If you hunt only rabbits, Tigers and dragons go uncaught. Li Bai (李白)
1. Introduction
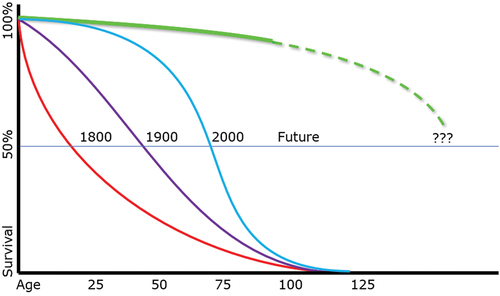
Until recently, longevity research has, as Li Bai might have put it, focused on ‘hunting rabbits.’ Although the mean human lifespan has tripled in the past two centuries (see ), this has been largely due to our knowledge of microbial theory and basic improvements in medical care. The maximum human lifespan, in contrast, has not budged. Much the same might be said, with some cautious qualifications, of our ability to effectively intervene in age-related diseases. Even when we have made inroads in treating such diseases, the improvements have generally been partial improvements in the morbidity and mortality. We postpone or slow the progression of some of the more obvious clinical problems, but without stopping or preventing the underlying aging processes that drive such diseases. We can offer statins and stents for age-related cardiovascular disease, but we do nothing to improve the aging of vascular endothelial cells. We replace osteoarthritic knees, but we do nothing to improve the aging of the chondrocytes that line those joints. In both these cases – as in so many other tissues – we treat the worst of the clinical problems (and at substantial costs, both financially and in terms of patient pain and risks) without addressing aging itself. In short, we have focused on the ‘rabbits’ rather than the ‘tigers and dragons’ of aging.
Figure 1. Historical changes to the human lifespan: mean versus maximum.
2. Why we fail
Why have we made so little basic progress in reversing human aging and in providing effective interventions to cure and prevent age-related disease? Part of the problem has been a series of conceptual barriers that are all-but universal. The two biggest obstacles consist of two unconscious assumptions:
1 - aging is equivalent to entropy and
2 - aging is equivalent to biomarkers.
As a result, we infer that aging cannot be reversed and that effective interventions consist of ‘band aid’ approaches, i.e. those targeting the biomarkers of aging rather than aging itself.
Many of us – despite clearly apparent contradictory evidence – unconsciously view aging as mere entropy, or wear-and-tear, and consequently immutable. After all, isn’t entropy ubiquitous and immutable? Yet we are equally well aware that different species have different lifespans, forcing us to recognize that genetic (and epigenetic) differences clearly play a role. So much for aging being no more than simple entropy alone. To the contrary, aging is, at the very least, forcibly and significantly modified by genetic variables. However, knowing that our genes modify the role of entropy in comparing the different rates of aging in different species is no more an explanation of those differences than is knowing that a single gene (LMN-A) modifies the rate of aging (and age-related disease) in our own species when a child has Hutchinson-Gilford Progeria. Merely pointing to a gene (LMN-A) or a host of genes as a modulator of the rate of aging does not provide an explanatory mechanism and is mere conceptual hand-waving. Genes adjust the rates of aging, but how? In such cases, we recognize that genes modify the aging process and alter the likelihood of age-related disease, but our belated recognition of such a role is not an explanation for how that modification occurs. We need to dig deeper to understand the fundamental relationships. Much the same solecism occurs when we naively attribute aging to mitochondrial dysfunction without addressing the question why mitochondrial dysfunction occurs in the first place. Blaming aging on mitochondrial changes merely begs the question. We cannot wave our hands and attribute aging to mitochondrial failure without explaining how we manage to inherit maternal mitochondria with a provenance of 1.7 billion years, yet those same inherited maternal mitochondria are entirely functional despite their impressive biological age. We cannot place the onus on mitochondria without explaining how 1.7-billion-year-old mitochondria are intact, yet the merely decades-old (measured from fertilization of the ova) mitochondria in our somatic cells clearly and demonstrably are not. Why do billion-year-old mitochondria show no evidence of aging, yet the mitochondria in our somatic cells evince aging in a mere handful of decades? Even our cells themselves, derived from a maternal line of ova going back perhaps 3.5 billion years, are intact at fertilization, yet our somatic cells show obvious aging changes over a mere handful of decades post fertilization. In short, we cannot wave our hands and blame ‘entropy,’ but nor can we simply be content with blaming ‘genes.’ Again, only a more sophisticated understanding of aging can allow us to make progress in changing human aging, human longevity, or age-related disease.
