Abstract
Objectives
Congenital hemangiomas are rare benign vascular tumors but can lead to serious adverse pregnancy outcomes. Its prenatal diagnosis is a challenge. We explored the clinical applications of prenatal ultrasound for evaluating fetal cutaneous hemangioma and associated complications.
Methods
A retrospective observational study was conducted comprising a population of pregnant women with fetal cutaneous hemangioma, the latter diagnosed by prenatal ultrasound, between January 2016 and December 2020. The clinical characteristics, sonographic images, complications, and pregnancy outcomes were documented and analyzed.
Results
We identified 20 cases of fetal cutaneous hemangioma diagnosed by prenatal ultrasound and confirmed by postpartum examinations. Most hemangiomas were in the head and neck (55%), with either solid isoechoicity (25%) or solid mildly hyperechoic (25%), and well-circumscribed (80%) mass. Eight (40%) fetuses experienced complications, which often occurred in fetuses with large hemangiomas (67% with maximum diameter ≥5 cm; 100% with a volume ≥40 cm3). The most common complications were cardiac-related (88%), including elevated cardiothoracic area ratio, atrioventricular valve regurgitation, and fetal hydrops. A large hemangioma was usually associated with advanced gestational age and a fast hemangioma growth rate. In five (25%) cases, the pregnancy was terminated; these involved hemangioma of the head or neck. One newborn developed Kasabach–Merritt phenomenon, pulmonary hemorrhage and respiratory distress, and died 3 days after birth. Among the 14 (70%) fetuses that survived birth, all hemangiomas disappeared or regressed after treatments with propranolol, interventional surgery, or observed routinely.
Conclusions
Prenatal ultrasound examination can accurately diagnose fetal cutaneous hemangioma and related complications to facilitate appropriate management during the pregnancy.
Rationale
Prenatal diagnosis of cutaneous hemangiomas is a clinical challenge. Prenatal ultrasound examination could be a method to accurately diagnose and monitor these hemangiomas.
Introduction
Hemangiomas are vascular tumors formed by rapid endothelial cell proliferations, with unclear pathogenesis [Citation1]. Congenital hemangiomas commonly appear in the head, neck, trunk, or extremities and are fully formed at birth [Citation2]. Congenital hemangiomas can cause serious intrauterine complications, such as fetal high output heart failure, hydrops, anemia, thrombocytopenia, and even death [Citation3–7]. During the delivery, fetuses with large hemangiomas in the head and neck may have airway obstruction that requires immediate ex-utero intrapartum treatments or postpartum management. Therefore, early prenatal diagnosis of congenital hemangiomas is essential to improve pregnancy outcomes [Citation8].
The prenatal diagnosis of congenital hemangiomas is difficult, which often depends on imaging evaluations, such as ultrasound examinations and magnetic resonance imaging (MRI) [Citation9]. MRI scanning is expensive and cannot provide real-time evaluations, whereas ultrasound is a noninvasive convenient bedside test with relatively low cost. However, there have been few reports regarding the prenatal ultrasound diagnosis and monitoring of fetal cutaneous hemangiomas [Citation5,Citation9–14].
Here, we present our retrospective study that analyzed 20 cases of fetal cutaneous hemangiomas, diagnosed by prenatal ultrasound, and confirmed by postpartum evaluations. The prenatal ultrasound characteristics of fetal cutaneous hemangioma are described. The fetal structural complications and pregnancy outcomes are also reported.
Materials and methods
Study design and participant selection
A retrospective study was conducted based on the medical records of pregnant women who received prenatal care at Guangdong Women and Children Hospital in Guangzhou, China between January 2016 and December 2020. The study protocol was approved by the hospital ethics committee (approval number 202101328).
In our hospital, prenatal ultrasound is routinely performed for all pregnant women during gestational weeks 22 through 26, to evaluate fetal development and pregnancy progress. Pregnant women who received a diagnosis of fetal cutaneous hemangiomas, as diagnosed by the prenatal ultrasound screening and confirmed by the postpartum examinations, were included in the present study. Women with incomplete data on the prenatal examinations or follow-up outcomes were excluded.
