other mAbs are currently under investigation in MM. Teclistamab (JNJ-64007957), a fully humanized IgG4 anti-BCMA/CD3 bispecific mAb, demonstrated to be effective within phase I-II studies including RRMM patients with an ORR of 63% and a median PFS of 11.3 months. Ciltacabtagene autoleucel and idecabtagene vicleucel are the currently approved CAR-T cell therapies. The first product showed an ORR of 88%, a median PFS of 20 months for all patients, and a negative, durable MRD status in 63% of cases. The second one demonstrated an ORR of 85% and a PFS of more than 11 months [Citation2].
DREAMM-1 trial [Citation3,Citation4] showed the optimal efficacy of belantamab mafodotin, an anti-BCMA immunoconjugate, in RRMM patients, with an ORR of 60%, a median duration of response of 14.3 months and a median PFS of 12 months.
DREAMM-2 evaluated the efficacy of the anti-BCMA antibody in heavily pre-treated patients with satisfied ORR. Increasingly frequent in literature is real-world experience. In the most relevant one, 106 RRMM patients were treated with belantamab mafodotin. Patients were heavily pre-treated with a median of six previous lines of therapy. At a median follow-up of 11.9 months, ORR was 45.5%, and the median PFS was 8.8 months among responders. The rate of AEs was comparable with those registered in main clinical trials [Citation16].
The safety profile of belantamab mafodotin is most of the time manageable. Ocular toxicity is the most common and critical AE. It includes keratopathy (microcyst-like epithelial changes [MECs], best-corrected visual acuity (BCVA) changes, blurred vision, and dry eye). The pathogenic mechanism consists of the apoptosis of epithelial cells due to microtubulin inhibition caused by MMAF’s off-target effect. In particular, the drug could be internalized in the limbal epithelial stem cells after reaching corneal cells throws limbus vessels; once inside the cells, through the micropinocytosis process, belantamab mafodotin determines apoptosis and, consequently, migration of the remaining alive epithelial stem cells toward the peripheral zone of the cornea. Ocular symptoms, such as BCVA changes or blurred vision, can occur when the migration involves the central area of the cornea, crossing the visual axis. After a median time of 14 days, new corneal epithelial replace dead cells, determining the resolution of the symptoms [Citation17].
Safety results from the DREAMM-1 trial demonstrated how ocular toxicity occurred more frequently at higher doses of belantamab. In DREAMM-2, keratopathy of all grades was the most common ocular side effect with a presence in nearly 73% of patients with the highest incidence in the subgroup who underwent the dosage of 3.4 mg/Kg. In particular, blurred vision and dry eyes represented the most complained adverse events, with patients with a prior history of dry eyes having a major risk to develop corneal changes. Other ocular side effects included photophobia, eye pain, and keratitis.
It is, therefore, important that patients must undergo corneal slit lamp examination before and during the treatment to monitor those expected AEs. Indeed, the occurrence of asymptomatic corneal toxicity may need ophthalmic examination before each dose modification to detect early corneal changes. The administration of quality-of-life questionnaires, such as NEI-VFQ-25 and OSDI, as well as the Keratopathy and Visual Acuity (KVA) scale may be useful for the clinician. The scale can identify mild, moderate, and severe superficial keratopathy as well as corneal epithelial defects on slit lamps which correspond to G-1 to G-4 toxicity, respectively, guiding treatment-related decisions. Supportive local therapy with lubricating eye drops is recommended, while the use of steroid eye drops has been shown to lead to the development of secondary cataracts and glaucoma. The role of cooling eye masks and vasoconstrictors is controversial. Lower doses and more prolonged treatment intervals could effectively minimize the incidence of such AEs [Citation18]. Recently, an ocular sub-study of DREAMM-2 demonstrated that a treatment delay of more than 60 days could reverse ocular AEs without negatively impacting the responses obtained with the therapy. In particular, 38% of 16 patients analyzed in the study deepened their response [Citation2].
