Abstract
Objective
Accurate prenatal diagnosis of congenital duodenal obstruction (CDO) is challenging. We aimed to determine new ultrasound metrics for accurate prenatal diagnosis of fetal CDO.
Methods
Data pertaining to 46 fetuses with suspected small intestinal obstruction (26 CDO; 16 high jejunal obstructions) were retrospectively analyzed. Prenatal ultrasonographic features including dilated intestinal length, stomach length, maximum intestinal dilatation, ratio of dilated intestinal length at late gestation and dilated stomach length (I/S ratio), and location of distal end of dilated bowel segment relative to spine were compared between CDO and high jejunal obstruction groups. The diagnostic performance of ultrasound indices was evaluated using receiver operating characteristics curve analysis.
Results
In 25 out of 26 CDO cases, the distal end of the dilated small intestine segment was located on the right side of spine, while that in the high jejunal obstruction group was located on the left side of spine. The dilated intestinal length and I/S ratio in CDO group were significantly smaller than those in high jejunal obstruction group (p < .05). Dilated intestinal length <51 mm or I/S ratio <1 showed high sensitivity (100, 100%) and specificity (96.1, 92.3%) for CDO (area under the curve: 0.995 and 0.988, respectively). There were no significant differences in the AUCs of dilated intestinal length and I/S ratio. Significant correlation of the site of obstruction in CDO with fetal dilated intestinal length and I/S ratio (r = 0.686; 0.660, p < .001, respectively) were noted.
Conclusion
Location of the distal end of the dilated small intestine segment relative to the spine, dilated intestinal length, and I/S ratio may help differentiate fetal CDO from high jejunal obstruction. The latter two metrics were associated with the site of obstruction in CDO patients.
Introduction
Congenital duodenal obstruction (CDO) is one of the most common congenital gastrointestinal anomalies, with an estimated incidence of 1 per 2500–10,000 live births [Citation1–3]. CDO is caused by intrinsic or extrinsic factors. Duodenal diaphragm is the most common intrinsic cause and annular pancreas, volvulus, and malrotation are the common extrinsic causes. CDO requires surgery in the neonatal period. Previous studies have demonstrated that early diagnosis can reduce the mortality and morbidity, including duodenal obstruction and intestinal failure [Citation4,Citation5]. Approximately 5% of patients with Down syndrome have duodenal atresia, and 25–50% of patients with duodenal atresia have Down syndrome. Other associated anomalies included VACTERL (vertebral, anorectal, tracheo-oesophageal, renal, limb) association and cardiac anomalies [Citation6–8]. Therefore, accurate prenatal diagnosis of CDO is a key imperative to ideally prompt appropriate prenatal counseling and facilitate further work up and timely treatment.
There is a paucity of studies on prenatal detection of CDO, and in most of these studies, the diagnosis was based on qualitative imaging signs, such as “double-bubble” sign [Citation9], “rat tail” sign, and the “pliers” sign [Citation10]. In particular, the “double-bubble” sign has been shown to be strongly indicative of duodenal obstruction [Citation11]. Although the association between double bubble sign and CDO is well established, its positive predictive value is yet unclear, with some studies reporting false-positives [Citation9,Citation11,Citation12]. Moreover, double bubble sign on prenatal screening has also been reported in cases with other gastrointestinal defects, such as fetal intestinal volvulus, high jejunal atresia, ileal atresia, and abdominal duplication cysts [Citation13–16]. A better identification of true cases of CDO is a clinical need for improving parental counseling and treatment strategies. To the best of our knowledge, no previous study has focused on prenatal diagnosis of CDO by using quantitative indices. The aim of this study was to explore new quantitative indices based on prenatal ultrasound to improve the accuracy of diagnosis of CDO, contributing to proper prenatal counseling and surgical intervention.
