1. Introduction
Thanks to biomedical research, significant advances have been made in preventing deaths caused by infectious agents and chronic diseases. Scientific progress has contributed to a remarkable increase in life expectancy, soaring from an estimated 30 years around 1800 to nearly 73 years by 2019 [Citation1]. This unprecedented global rise in life expectancy has resulted in a noticeable shift in the age distribution toward older demographics. However, even with these advancements, we still experience frailty as we age, accompanied by disabilities that reduce our quality of life. While the prospect of an extended lifespan is generally viewed positively, it is essential to recognize that living longer does not automatically guarantee good health. Therefore, developing interventions aimed at promoting healthy longevity has become imperative.
Achieving healthy longevity and mitigating the progressive loss of physiological integrity is a complex endeavor influenced by various factors that contribute to the development of vulnerability and diseases over time [Citation2]. In 2013, Lopez-Otin et al. proposed nine hallmarks of aging, sparking the publication of more than 300,000 articles with the aim of dissecting each hallmark to understand the molecular changes and develop interventions to mitigate aging and aging-associated diseases [Citation3]. Recently 12 hallmarks of aging were described, taking into account age-associated alterations in molecular, cellular, and systemic processes related to the deterioration of biological function over time. These hallmarks include primary (genomic instability, telomere attrition, epigenetic alterations, loss of proteostasis, and disabled macroautophagy), antagonistic (deregulated nutrient-sensing, cellular senescence, and mitochondrial dysfunction), and integrative (stem cell exhaustion, altered intercellular communication, chronic inflammation, and dysbiosis) [Citation3]. Notably among these hallmarks is mitochondria, which are interconnected with all of them, potentially offering a pathway to healthy longevity.
Today, our understanding of mitochondria extends beyond cellular boundaries, as they have been identified as viable entities outside of cells existing in circulation [Citation4–6]. Mitochondria transfer seems to play crucial roles in energy management and facilitating inter-organ metabolic adaptation, particularly in response to nutrient stress and possibly caloric restriction [Citation7]. The phenomenon of natural (natural mitochondrial transfer, NMT) and artificial mitochondrial transfer/transplant (AMT/T) between cells opens up the possibility of modifying the metabolism, mitochondrial DNA (mtDNA), and phenotype of recipient cells, particularly those that could be affected by the aging process [Citation8,Citation9]. This suggests that mitochondria, with their multifaceted roles in intracellular molecular interactions, cellular function, and systemic effects by their transfer between cells, may occupy a central position in the study of the hallmarks of aging and the development of interventions (). We focus on uncovering and proposing an untested link between the hallmarks of aging and mitochondrial transfer to promote healthy longevity.
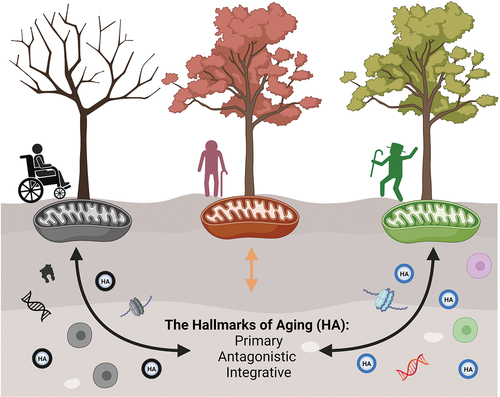
Figure 1. Mitochondria exert an influence on the hallmarks of aging, while the hallmarks, in turn, reciprocally impact mitochondria, shaping the aging process. Trees symbolize the diverse ways we experience aging, and they are intricately linked to mitochondrial function. Mitochondrial function, in turn, is influenced by the hallmarks of aging, which can also be influenced by mitochondria. The hallmarks of aging are categorized into three groups: primary (genomic instability, telomere attrition, epigenetic alterations, loss of proteostasis, and disabled macroautophagy), antagonistic (deregulated nutrient-sensing), and integrative (cellular senescence, stem cell exhaustion, altered intercellular communication, chronic inflammation, and dysbiosis). The hallmarks of aging, much like soil, can be differently enriched, leading to changes in mitochondrial function. Created with BioRender.com.