Less is known about ADC resistance from in vitro studies. Several are the mechanisms postulated. They may consist of changing the signaling pathways and drug efflux pumps. Biallelic or monoallelic BCMA loss on chromosome 16p or point BCMA mutations have been found in patients who underwent anti-BCMA CAR-T cells. Reduction of antigen levels, mediated by a cleavation operated by a γ-secretase, was also observed [Citation19]
The recent introduction of new target therapies revolutionized clinicians’ approach to MM. A matching-adjusted indirect treatment comparison was recently developed to assess the efficacy of ciltacabtagene autoleucel in CARTITUDE-1 vs belantamab mafodotin in DREAMM-2, selinexor-dexamethasone in STORM Part 2, and melphalan flufenamide-dexamethasone in HORIZON for the treatment of triple refractory MM patients. After adjustment, patients treated with cilta-cel achieved at least a 3.1-fold and a 10.3-fold increase in overall response compared to the other treatments analyzed [Citation20]. CAR-T and bispecific antibodies share with belantamab the same antigen target and they could often represent an unsuitable option for those RRMM patients who are unfit for conditioning regimens. One of the new challenges is combining drugs with different mechanisms of action and using them properly to maximize their efficacy. Indeed, the best timing should yet be identified to obtain the highest synergistic anti-tumor effects and to decrease relapse rates. To date only daratumumab is used as first line in the therapeutic algorithm, both in the setting of transplant-eligible and ineligible MM patients, and it is precisely in its early use that we could achieve higher rates of response. A preserved immune system functionality represents the most fertile ground for a good therapeutic outcome in the age of new drugs. For this very reason, and earlier use of belantamab, currently approved only for pluri-refractory patients, or other cellular therapies, is desirable.
Declaration of Interest
The authors have no relevant affiliations or financial involvement with any organization or entity with a financial interest in or financial conflict with the subject matter or materials discussed in the manuscript. This includes employment, consultancies, honoraria, stock ownership or options, expert testimony, grants or patents received or pending, or royalties.
Reviewer disclosures
Peer reviewers on this manuscript have no relevant financial or other relationships to disclose.
Additional information
Funding
References
- Abramson HN. Immunotherapy of multiple myeloma: promise and challenges. Immunotargets Ther. 2021 Sep 9;10:343–371. PMID: 34527606; PMCID: PMC8437262. DOI:10.2147/ITT.S306103
- Leong S, Lam HPJ, Kirkham Z, et al. Antibody drug conjugates for the treatment of multiple myeloma. Am J Hematol. 2023 Mar;98(Suppl 2):S22–S34. Epub 2022 Oct 24. PMID: 36199262. DOI:10.1002/ajh.26750
- Trudel S, Lendvai N, Popat R, et al. Targeting B-cell maturation antigen with GSK2857916 antibody-drug conjugate in relapsed or refractory multiple myeloma (BMA117159): a dose escalation and expansion phase 1 trial. Lancet Oncol. 2018;19(12):1641–1653.
- Trudel S, Lendvai N, Popat R, et al. Antibody–drug conjugate, GSK2857916, in relapsed/refractory multiple myeloma: an update on safety and efficacy from dose expansion phase I study. Blood Cancer J. 2019;9(4):37.
- Lonial S, Lee HC, Badros A, et al. Belantamab mafodotin for relapsed or refractory multiple myeloma (DREAMM-2): a two-arm, randomised, open-label, phase 2 study. Lancet Oncol. 2020;21(2):207–221. DOI:10.1016/S1470-2045(19)30788-0
- Nooka AK, Cohen A, Lee HC. Single-agent belantamab mafodotin in patients with relapsed or refractory multiple myeloma: final analysis of the DREAMM-2 Trial. Blood. 2022;140(Supplement 1):7301–7303. DOI:10.1182/blood-2022-164877