Diagnosis criteria of cutaneous hemangiomas
The 2018 ISSVA classification assigned vascular diseases to 2 categories: vascular tumors and vascular malformations [Citation15]. Vascular tumors were further categorized as benign (locally aggressive or borderline) or malignant. Congenital hemangioma is a benign vascular tumor. During a prenatal ultrasound examination, when a mixed or homogeneous solid mass on the fetal body surface was detected and showed blood flow in the color Doppler, with or without dilated venous sinuses, cutaneous hemangioma was diagnosed [Citation15,Citation16]. The diagnosis of postpartum cutaneous hemangiomas was based on the clinical examinations [Citation15,Citation16].
Prenatal evaluations
All fetuses underwent the prenatal ultrasound evaluations. The ultrasound system was the GE Voluson E8 and E10 and Samsung WS80A (GE Healthcare, Chicago, USA), with a transabdominal 2-dimensional (2D) convex array probe, 3-dimensional (3D) volume probe, and high-frequency linear array probe, at frequencies 2.0–5.0 MHz, 6.0–8.0 MHz, and 3.0–12.0 MHz, respectively.
During the prenatal ultrasound examination, if a mass was identified in the fetal skin, a 2D ultrasound with a high-frequency linear array probe was used to determine the mass location, size, shape, echogenicity, boundary, internal blood flow, and relationship between the mass and surrounding tissues. A 3D ultrasound surface imaging was applied to identify the mass location and shape. Hemangiomas were grouped as <5 cm or ≥5 cm based on the maximum diameter, and <40 cm3 or ≥40 cm3 in volume.
In addition to the ultrasound evaluations, some fetuses also underwent MRI scanning (FIESTA, SSFSE, or SSFSE Thickslab sequences) and genetic testing, according to the judgment of their treating physicians. MRI scanning was performed with a Brivo MR355 1.5 T MRI scanner with 8-channel coil system (GE Healthcare, Chicago, IL, USA). Two experienced radiologists jointly gave the MRI reports, which included tumor location, size, signal intensity, and concurrent morbidities. Genetic testing was performed using umbilical cord blood lymphocytes. G-banded chromosome analysis (320 bands) and chromosomal microarray analysis (gene chip Affymetrix Cytoscan 750 K, Thermo Fisher Scientific, Santa Clara, CA, USA) were conducted.
Outcome measurements
Outcome measurements included intrauterine complications and pregnancy outcomes. During the prenatal ultrasound evaluations, possible complications such as an enlarged fetal heart size, atrioventricular valve regurgitation, or fetal hydrops, were determined. Cardiothoracic area ratio was calculated, with the diagnostic criterion for a normal heart no larger than one third of the area of the chest [Citation17]. Fetal hydrops was diagnosed when the fetus had effusions in 2 or more cavities. Anemia, abnormal cardiopulmonary measurements, and polyhydramnios were also recorded. Fetal anemia was defined as an increase in the middle cerebral artery peak systolic velocity (PSV) >1.5 multiples of the median (MoM) [Citation18].
Pregnancy outcomes included death or survival. We also investigated the treatments received and prognosis of the hemangiomas.
Statistical analysis
Continuous data are shown as mean and standard deviation or median, as appropriate. Categorical data are shown as number and percentage. Associations between the size of the hemangiomas and gestational age, and growth rate, were analyzed by paired t-test. All statistical analyses were performed using SPSS software (version 19.0, IBM, NY, USA).
Results
Clinical characteristics of study participants
Overall, 21 fetal cutaneous hemangiomas were diagnosed via prenatal ultrasound. Among them, 20 (95.2%) were confirmed as cutaneous hemangiomas through the postpartum examinations. One case had thickened skin in the dorsal side of foot, with no other structure abnormalities. This case was not followed. In 20 cases with the cutaneous hemangiomas, the median maternal age was 28 (ranging from 23 to 35) years old. The mean gestational weeks at the diagnosis of fetal cutaneous hemangioma was 28 (ranging from 23 to 35 + 1) weeks. All these 20 patients underwent prenatal ultrasound examinations. Their clinical courses are listed in Supplementary Table S1.
Among these 20 cases of fetal hemangiomas, 4 (25%) cases had initial misdiagnoses. These included one misdiagnosis of neck skin thickening, and one each of placental hemangioma, teratoma, and possible hemangioma or meningocele. Twelve (60%) patients underwent prenatal MRI scanning, which reported hemangiomas and vascular malformations in 9 and 3 fetuses, respectively. The 3 cases diagnosed as vascular malformations by MRI were diagnosed as hemangiomas after prenatal ultrasound evaluations performed within the same gestational weeks.