The second assumption occurs when we conflate biomarkers with aging. Biomarkers are no more aging than symptoms are a disease. We blithely talk of the biomarkers or the hallmarks of aging as though they constitute aging, a mistake we would never make in talking about Covid infections: biomarkers are no more aging than dyspnea and leukocytosis are Covid. Biomarkers are remarkably useful in defining the degree of aging – just as symptoms and signs are critical in diagnosing Covid – but we should not confuse biomarkers with the complex cascade of cellular events that constitute aging. Measures of aging are necessary and valuable, but they are not the process itself. Biomarkers are not aging, they are (at best) only measures of aging. When we confuse biomarkers with the process, we get lost in a side issue and mistakenly target outcomes, symptoms, syndromes, and downstream events rather than targeting fundamental processes. Only when we go beyond biomarkers can we alter the aging process.
The first conceptual error can be addressed simply: aging is not the outcome of entropy, but the outcome of entropy in the face of failed maintenance. Biology is exactly that: the ability to maintain complex systems despite entropy. Life has managed that feat sufficiently to result in an uncountable number of living cells (and species) over the past several billion years. Aging occurs not simply due to entropic damage, but rather it occurs when maintenance fails to keep up with entropic damage. We recognize that maintenance keeps up retrospectively in the unbroken germ cell lineage – every one of your cells is, biologically speaking, 3.5 billion years old – yet maintenance fails to keep up in the somatic cell lineage and this failure becomes progressively more apparent in somatic cells post fertilization. This latter progressive failure of maintenance is cell aging. It lies at the heart of all organism aging as well as all age-related diseases. Once we clearly identify the failure of maintenance in cell aging, we can begin to target an effective point of intervention: not entropy, but the down-regulation of maintenance that defines cell aging.
The second conceptual error can also be addressed simply: aging is not biomarkers, hallmarks, or outcomes, but the complex cascade of processes that results in such biomarkers, hallmarks, or outcomes. Again, once we clearly identify the aging process as our target, rather than targeting biomarkers we can target the central process itself: cell aging. We cannot target mitochondrial enzymes, DNA repair enzymes, methylation, acetylation, mutation rates, or glycation when we should be targeting the underlying processes that drive these and myriad other biomarkers of cell aging. Biomarkers can enable us to measure success, they cannot provide an effective target for success.
3. Cell aging
Cell aging is not only the optimal target for age-related diseases and human longevity, but has proven to be an effective target for more than two decades. The first research to demonstrate reversal of cell aging in human cells in vitro was published by the Geron group in a classic paper in Science in 1998 [Citation1]. This established that to the extent we were technically capable of resetting telomere lengths in human cells, we were capable of reversing cell aging. The first research to demonstrate reversal of cell aging in human tissues ex vivo was published by the same group in 2000 [Citation2]. This established that to the extent we were technically capable of resetting telomere lengths in human cells, we were capable to reverse tissue aging. This paper, targeting human skin, was followed by several papers targeting bone [Citation3], vascular endothelial cells [Citation4], etc. The question of whether such interventions could be effective on the organismal, rather than merely the cell or tissue, level was addressed in multiple papers over the past decade, including Blasco’s work using an AAV vector and mouse TERT, showing that to the extent they could extend telomeres, they could reverse behavioral, skin, immune system, bone, and other age-related outcomes in mice [Citation5]. These results were in keeping with the magisterial review of the field, predicting precisely such results in a 2004 medical textbook on this topic [Citation6].