- Quach H, Gironella M, Lee C, et al. Safety and clinical activity of belantamab mafodotin with lenalidomide plus dexamethasone in patients with relapsed/refractory multiple myeloma (Rrmm): dreamm-6 arm-a interim analysis. JCO. 2022;40(16_suppl):8017. DOI:10.1200/JCO.2022.40.16_suppl.8017
- Trudel S, McCurdy A, Sutherland H, et al. Part 1 results of a dose-finding study of Belantamab Mafodotin in combination with Pomalidomide and dexamethasone for the treatment of relapsed/refractory multiple myeloma (RRMM). Blood. 2021;138(Supplement 1):1653. DOI:10.1182/blood-2021-147101
- Popat R, Nooka A, Stockerl-Goldstein K, et al. DREAMM-6: safety, tolerability and clinical activity of Belantamab Mafodotin (Belamaf) in combination with Bortezomib/dexamethasone (BorDex) in relapsed/refractory multiple myeloma (RRMM). Blood. 2020;136(Supplement 1):19–20. DOI:10.1182/blood-2020-139332
- Trudel S, Nooka A, Fecteau D, et al. 1105tiP - DREAMM 4: a phase I/II single-arm open-label study to explore safety and clinical activity of belantamab mafodotin (GSK2857916) administered in combination with pembrolizumab in patients with relapsed/refractory multiple myeloma (RRMM). Ann Oncol. 2019;30(v447):v447. DOI:10.1093/annonc/mdz251.039
- Nooka AK, Weisel K, van de Donk NW, et al. Belantamab mafodotin in combination with novel agents in relapsed/refractory multiple myeloma: dreamm-5 study design. Future Oncol. 2021;17(16):1987–2003. DOI:10.2217/fon-2020-1269
- Weisel K, Hopkins TG, Fecteau D, et al. Dreamm-3: a phase 3, open-label, randomized study to evaluate the efficacy and safety of Belantamab Mafodotin (GSK2857916) monotherapy compared with Pomalidomide plus low-dose dexamethasone (Pom/Dex) in participants with relapsed/refractory multiple myeloma (RRMM). Blood. 2019;134(Supplement_1):1900.
- Rifkin RM, Boyd K, Grosicki S, et al. DREAMM-7: a phase III study of the efficacy and safety of Belantamab Mafodotin (Belamaf) with Bortezomib, and dexamethasone (B-Vd) in patients with relapsed/refractory multiple myeloma (RRMM). Blood. 2020;136(Supplement 1):53–54. DOI:10.1182/blood-2020-139181
- Usmani SZ, Alonso Alonso A, Quach H, et al. DREAMM-9: phase I study of Belantamab Mafodotin plus standard of Care in Patients with transplant-ineligible newly diagnosed multiple myeloma. Blood. 2021;138(Supplement 1):2738. DOI:10.1182/blood-2021-153315
- Gandhi UH, Cornell RF, Lakshman A, et al. Outcomes of patients with multiple myeloma re-fractory to CD38-targeted monoclonal antibody therapy. Leukemia. 2019 Sep 1;33(9):2266–2275. DOI:10.1038/s41375-019-0435-7
- Shragai T, Magen H, Lavi N, et al. Real-world experience with belantamab mafodotin therapy for relapsed/refractory multiple myeloma: a multicentre retrospective study. Br J Haematol. 2023 Jan;200(1):45–53.
- Farooq AV, Degli Esposti S, Popat R, et al. Corneal epithelial findings in patients with multiple myeloma treated with antibody–drug conjugate belantamab mafodotin in the pivotal, randomized, DREAMM-2 study. Ophthalmol Ther. 2020;9(4):889–911. DOI:10.1007/s40123-020-00280-8
- Cohen AD, Lee HC, Trudel S, et al. Impact of prolonged dose delays on response with belantamab mafodotin (Belamaf; GSK2857916) treatment in the in the DREAMM-2 study: 13-month follow-up. Clin Lymphoma Myeloma Leuk. 2020;20(Suppl. 1):abstract MM 250. DOI:10.1016/S2152-2650(20)30949-6
- De Novellis D, Fontana R, Giudice V, et al. Innovative Anti-CD38 and Anti-BCMA targeted therapies in multiple myeloma: mechanisms of action and resistance. Int J Mol Sci. 2022 Dec 30;24(1):645. DOI:10.3390/ijms24010645
- Weisel K, Krishnan A, Schecter JM. Matching- adjusted indirect treatment comparison to assess the comparative efficacy of ciltacabtagene autoleucel in CARTITUDE-1 versus Belantamab Mafodotin in DREAMM-2, Selinexor-Dexamethasone in STORM Part 2, and melphalan flufenamide-dexamethasone in HORIZON for the treatment of patients with triple-class exposed relapsed or refractory multiple myeloma. Clin Lymphoma Myeloma Leuk. 2022 Sep;22(9):690–701.