Ultrasound characteristics of congenital hemangiomas
The earliest gestational age at which hemangioma was diagnosed via ultrasound was 23 weeks. Most hemangiomas were in the head and neck (55%), with either solid isoechoicity (25%) or solid mildly hyperechoic (25%), and well-circumscribed mass (80%). Most of them (12 cases) had a peripheral blood supply. Central blood flow was mostly observed in cases with head and neck lesions.
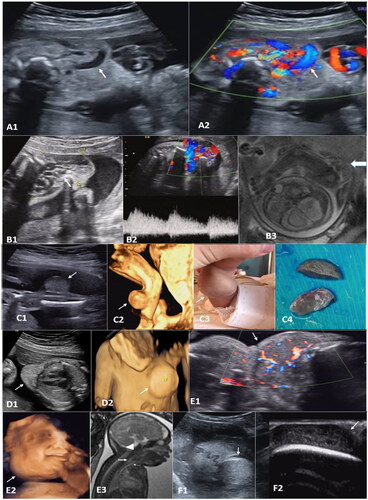
Some hemangiomas showed tortuous tubular anechoic signals, which were dilated vascular sinuses (), or honeycomb structures without obvious capsules (). Hemangiomas appeared as a well-circumscribed tumor-like growth (), while cirsoid angioma was poorly circumscribed (). Hemangioma with arteriovenous leakage had multicolored mosaic blood flow signals at the site of leakage, with speculated spectrums (). On the 3D ultrasound, the location and shape of the mass could be observed stereoscopically (). The relationship between the mass and the fetal body surface could be determined clearly under the ultrasound.
Figure 1. A: (case 17) cervical cutaneous hemangioma at 27 weeks gestation. A1, a mixed mass mainly in the front neck, with a size of about 56 × 33 × 56 mm and internal tubular anechoic signals (arrow). A2 shows abundant blood flow signals within the mass by the color Doppler (arrow). B: (case 5) B1, a mixed echogenic mass was seen in the left shoulder, left axillary area, and left chest wall, with a size of about 58 × 41 × 40 mm, with internal honeycomb echoes (arrow). B2, the rich blood flow signal can be detected internally, with burr-like blood flow spectrum. B3, unenhanced MRI SSFSE sequence shows multiple tortuous vessels with low signal intensities (arrow). C: (case 1) C1, at 23 weeks of gestation, a solid uniform echogenic mass, with a size of 22 × 17 × 19 mm and clear borders, was detected in the dorsal side of right lower leg (arrow). C2, three-dimensional reconstruction (arrow). C3, after birth. C4, surgical resected specimens (arrow). D: (case 12) D1, a hyperechoic well-circumscribed mass in the precordial area of the left chest wall, about 27 × 18 × 33 mm and regular shape (arrow). D2, three-dimensional reconstruction (arrow). E: (case 14) at 35 weeks of gestation, hemangioma from the right buccal extending to the frontal chest wall. E1, internal blood supply is shown in Doppler images (arrow). E2, three-dimensional reconstruction (arrow). E3, unenhanced MRI FIESTA sequence shows a mass (triangle arrow) compressing the esophagus (large arrow) and traches (small arrow). F: (case 2) At 30 weeks of gestation. F1, a cutaneous hemangioma was observed on the top of the head (arrow). F2, the internal structure of the mass was clearly shown by a high frequency line array probe (arrow).
There were 15 cases with the repeated ultrasound evaluations (). The size of hemangioma mass increased with the gestational week (p = .003). Larger hemangiomas had a faster growth rate (p = .001).
Table 1. Size and growth rate of fetal cutaneous hemangioma diagnosed at different gestational weeks in 15 cases who chose to continue pregnancy.
Complications in fetuses with congenital hemangiomas
All the fetuses with hemangiomas in the <5 cm group were without complications. Among the fetuses with hemangiomas ≥5 cm, 8 (67%) had complications. Fetuses with hemangiomas <40 cm3 were without complications. All 8 fetuses with hemangiomas ≥40 cm3 showed complications.