2. Mitochondrial dysfunction: a central hub linking all other hallmarks of aging
Mitochondria serve as a central hub connecting all other hallmarks of aging and encompassing both their well-understood functions and those crucially remain to be described, such as mitochondrial transfer between cells [Citation10,Citation11]. In light of recent advances in interconnected pathways that may contribute to longevity and reduced age-associated diseases, mitochondria emerge as a key factor of aging decay. Furthermore, it is crucial to delve into how these factors could influence the release and uptake of our ‘nomad’ mitochondria as they migrate outside the cell and from one cell to another, enriching recipient cells with healthy mitochondria and potentially reducing effects on the aging hallmarks.
Single nucleotide polymorphisms (SNPs) in mtDNA have been associated with healthy longevity in centenarians, providing insights into their key role in the aging process and the importance of genomic stability [Citation12]. In contrast, genomic instability is related to the accumulation of somatic mutations in nuclear and mitochondrial DNA affecting essential genes and transcriptional pathways, leading to cellular dysfunction and compromising our health. Depletion in mtDNA content and mitochondrial number is reported during aging [Citation13]. Low mtDNA copy number correlates with frailty and all-cause mortality. Recent research indicates an average loss of four mtDNA copies per decade in humans, linked to age-related physiological changes [Citation14–16]. Additionally, numerous studies affirm the connection between reduced mtDNA content and aging and aging-related diseases [Citation13]. Since the pioneering study conducted by Clark and Shay in 1982, AMT/T has enabled the selective enrichment of a cell’s mitochondrial content with a specific type conferring a selective advantage to cells that are less fit or have become unhealthy as a result of the aging process [Citation6,Citation17].
Among the primary hallmarks of aging, the connection between telomere damage and mitochondrial dysfunction is pivotal to understanding cellular decay over time. The relationship between telomere attrition and mitochondrial metabolic malfunction, particularly through the p53-PGC-1α-NRF-1 axis, remains a subject requiring further research [Citation18]. Interestingly, during the reprogramming of induced pluripotent stem cells (iPSCs), which is associated with potential rejuvenation, telomerase is upregulated, resulting in the lengthening of telomeres to embryonic stem cell (ESC)-like lengths. However, mitochondria that have accumulated mutations during the previous somatic life of the iPSCs exhibit diverse responses, leading to the generation of various mtDNA variants and mitochondrial heterogeneity [Citation19]. Some iPSCs appear to be better suited than others, with specific mtDNA variants improving reprogramming efficiency [Citation20]. The question arises: Is it possible to enhance the reprogramming process using specific AMT/T of mitochondria with mtDNA variants to effectively reset the cellular clock and rejuvenate cells? This intriguing avenue of research holds promise for unlocking new insights into cellular rejuvenation and healthy aging.
The other primary, antagonistic and the integrative hallmarks,—altered intercellular communication, stem cell exhaustion, and chronic inflammation – could also be mediated by NMT and AMT/T of healthy mitochondria. Mesenchymal stem cells (MSCs), along with the hematopoietic niche, are key components of tissue stability and function. Currently, it is unknown how MSCs are affected with time and whether the aging process could change their capacity to transfer mitochondria naturally to other cells. NMT and AMT/T could have strong implications for how cells communicate with each other to maintain their function over time and preserve the health of the immune system (). Furthermore, observations indicate that the transfer of mitochondria to immune cells, whether through natural processes or via AMT/T from MSCs, can induce an immunoregulatory profile and potentially reduce chronic inflammation. Indeed, Singh et al. (2018) have shown that restoring mitochondrial function can mitigate the aging process in mice [Citation13].
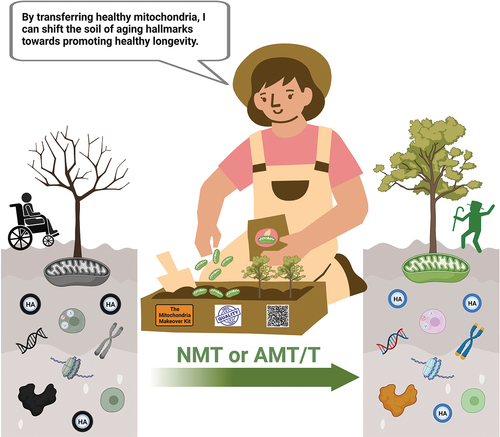
Figure 2. Mitochondria, playing critical roles in molecular, cellular, and systemic aspects of aging, have the potential to profoundly impact the hallmarks of aging (HA) through both natural mitochondrial transfer (NMT) and artificial mitochondrial transfer/transplant (AMT/T). Mitochondria play a pivotal role in governing the molecular, cellular, and systemic processes associated with aging, both in accelerating and potentially reversing the aging process for healthy longevity. In addition to their vital intracellular functions, both NMT and AMT/T hold promise as tools for gaining deeper insights into the hallmarks of aging and for the development of therapeutic interventions. Created with BioRender.com.