The key question is not ‘can we reverse aging?,’ but the more practical and realistic question of ‘to what extent can we reverse aging effectively, given our current technical limitations?’ By analogy, our current state of technical ability is much like that of the Wright brothers in 1903: they had proven powered flight was possible, but this technology was not yet capable of safely and reliably carrying hundreds of passengers across the ocean in relatively comfortable pressurized cabins at several miles altitude. We are in a parallel situation in that the reversal of cell aging has proven to be feasible, but we are not yet capable of safely and effectively reversing aging and age-related disease in human trials. Our current technical limitations and uncertainties include:
Identifying the optimal form of telomerase (e.g. gene, mRNA, protein, etc.)
Identifying an optimal vehicle (e.g. AAV, LNP’s, etc.) and route (e.g. IT, IV, etc.)
Ensuring delivery to targeted cells (e.g. microglia, vascular endothelial cells, etc.)
Optimizing transduction of targeted cells (e.g. percentage of target cells affected)
Optimizing telomere extension (e.g. a few base pairs versus 10KBP)
Optimizing range of cell aging reversal (e.g. rescuing partially v fully senescent cells)
Establishing clinical limits of tissue and organ age reversal (e.g. extent of clinical cure)
While the reversal of human aging and cures for age-related disease are possible, the approach has not yet undergone rigorous human trials nor has it proven to be either safe or effective.
In addition to the above, a number of caveats pertain. The one most commonly cited in the literature is that of cancer, with the naïve claim that telomerase causes cancer. Telomerase is not causal, although in some cases it can be permissive. The literature is, unfortunately, full of poorly constructed arguments and ill-considered data. The reality is that the relationship between telomerase, telomere lengths, and cancer is complex and decidedly non-linear [Citation7]. One common issue in discussing both age-related disease generally and cancer specifically is the reliance on irrelevant data. This generally consists of measuring peripheral leukocyte telomeres rather than the relevant cells. While such cells are readily obtainable, their relevance to other cell types – those of the CNS, cardiovascular system, liver, kidneys, lungs, joints, etc. – is almost non-existent. This point has been made repeatedly [Citation8,Citation9], but the error persists and is generally unappreciated. In addition, most telomere measurements are done using the mean length (largely irrelevant to cell aging) rather than the shortest telomere (or shortest decile, etc.), which is the operative variable in cell aging. Peripheral leukocytes also divide in response to myriad parameters (infection, stress, etc.) and are unreliable indicators of immune senescence, let alone cell aging in other tissues. A final point is that telomere shortening is implicated in slowing DNA repair and telomere lengthening may well accelerate the repair of DNA damage, hence the suggestion that telomerase therapy may even be clinically protective against cancer, as well as upregulating mitochondrial function and decreasing free radical damage.
4. Potential and barriers to success
The implications of our potential ability to reverse aging and to both cure and prevent age-related disease are without social, financial, or clinical precedent. The closest historical parallel might be that of microbial theory. Note that, as with the case of aging theory, microbial theory was essentially a conceptual rather than a technical advance, and was all the more important for that difference. In both cases – microbial theory and a fundamental understanding of aging – the requirement for making headway depends upon our ability to reexamine and accurately recognize the fundamental causes of diseases. In the case of microbial disease, it required that we accept the ability of ‘invisible’ microorganisms to cause disease. In the case of aging disease, it requires that we reexamine our assumptions about the role of entropy and broaden our conceptual reach to (at the very least) include the germ cell lineage, multiple species, and human progerias. While both microbial and aging theory benefit from technical advances, the key advance is – in both cases – a conceptual one. As in the case of microbial theory, so in the case of aging theory: the ultimate goal is to cure and prevent human diseases, whether the etiology is microbial or cell aging.