Most of the complications were cardiac-related (7/8, 88%; ). Six fetuses had elevated cardiothoracic area ratio (case 3, 5, 9, 13, 17, 18; 6/20, 60%) and 4 fetuses had tricuspid regurgitation (case 3, 5, 9, 17; 4/20, 20%). One fetus (case 14) had anemia, with increased PSV (80.8 cm/s) to >1.5 MoM in the middle cerebral artery.
Table 2. Prenatal ultrasound findings and pregnancy outcomes in 8 fetuses with complications.
Five fetuses received the prenatal chromosomal examination, including G-banded karyotype and chromosomal microarray analyses, with no obvious abnormalities found.
Pregnancy outcomes
Fifteen fetuses (75%) reached delivery (including one death at day 3 after the birth). Their gestational weeks were 36+1 to 40+6 weeks (mean 38+1 weeks) and the birth weights ranged from 1.96 to 3.85 kg (mean 3.13 kg; (). Five (25%) pregnancies were terminated after discussion between the parents and treating physicians.
All the fetuses in which pregnancy was terminated had cutaneous hemangiomas in the head and neck (4 with complications; ). There was one fetus with occipital scalp hemangioma and without complications, and the pregnancy was terminated as requested by the mother). All masses had significant blood flow signals, with a PSV of 18.3–91.1 (median 43.1) cm/s and resistance index (RI) 0.3–0.8 (median 0.6).
Among the 14 newborns who survived after birth, 5 (25%) underwent postnatal surgery or interventional treatments for hemangioma. One (5%) received propranolol treatment. Eight parents (40%) opted for no surgery and their children were continually followed by the clinic. At this writing, the oldest child is 6 years old and the youngest child is 1 year old. All the hemangiomas disappeared or regressed regardless of whether they were treated with drugs, interventional surgery, or routine observations.
In 8 fetuses with intrauterine complications (), 4 underwent termination of pregnancy and 4 were delivered by cesarean section. MRI showed compressions on the esophagus and trachea from the mass (). One case (case 14) had a large mass extending from neck to the chest, skin edema, pleural effusion, and polyhydramnios. Fetal distress occurred at 36+1 weeks. During the emergency cesarean section, the Apgar scores were 2, 5, and 8 at 1, 5, and 10 min, respectively. A large hemangioma in the neck and jaw (10 × 15 cm) was identified at birth. During the difficult endotracheal intubation, the fetus demonstrated weak breathing, and abnormal development of the oral cavity and airway. Laboratory tests showed anemia and abnormal coagulation functions. The neonate had Kasabach–Merritt phenomenon (KMP) after birth and died 3 days later.
Discussion
This retrospective study explored the applications of prenatal ultrasound in the diagnosis of fetal cutaneous hemangioma. We also reviewed the structural complications and pregnancy outcomes in these cases.
In the present study, cutaneous hemangiomas were found in the head and neck (55%), which was consistent with the previous studies [Citation19,Citation20]. Hemangiomas in these locations suggest a poor outcome. However, we found that more hemangiomas were in the lower limbs than the trunk, which was differed from previous reports [Citation3,Citation21].
The main prenatal ultrasonic characteristics of cutaneous hemangiomas were thickening of the subcutaneous soft tissue, with solid or solid-cystic mixed masses. Color Doppler ultrasound could detect multiple blood flow bands, which were important toward diagnosing hemangioma. A hemangioma of large size was usually associated with advanced gestational age and a fast growth rate.
Cutaneous hemangiomas are easily misdiagnosed as teratoma, placental hemangioma, meningocele, or meningoencephalocele, which occurred in 5 cases in the present study. Teratomas are mostly located in the sacrococcygeal region, head, and neck, with lipid stratification and calcified hyperechoic mass. Placental hemangiomas are mostly located on the fetal surface of the placenta, close to the entrance of the umbilical cord placenta and protruding into the amniotic cavity. The relationship between the mass and surrounding tissues and organs should be monitored to determine its origin, in order to differentiate between placental and cutaneous hemangioma. Meningocele or meningoencephalocele is the interruption of the skull continuity, with a bulging mass at the skull defect, whereas the hemangiomas has intact skull, with the mass not connected with the intracranial tissue.