It’s worth noting that, except for a few exceptions, the spectrum, precise nature and extent of mitochondrial dysfunction concerning the hallmarks of aging remain largely unknown across various cell types and tissues. The factors primarily responsible for the decline in the common core components of the mitochondrial metabolic machinery, leading to dysfunction and subsequent aging in different tissues, organs, and the entire organism, remain unclear. Intriguingly, not all tissues age in the same manner, adding further complexity to this phenomenon. It could be possible to stimulate NMT between cells in a particular tissue or harness AMT/T with tissue-specific administration of mitochondria. This approach should lead to the utilization of specific mitochondrial transfers in tissues that are particularly affected, offering innovative strategies to rejuvenate and mitigate tissue-specific aging ().
Targeting mitochondria and facilitating NMT or AMT/T as a primary approach to extend lifespan and delay aging-associated disease onset necessitates more research. This research should span from basic science to translational and clinical validation. The identification and understanding of core similarities and tissue-specific differences in the nature and scope of mitochondrial dysfunction, along with its connections to the hallmarks of aging, hold the potential to significantly advance our knowledge in the field of healthy longevity ().
3. Expert opinion
In the pursuit of achieving healthy longevity, we have discovered that intricate interactions among aging hallmarks converge upon a central player – mitochondria. This dynamic organelle, long recognized for its pivotal role in energy production, management, and metabolism, has now emerged as a linchpin connecting the dots among distinct aging hallmarks. From genomic instability to telomere attrition, from cellular senescence to chronic inflammation, mitochondria stand at the nexus of these age-related processes. Recent breakthroughs in our understanding of NMT and AMT/T have illuminated a path toward restoring mitochondrial function, regenerating cellular vitality, and rejuvenating aging cells, tissues, and organs. These ‘nomad’ mitochondria, migrating between cells, enriching recipient cells with essential functions, and potentially mitigating the effects of other aging hallmarks, present new strategies for extending healthy lifespans (). While it is known that the restoration of mitochondrial function can reverse various aging phenotypes [Citation13], it is currently unknown whether cellular rejuvenation through mitochondrial transfer/transplantation can fully reverse the effects of aging. As research advances and synthetic biology opens up new possibilities, we may harness the power of custom-designed mitochondria to re-energize aging tissues and bolster our health over time. In the ever-evolving landscape of aging research, mitochondria must take center stage, offering the prospect of a healthier, more resilient journey through the passage of time.
Declaration of interest
A Caicedo is the leader of Dragon BioMed. KK Singh is the scientific founder of Yuva Biosciences and serves as Chief Scientific Advisor. The authors have no other relevant affiliations or financial involvement with any organization or entity with a financial interest in or financial conflict with the subject matter or materials discussed in the manuscript apart from those disclosed.
Reviewer disclosures
Peer reviewers on this manuscript have no relevant financial or other relationships to disclose.
Author contributions
A Caicedo and KK Singh wrote the manuscript, cured, analyze and conveyed the information in the manuscript. A Caicedo and KK Singh proposed natural mitochondrial transfer (NMT) and artificial mitochondrial transfer/transplant (AMT/T) as key mitigators of aging hallmarks. A Caicedo made the figures with comments from KK Singh. A Caicedo and KK Singh conceived this work.
Acknowledgments
We would like to express our gratitude to the School of Medicine at the Universidad San Francisco de Quito (USFQ), the ‘Instituto de Investigaciones en Biomedicina, USFQ,’ and the Mito-Act Research Consortium in Quito, Ecuador, for their unwavering support of our work and initiatives. Andres Caicedo extends his gratitude to Luisa Páliz for her invaluable support in his personal development and career. AC and KKS thank their laboratory members for their participation in research targeting mitochondria to mitigate aging and aging-associated diseases. The authorship team thanks Reema Azar for her help in ensuring the appropriate use of the English language in the manuscript.