Materials and methods
We retrospectively reviewed the records of all pregnant women with suspected fetal intestinal obstruction treated at our center between January 2017 and February 2021. Suspected intestinal obstruction was identified based on prenatal ultrasound findings of dilated small intestine and/or “double-bubble” sign. All included patients were singleton pregnant women with one or more prenatal ultrasound and complete postnatal follow-up at our center. Women for whom detailed prenatal ultrasound data or follow-up including baseline features and details of surgical records after birth were not available at our center or those with poor sonographic image quality were excluded from the study. The diagnosis of CDO or high jejunal obstruction was based on postnatal surgery records. High jejunal obstruction refers to obstruction in the small intestine <30 cm below the Treitz ligament.
Data pertaining to maternal characteristics, gestational age at the time of diagnosis, sonographic findings, and postnatal follow-up were recorded. Gestational age was calculated from the last menstrual period and confirmed by the first trimester crown-rump length measurement. Down syndrome was diagnosed either by prenatal or postnatal chromosomal analysis or based on clinical diagnosis by a pediatrician. The newborns were transferred to neonatal surgery department immediately after birth and the postoperative diagnosis was determined based on the intraoperative findings. Postnatal data including postoperative diagnosis and length of hospital stay were also noted. The study was approved by the Institutional Review Board of our institution (No: 2021053A01). The requirement for informed consent was waived off due to the retrospective analysis. The study protocol complied with the principles of the Declaration of Helsinki.
Ultrasound examination
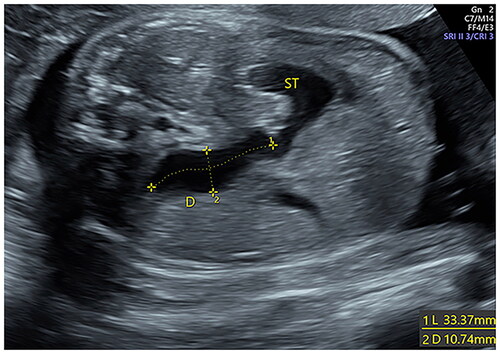
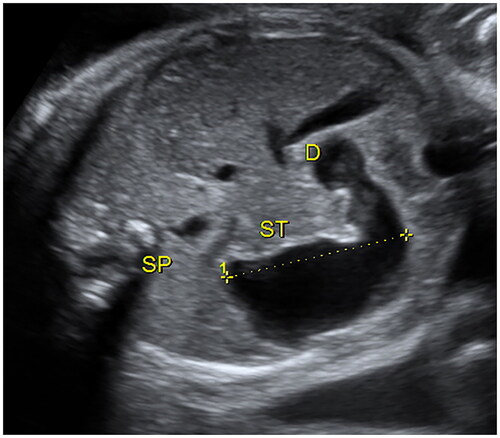
Ultrasound examinations were performed using standard obstetric ultrasound machines (GE Healthcare E10, E8, E6) with a 5.0 MHz trans-abdominal transducer. Two senior obstetricians retrospectively reviewed each scan and independently evaluated the sonographic characteristics of the included participants. Once “double-bubble” sign or the dilated small intestine (>7 mm) [Citation17] was found, the sonographers made a multi-sectional scan of the abdomen to observe the length, inner diameter, distribution, shape, wall, and peristalsis of the dilated bowel, distinguish whether the dilated bowel was connected to the stomach bubble, and observe the distal small intestine, colon, and rectum. In addition, due attention was paid to amniotic fluid, abdominal calcification, ascites, and any cystic mass. Polyhydramnios was defined as a maximum vertical pocket >8 cm or an amniotic fluid index exceeding 25 cm. The length of the dilated small intestine was measured by tracing the length of the midline of the dilated bowel from the beginning of the duodenal bulb to the junction of the dilated segment with the distal narrow segment (). If the dilated small intestine was not seen completely in a single figure, the measurements were made separately in different consecutive parts and were added up to determine the total length of the dilated small intestine. The maximum transverse diameters of the small intestine were measured from inner wall to inner wall (). The longitudinal dimension of the dilated stomach was measured [Citation18] (). All measurements were made for three times and the average value was used in the analysis. In addition, the ratio of the dilated intestinal length and dilated stomach length (I/S ratio) was calculated. The two different examiners analyzed 10 randomly selected patients for the evaluation of intra-observer and inter-observer variability. The same examiner A (J. L.), measured the length and transverse diameters of the small intestine twice. Examiner B (Y. C.) performed the measurements once, and the results were then compared with those of examiner A for inter-observer reproducibility. The examiners were blinded to the postnatal outcomes of patients and the results of the other examiner.