Such fundamental conceptual advances require not mere knowledge, but understanding.
The current barriers to such an advance have been alluded to above: we need to revise our understanding to aging to grasp the complex cascade of events in aging and take a unified, systems view of the processes. This lack of understanding has not only hampered effective clinical research, but has hampered investor interest as well. In parallel with this, the IP world is changing dramatically from that of small molecular approaches (in which the molecule is patented) to that of biological interventions, such as gene therapy, in which we patent not molecules (or genes) but protocols. Many legal firms and regulatory agencies have adapted to this sea change in what biotechs ‘own’ as their intellectual property, but many potential investors and VC’s have not yet caught up, again undermining investor willingness to fund the relevant human trials. Such human trials themselves are still barriers that we need to address, as alluded to above. We have yet to define the optimal intervention that will permit safe and effective reversal of cell aging in human patients.
5. Expert opinion: where are we going?
Historically, the single most important medical advance was not technical, but conceptual. Microbial theory did more to improve human lives than anything else in history. The act of hand-washing alone – prior to delivery, prior to surgery, in daily patient care – saved uncountable neonatal and maternal lives, while lowering the costs of medical care. Compare this to purely technical advances such as heart transplants, joint replacement, or robotic surgery. In each case, these resulted in marginal improvements in the quality of life, albeit balanced by increased costs in medical care. Technical advances are necessary and laudable, but conceptual advances outstrip technical advances in their stunning impact on both human welfare and mean lifespan.
Microbial theory was the first conceptual revolution in medicine; we are about to undergo a second conceptual revolution, that of understanding the aging process, thereby enabling us to cure and prevent age-related diseases. If our understanding of microbial theory was the most important past change in medicine, then our understanding of aging will become the most important future change in medicine. The former drastically increased the mean human lifespan, the latter will dramatically increase the maximum human lifespan, and both affect not merely the length, but the quality of human lives.
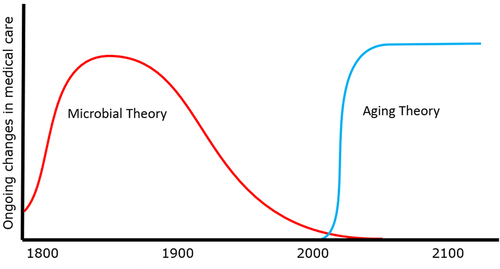
To echo our previous analogy, consider the inadequacy were we to attempt to treat Covid infections with no concept of microbial disease. Given the conceptual limitations, we would target downstream biomarkers (hallmarks, symptoms, signs, etc.) such as cough, discomfort, or fever, using antitussives, anti-inflammatories, and antipyretics. While these approaches might improve the patient’s experience, none of them address the underlying cause of Covid, nor would they significantly improve the Covid mortality rate. Currently, we are in much the same circumstance with regard to age-related diseases: given our current conceptual limitations, we target downstream biomarkers, including amyloid plaques, cholesterol plaques, joint inflammation, and the pathognomonic findings of other common age-related diseases. While these approaches may offer marginal value, none of these approaches address the underlying causes of aging, nor the fundamental drivers of age-related disease. Only by truly understanding the complex cascades of pathology involved in cell aging can we identify and target the optimal point of intervention, both clinically and financially ().
Figure 2. Conceptual revolutions induce transformational change in medicine.