There were no complications among cases in which the maximum diameter of the hemangioma was <5 cm or volume <40 cm3. The complication rates in cases in which the maximum diameter was ≥5 cm or volume ≥40 cm3 were 66.7 and 100%, respectively. Most complications were cardiac-related (87.5%), mainly indicated by an increased cardiothoracic area ratio and atrioventricular valve regurgitation. This suggested that ultrasound should be used to rule out structural abnormalities in fetuses when the cutaneous hemangioma has a maximum diameter ≥5 cm or mass volume ≥40 cm3.
KMP can be life-threatening, with significant thrombocytopenia, decreased fibrinogen level, increased fibrin degradation products, and accelerated tumor growth. Successful management can resolve KMP and make angioma regress [Citation4]. The only case of postnatal death in this study involved a newborn with congenital clustered hemangioma or kaposiform hemangioendothelioma (KHE) related to KMP. It is necessary to rule out cluster hemangiomas and KHE in cases with KMP.
Polyhydramnios can occur in up to 40% of fetuses with prenatal diagnosis of large neck masses [Citation22]. When there is polyhydramnios, the possibility of esophagus and airway obstruction should be highly suspected. Real-time ultrasound can evaluate these functions in the fetus. Ultrasound is the first choice and highly accurate for prenatal examination of fetal cutaneous hemangiomas. MRI can be an important supplement to the ultrasound examination. MRI has special clinical applications when to evaluate the blood vessels in the mass () and assess the compressions on the esophagus and trachea from the mass. Prenatal ultrasound and MRI may also help delivery planning and immediate postnatal care [Citation23].
In the present study, chromosome examination was performed in 5 patients (case 3,5,8,10,12) before delivery, with no obvious abnormalities found. This result was similar to a previous study reported by Zheng et al. [Citation9].
If the newborn survives, most congenital hemangiomas regress rapidly during the first year of life [Citation24,Citation25]. Some of them may remain stable or partially regress [Citation2]. In the present study, the hemangiomas of newborns who were not give surgery or interventional treatments regressed to varying degrees during the follow-ups of up to 6 years.
The strengths of our study included its large sample size. We also studied the association between the size of hemangioma and complications, and proposed a cutoff value of diameter ≥5 cm and a volume ≥40 cm3. The limitations of the study included its retrospective study and single-center research, which could bring biases into our study. We were not able to identify the prevalence of fetal hemangiomas. The ultrasound examination results could be examiner-dependent, which might limit the generalizability of our study conclusions. We also did not follow up the neonates to study their potential long-term complications. Further prospective studies with a large sample size should be performed to validate our results.
Conclusion
Congenital hemangiomas can cause serious intrauterine complications or even fetal death. Its accurate prenatal diagnosis is always a challenge. Here, we showed that the prenatal ultrasound examination is an important tool in the diagnosis of fetal cutaneous hemangioma and pregnancy monitoring. It can measure not only the size and growth rate of hemangiomas but also detect fetal structural complications to facilitate postpartum management.
Author contributions
ML designed the clinical study and drafted and revised the manuscript. BH performed the data analysis and processed the Figures. ZX collected data and performed the initial data analysis. LW, YC, and XL collected and analyzed the image data. SS prepared the Tables. NS designed the clinical study and reviewed and revised the manuscript.
Supplemental Material
Download MS Word (16.6 KB)Disclosure statement
No potential conflict of interest was reported by the author(s).
References
- Blatt J, Powell CM, Burkhart CN, et al. Genetics of hemangiomas, vascular malformations, and primary lymphedema. J Pediatr Hematol Oncol. 2014;36(8):587–593.
- Wassef M, Blei F, Adams D, ISSVA Board and Scientific Committee, et al. Vascular anomalies classification: recommendations from the International Society for the Study of vascular anomalies. Pediatrics. 2015;136(1):e203-14–e214.
- Enjolras O, Mulliken JB, Wassef M, et al. Residual lesions after Kasabach–Merritt phenomenon in 41 patients. J Am Acad Dermatol. 2000;42(2 Pt 1):225–235.
- Mahajan P, Margolin J, Iacobas I. Kasabach-Merritt phenomenon: classic presentation and management options. Clin Med Insights Blood Disord. 2017;10:1179545x17699849.