Statistical analysis
Normally distributed continuous variables were described as mean ± standard deviation and between-group differences were assessed using the Student t-test. Non-normally distributed continuous variables were described as median (25–75% interquartile range) and between-group differences were assessed using Mann–Whitney U test. Categorical variables were presented as frequency (%) and between-group differences were assessed using the Pearson χ2 test or continuity correction. The predictive values of independent variables were assessed using receiver operating characteristic (ROC) curve analysis. The optimal cut-off values for the diagnosis of CDO were determined based on the maximum Youden index (sensitivity + specificity − 1). The sensitivity, specificity, positive predictive value, and negative predictive value was calculated using the optimal cut-off level. The intra- and interobserver reproducibility of the measurements were assessed by calculating the intraclass correlation coefficient (ICC) and 95% confidence interval (CI). ICC > 0.80 was considered as excellent and 0.60–0.80 as good. p-Values <.05 were considered indicative of statistical significance. The Spearman rank correlation test was used to analyze the correlation between the location of CDO and sonographic measurements. All statistical analyses were performed using IBM SPSS statistical software package version 25.
Results
Sixty-five patients with prenatal suspicion of CDO and high jejunal obstruction were included in this study. Ten patients opted for termination of pregnancy while eight cases had poor prenatal image quality. Three patients were lost to postnatal follow-up and two newborns did not undergo an operation at our center. Thus, the total number of included participants with complete follow-up was 42 (26 with CDO and 16 with high jejunal obstruction). Out of the 26 CDO, 12 cases had annular pancreas while the others were diagnosed as duodenal diaphragm (three in the superior part, eight in the descending part, two in the horizontal part, and one in the ascending part of duodenum), which were determined by postnatal surgery. All 16 cases with high jejunal obstruction had high jejunal atresia.
compares the baseline features and neonatal outcomes in the CDO and high jejunal obstruction groups. There were no significant differences with respect to gestational age at detection, gravidity of pregnant women, as well as the percentage of patients with polyhydramnios between CDO group and high jejunal obstruction group. Pregnant women in the high jejunal obstruction group were more likely to be parous compared to CDO group. Preterm birth was more common in the high jejunal obstruction group than in CDO group. Additionally, infants in the high jejunal obstruction group had significantly lower gestational age at delivery, birth weight, and Apgar score at 5 min. Besides, the length of hospital stay in the high jejunal obstruction group was much longer than that in the CDO group. In the high jejunal obstruction group, the distal end of the dilated small intestine of the fetus was on the left side of the spine, while that of most fetuses in the CDO group was on the right side of the spine (, ). In one fetus in the CDO group, the distal end of the dilated small intestine was on the front side of spine; this fetus was found to have diaphragm in the ascending part of the duodenum after birth. The dilated intestinal length and I/S ratio in the CDO group were much smaller than those in the high jejunal obstruction group, while there were no significant between-group differences with respect to the dilated stomach length or maximum transverse diameter of the dilated small intestine (). The ICC values indicated good intra-observer and interobserver repeatability (Supplementary Table 1).