The importance of identifying an optimal point of clinical intervention are manifest, but what of the cost? In 1950, prior to the availability of an effective polio vaccine, there were concerns that the costs of treating polio victims – rehabilitation, braces, nursing care, iron lungs – would result in medical bankruptcy for many countries by the year 2000, yet by the year 2000 the costs of treating polio were approximately 10 cents per patient. So much for the predictions of 1950. Currently, there are concerns that the costs of treating Alzheimer’s disease – as merely one example among many – will result in medical bankruptcy for many countries within the next few decades. For example, the cost of treating a typical Alzheimer’s patient in the last year of life – with full time nursing care – is routinely above $100k. Yet current per patient estimates for the cost of a curative or preventive intervention – telomerase gene therapy – is less than a third of that. Effective therapies for age-related diseases – the dementias [Citation10], cardiovascular disease [Citation11], osteoarthritis, chronic kidney disease, etc [Citation12]. – are not only unlikely to raise medical costs, but (like microbial theory before it) lower the costs of medical care personally, nationally, and globally.
We are on the verge of a transformation in global medicine. Understanding the fundamental aging process, the fundamental driver of all age-related diseases, promises a major revolution in medical care, one with (perhaps excepting microbial theory) no historical precedent. Such a revolution has the potential to radically improve human health, extend the healthy lifespan, and lower medical costs.
As the introductory poem suggests above, we now have the chance to catch not merely the rabbits, but the tigers and dragons of medicine. It is a chance to give the gift of hope to those who have, until now, had none.
Disclosure of interest
The author is president and founder of Telocyte LLC, a startup biotechnology firm targeting Alzheimer’s disease. He has received no salary or other income from the firm. The author has no other relevant affiliations or financial involvement with any organization or entity with a financial interest in or financial conflict with the subject matter or materials discussed in the manuscript apart from those disclosed.
Reviewer disclosures
Peer reviewers on this manuscript have no relevant financial or other relationships to disclose.
Additional information
Funding
References
- Bodnar AG, Ouellette M, Frolkis M, et al. Extension of life-span by introduction of telomerase into normal human cells. Science. 1998;279(5349):349–2. doi: 10.1126/science.279.5349.349
- Funk WD, Wang CK, Shelton DN, et al. Telomerase expression restores dermal integrity to in vitro-aged fibroblasts in a reconstituted skin model. Exp Cell Res. 2000;258:270–278. doi: 10.1006/excr.2000.4945
- Yudoh K, Matsuno H, Nakazawa F, et al. Reconstituting telomerase activity using the telomerase catalytic subunit prevents the telomere shorting and replicative senescence in human osteoblasts. J Bone Mineral Res. 2001;16(8):1453–1464. doi: 10.1359/jbmr.2001.16.8.1453
- Matsushita H, Chang E, Glassford AJ, et al. eNOS activity is reduced in senescent human endothelial cells: preservation by hTERT immortalization. Circ Res. 2001;89(9):793–798. doi: 10.1161/hh2101.098443
- de Jesus B B, Vera E, Schneeberger K, et al. Telomerase gene therapy in adult and old mice delays aging and increases longevity without increasing cancer. EMBO Mol Med. 2012;4:691–704. doi: 10.1002/emmm.201200245
- Fossel M. Cells, aging, and human disease. New York, NY, USA: Oxford University Press; 2004.
- Fossel M, Whittemore K. Telomerase and cancer: a complex relationship. OBM Geriatrics. 2012;5:18. doi:10.21926/obm.geriatr.2101156
- Fossel M. Use of telomere lengths as a biomarker for aging and age-related disease. Curr Tran Geriatr Exp Gerontol Rep. 2012;1:121–127.
- Semeraro MD, Almer G, Renner W, et al. Telomere length in leucocytes and solid tissues of young and aged rats. Aging. 2022;14(4):1713–1728. doi: 10.18632/aging.203922
- Fossel M. A unified Model of dementias and age-related neurodegeneration. Alzheimer’s Dementia. 2020;16(2):365–383. doi: 10.1002/alz.12012
- Fossel M, Bean J, Khera N, et al. A unified Model of age-related cardiovascular disease. Biology. 2022;11(12):1768. doi: 10.3390/biology11121768
- Fossel M, Editor. Aging: how aging works, how we can reverse aging, and prospects for curing aging diseases. (NY): Academic Press; in press.