Additional information
Funding
References
- Shaw-Taylor L. An introduction to the history of infectious diseases, epidemics and the early phases of the long-run decline in mortality†. Econ Hist Rev. 2020;73(3):E1–E19. doi: 10.1111/ehr.13019
- Campisi J, Kapahi P, Lithgow GJ, et al. From discoveries in ageing research to therapeutics for healthy ageing. Nature. 2019;571(7764):183–192. doi: 10.1038/s41586-019-1365-2
- López-Otín C, Blasco MA, Partridge L, et al. Hallmarks of aging: an expanding universe. Cell. 2023;186(2):243–278. doi: 10.1016/j.cell.2022.11.001
- Song X, Hu W, Yu H, et al. Existence of circulating mitochondria in human and animal peripheral blood. Int J Mol Sci. 2020;21(6):2122. doi: 10.3390/ijms21062122
- Al Amir Dache Z, Otandault A, Tanos R, et al. Blood contains circulating cell-free respiratory competent mitochondria. FASEB J. 2020;34(3):3616–3630. doi: 10.1096/fj.201901917RR
- Caicedo A, Zambrano K, Sanon S, Luis Vélez J, Montalvo M, Jara F, et al. The diversity and coexistence of extracellular mitochondria in circulation: a friend or foe of the immune system. Mitochondrion. 2021;58:270–284. doi: 10.1016/j.mito.2021.02.014
- Borcherding N, Jia W, Giwa R, et al. Dietary lipids inhibit mitochondria transfer to macrophages to divert adipocyte-derived mitochondria into the blood. Cell Metab. 2022;34(10):1499–1513.e8. doi: 10.1016/j.cmet.2022.08.010
- Balcázar M, Cañizares S, Borja T, et al. Bases for treating skin aging with artificial mitochondrial transfer/transplant (AMT/T). Front Bioeng Biotechnol. 2020;8:919. doi: 10.3389/fbioe.2020.00919
- Cabrera F, Ortega M, Velarde F, et al. Primary allogeneic mitochondrial mix (PAMM) transfer/transplant by MitoCeption to address damage in PBMCs caused by ultraviolet radiation. BMC Biotechnol. 2019;19(1):42. doi: 10.1186/s12896-019-0534-6
- Li Y, Berliocchi L, Li Z, et al. Interactions between mitochondrial dysfunction and other hallmarks of aging: paving a path toward interventions that promote healthy old age. Aging Cell. 2023;e13942. doi: 10.1111/acel.13942
- Berry BJ, Kaeberlein M. An energetics perspective on geroscience: mitochondrial protonmotive force and aging. Geroscience. 2021;43(4):1591–1604. doi: 10.1007/s11357-021-00365-7
- Castañeda V, Haro-Vinueza A, Salinas I, et al. The MitoAging project: single nucleotide polymorphisms (SNPs) in mitochondrial genes and their association to longevity. Mitochondrion. 2022;66:13–26. doi: 10.1016/j.mito.2022.06.008
- Singh B, Schoeb TR, Bajpai P, et al. Reversing wrinkled skin and hair loss in mice by restoring mitochondrial function. Cell Death Dis. 2018;9(7):735. doi: 10.1038/s41419-018-0765-9
- Filograna R, Mennuni M, Alsina D, et al. Mitochondrial DNA copy number in human disease: the more the better? FEBS Lett. 2021;595(8):976–1002. doi: 10.1002/1873-3468.14021
- Zhang R, Wang Y, Ye K, et al. Independent impacts of aging on mitochondrial DNA quantity and quality in humans. BMC Genomics. 2017;18(1):890. doi: 10.1186/s12864-017-4287-0
- Singh KK. Mitochondrial secrets of youthfulness. Plast Reconstr Surg. 2021;147(1S–2):33S–37S. doi: 10.1097/PRS.0000000000007619
- Clark MA, Shay JW. Mitochondrial transformation of mammalian cells. Nature. 1982;295(5850):605–607. doi: 10.1038/295605a0
- Gao X, Yu X, Zhang C, et al. Telomeres and mitochondrial metabolism: implications for cellular senescence and age-related diseases. Stem Cell Rev And Rep. 2022;18:2315–2327. doi: 10.1007/s12015-022-10370-8
- Wei W, Gaffney DJ, Chinnery PF. Cell reprogramming shapes the mitochondrial DNA landscape. Nat Commun. 2021;12(1):5241. doi: 10.1038/s41467-021-25482-x
- Latorre-Pellicer A, Lechuga-Vieco AV, Johnston IG, et al. Regulation of mother-to-offspring transmission of mtDNA Heteroplasmy. Cell Metab. 2019;30(6):1120–1130.e5. doi: 10.1016/j.cmet.2019.09.007