Figure 3. (a) A fetus was diagnosed annular pancreas by postnatal surgery. Prenatal ultrasound shown that the dilated bowel connected with the stomach, and the end of the enlarged intestine was located at the level of right side of spine at 35 weeks. White arrow indicates the stomach; yellow arrow indicates the end of the enlarged intestine; blue arrow indicates the spine. (b) A fetus was diagnosed high jejunal atresia by postnatal surgery. Prenatal ultrasound shown that the enlarged intestine, in a “C” shape, connected with the stomach, and extended from the right side of the abdominal cavity to the left side, and the end of the enlarged intestine significantly exceeded the level of left side of spine at 29 weeks. White Arrow indicates the stomach; yellow arrow indicates the end of the enlarged intestine; blue arrow indicates the spine.
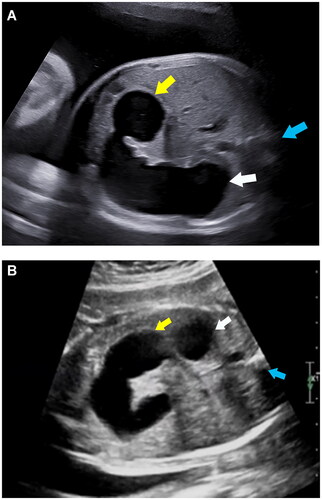
Table 1. Clinical characteristics of patients with CDO and high jejunal obstruction.
Table 2. Prenatal sonographic findings of patients with CDO and high jejunal obstruction.
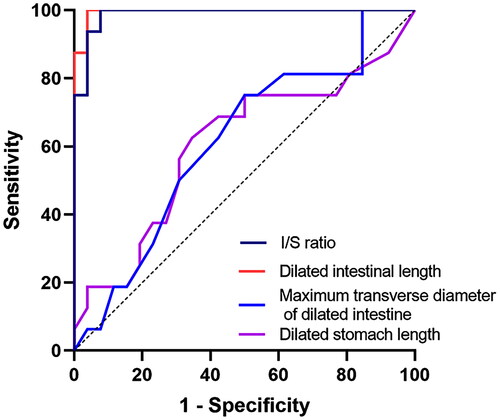
ROC curve analysis was conducted to test the ability of indicators to differentiate between CDO and high jejunal obstruction. shows the following metrics included in the ROC curve analysis: dilated intestinal length, dilated stomach length, maximum transverse diameter of dilated small intestine, and I/S ratio. The AUC values ranged from 0.606 to 0.995. The ROC curves of the above indices are shown in . The AUC value of dilated intestinal length and I/S ratio were 0.995 and 0.988, respectively, and the optimal cut-off values were <51 mm and <1, respectively, which differentiated CDO from high jejunal obstruction with 96.2%, 92.3% specificity, and 100% sensitivity, respectively (). Besides, no significant differences in the AUCs of dilated intestinal length and I/S ratio were noted (p = .33) ().
Table 3. Results of receiver operating characteristic curve analysis.
To further analyze the relationship between the location of CDO and the above ultrasound metrics, CDO patients were divided into four groups, according to the location of duodenal obstruction. The obstruction in the first, second, third, and fourth parts of the duodenum were respectively referred to as groups 1, 2, 3, and 4. Since the annular pancreas cases were characterized by partial or complete circumferential encasement of the second part of the duodenum by pancreatic tissue, these were considered as group 2. Spearman rank correlation analysis revealed that the location of obstruction in the CDO was significantly correlated with fetal dilated intestinal length and I/S ratio (r = 0.686; 0.660, p < .001, respectively), whereas it showed no obvious correlation with the length of the dilated stomach or the maximum transverse diameter of the dilated small intestine ().
Table 4. Correlation between the location of CDO and ultrasound metrics.