- Darouich S, Bellamine H, Mkaouar L, et al. Congenital large cutaneous hemangioma with arteriovenous and arterioarterial malformations: a novel association. Fetal Pediatr Pathol. 2019;38(1):85–90.
- Iacovella C, Chandrasekaran N, Khalil A, et al. Fetal and placental vascular tumors: persistent fetal hyperdynamic status predisposes to poorer long-term neurodevelopmental outcome. Ultrasound Obstet Gynecol. 2014;43(6):658–661.
- Baselga E, Cordisco MR, Garzon M, et al. Rapidly involuting congenital haemangioma associated with transient thrombocytopenia and coagulopathy: a case series. Br J Dermatol. 2008;158(6):1363–1370.
- Kamil D, Tepelmann J, Berg C, et al. Spectrum and outcome of prenatally diagnosed fetal tumors. Ultrasound Obstet Gynecol. 2008;31(3):296–302.
- Zheng W, Gai S, Qin J, et al. Role of prenatal imaging in the diagnosis and management of fetal facio-cervical masses. Sci Rep. 2021;11(1):1385.
- Viora E, Grassi Pirrone P, Comoglio F, et al. Ultrasonographic detection of fetal cranio-facial hemangioma: case report and review of the literature. Ultrasound Obstet Gynecol. 2000;15(5):431–434.
- Yoshida S, Kikuchi A, Naito S, et al. Giant hemangioma of the fetal neck, mimicking a teratoma. J Obstet Gynaecol Res. 2006;32(1):47–54.
- Tsukimori K, Hojo S, Kawarabayashi Y, et al. Fetal neck capillary hemangioma associated with Kasabach–Merritt syndrome. J Ultrasound Med. 2007;26(3):397–401.
- Li L, Qin P. Prenatal diagnosis and outcomes of fetuses with cutaneous hemangioma. Zhonghua fu Chan ke za Zhi. 2006;41(9):605–607.
- Shiraishi H, Nakamura M, Ichihashi K, et al. Prenatal MRI in a fetus with a giant neck hemangioma: a case report. Prenat Diagn. 2000;20(12):1004–1007.
- International Society for the Study of Vascular Anomalies. ISSVA classification for vascular anomalies; 2018. Available from: https://www.issva.org/classification.
- Li S, Luo G. Prenatal ultrasonographic diagnosis of fetal abnormalities. 2nd edition. (in Chinese). Beijing, P.R. China: China Science Publishing & Media Ltd. (CSPM); 2017.
- , Carvalho JS, Allan LD, Chaoui R, International Society of Ultrasound in Obstetrics and Gynecology, et al. ISUOG practice guidelines (updated): sonographic screening examination of the fetal heart. Ultrasound Obstet Gynecol. 2013;41(3):348–359.
- Mari G, Deter RL, Carpenter RL, et al. Noninvasive diagnosis by Doppler ultrasonography of fetal anemia due to maternal red-cell alloimmunization. Collaborative group for Doppler assessment of the blood velocity in anemic fetuses. N Engl J Med. 2000;342(1):9–14.
- Mansfield SA, Williams RF, Iacobas I. Vascular tumors. Semin Pediatr Surg. 2020;29(5):150975.
- Boye E, Jinnin M, Olsen BR. Infantile hemangioma: challenges, new insights, and therapeutic promise. J Craniofac Surg. 2009;20(Suppl 1):678–684.
- Dickison P, Christou E, Wargon O. A prospective study of infantile hemangiomas with a focus on incidence and risk factors. Pediatr Dermatol. 2011;28(6):663–669.
- Marwan A, Crombleholme TM. The EXIT procedure: principles, pitfalls, and progress. Semin Pediatr Surg. 2006;15(2):107–115.
- Kolbe AB, Merrow AC, Eckel LJ. Congenital hemangioma of the face-Value of fetal MRI with prenatal ultrasound. Radiol Case Rep. 2019;14(11):1443–1446.
- Mulliken JB, Enjolras O. Congenital hemangiomas and infantile hemangioma: missing links. J Am Acad Dermatol. 2004;50(6):875–882.
- Boon LM, Enjolras O, Mulliken JB. Congenital hemangioma: evidence of accelerated involution. J Pediatr. 1996;128(3):329–335.