Discussion
Accurate prenatal diagnosis of CDO allows for personalized antenatal and postpartum counseling and facilitates the timely formulation of an appropriate treatment strategy. Studies have demonstrated that prenatal diagnosis of duodenal atresia decreases the delay before operation and reduces the morbidity from metabolic complications [Citation19]. Several ultrasound indicators have been shown to potentially improve the prenatal diagnosis of CDO, including the “double-bubble” sign [Citation9], “rat tail” sign, and the “pliers” sign [Citation10]. However, differentiation of the CDO from high jejunal obstruction remains challenging. In this study, we explored the association between the location of the distal end of the dilated small intestine and the site of intestinal obstruction and developed new quantitative metrics, i.e. dilated intestinal length at late gestation and I/S ratio. We found that these parameters were significantly decreased in fetuses with CDO, compared to those with high jejunal obstruction.
The ligament of Treitz connects the end of the duodenum and beginning of the jejunum in the gastrointestinal tract. Since the ligament of Treitz stabilizes the duodenojejunal flexure and prevents its displacement, it is usually located on the left side of the second lumbar vertebra level. Similar to the anatomy of ligament of Treitz, we noticed that the distal end of the dilated duodenum was on the right side of the spine in most CDO cases (except in one case where it was on the front side of spine), while the distal end of the dilated small intestine was on the left side of spine in cases with high jejunal obstruction. Thus, we proposed that the location of the distal end of the dilated small intestine segment connected with the stomach can provide anatomical information to facilitate the identification of the location of intestinal obstruction. To be specific, if the distal end of the dilated small intestine segment connected with the stomach is located on the left side of the spine, it is likely to be a case of high jejunal obstruction while if it is located on the right or front side of the spine, it is likely to be CDO; this phenomenon corresponds to the anatomy of the ligament of Treitz. Thus, in the case of prenatal detection of the dilated small intestine, the location of the distal end of the dilated small intestine segment relative to the fetal spine may facilitate the sonographers in providing the initial impression of the location of intestinal obstruction.
The double-bubble sign has been shown to be a predictive marker of CDO [Citation9]. However, the double bubble sign can be seen in other gastrointestinal anomalies [Citation13–16]. Accurate prenatal counseling for CDO provides families with valuable insights into postnatal treatment and outcomes. The low sensitivity of this marker together with potential differential diagnosis (such as high jejunal obstruction) often makes the counseling unfocused and possibly increases parental anxiety. To date, no studies have quantified the detailed dilated small intestine length to predict CDO in utero. We demonstrated that the dilated intestinal length (<50 mm) at late gestation can help distinguish between CDO cases and high jejunal obstruction with 96.1% specificity and 100% sensitivity. However, the length of the dilated gastrointestine may change due to gastrointestinal peristalsis. The stomach and bowel dilation may even change during the same scan, which makes the accurate prediction of CDO at late gestation using the length of dilated small intestine challenging. Thus, we further explored a new indicator I/S ratio on the evaluation of the location of intestinal obstruction. Of note, I/S ratio achieved good separation of the cohort with CDO from high jejunal obstruction. Fetal I/S ratio (<1) predicted CDO with 92.3% specificity and 100% sensitivity. Despite, the AUC value for dilated intestinal length was greater than that of the I/S ratio, the difference was not statistically significant. Assessments of the I/S ratio is a simple and convenient method. It is feasible in patients with suspected dilated bowel. A recent study by Chen et al. found a novel sonographic marker, C-sign, resembling the shape of the dilated duodenum according to the anatomical location of the obstruction. It was shown to enable early detection of jejunal atresia, particularly high jejunal atresia [Citation20]. Although this sign is useful in detecting high jejunal atresia, the analysis was limited to individuals with non-duodenal small bowel atresia. The C sign can be observed in some cases with obstruction in the distal part of the duodenum because of the dilation of almost the entire duodenum. To the best of our knowledge, our study is the first study to demonstrate the use of I/S ratio in predicting CDO with high sensitivity and specificity. I/S ratio could be a useful additional diagnostic imaging marker for fetuses with suspected CDO with the dilated small intestine.
What’s more, fewer studies have assessed the quantitative ultrasound characteristics of CDO prenatally [Citation21]. A study by Kim et al. suggested that maximum duodenal dilation may be helpful in distinguishing the cause of CDO, but not adverse outcomes [Citation21]. In contrast, we found no association between maximum transverse intestinal dilation with the location of CDO. Besides, it failed to achieve the good distinction of fetuses with CDO from those with high jejunal obstruction. Part of this discrepancy may be explained by the different composition of the study population (only annular pancreas and duodenal diaphragm in CDO group in this study), operator variability, and the effect of measurements at different gestational ages. Additionally, the dilated intestinal length at late gestation and I/S ratio showed a significant positive association with the location of CDO. These observations are in line with the anatomy of duodenum. The dilated intestinal length at late gestation and the I/S ratio directly reflect the duodenal dilation above the obstructive site, which may probably contribute to further prediction of the location of CDO in cases with suspected CDO. However, in this study, the small number of patients with obstruction in the ascending part of duodenum limited further subgroup analysis. Further studies are required to assess the usefulness of these markers in the prenatal diagnosis of CDO.
Some limitations of our study should be acknowledged. This was a single-center retrospective study. The incidence of CDO in our center is about 7.25:10,000 which is higher than the reported incidence of CDO (1 per 2500–10,000) [Citation1] since our center, a tertiary specialized hospital for women and children, provides the referral system for complicated cases and intensive care for the secondary care facilities and some tertiary general hospitals. Most of the patients were referred because of prenatal findings of the dilated fetal intestine and some patients chose to terminate the pregnancy which led to relatively small numbers with complete follow-up. Furthermore, the predictive value of the metrics reported in this study might be biased by the fact that it was extrapolated from the CDO cases only with annular pancreas and duodenal diaphragm; thus our study may not entirely reflect the true diagnostic performance of these indices for prenatal detection of CDO. Therefore, a larger multicenter study is warranted to validate these indicators.
Conclusion
The location of the distal end of the dilated small intestinal segment connected with the stomach relative to spine, dilated intestinal length, and I/S ratio may help distinguish cases of fetal CDO from those with high jejunal obstruction in the cohort of patients with bowel dilation. Dilated intestinal length and I/S ratio were also associated with the location of CDO. Larger prospective studies are required to validate our findings.
Supplemental Material
Download MS Word (12.3 KB)Disclosure statement
No potential conflict of interest was reported by the author(s).
Additional information
Funding
References
- Escobar MA, Ladd AP, Grosfeld JL, et al. Duodenal atresia and stenosis: long-term follow-up over 30 years. J Pediatr Surg. 2004;39(6):867–871.
- Bethell GS, Long AM, Knight M, et al. Congenital duodenal obstruction in the UK: a population-based study. Arch Dis Child Fetal Neonatal Ed. 2020;105(2):178–183.
- Lupo PJ, Isenburg JL, Salemi JL, et al. Population-based birth defects data in the United States, 2010–2014: a focus on gastrointestinal defects. Birth Defects Res. 2017;109(18):1504–1514.
- Burjonrappa S, Crete E, Bouchard S. Comparative outcomes in intestinal atresia: a clinical outcome and pathophysiology analysis. Pediatr Surg Int. 2011;27(4):437–442.
- Chen QJ, Gao ZG, Tou JF, et al. Congenital duodenal obstruction in neonates: a decade’s experience from one center. World J Pediatr. 2014;10(3):238–244.
- Choudhry MS, Rahman N, Boyd P, et al. Duodenal atresia: associated anomalies, prenatal diagnosis and outcome. Pediatr Surg Int. 2009;25(8):727–730.
- Dalla Vecchia LK, Grosfeld JL, West KW, et al. Intestinal atresia and stenosis: a 25-year experience with 277 cases. Arch Surg. 1998;133(5):490–496; discussion 496–497.
- Spahis JK, Wilson GN. Down syndrome: perinatal complications and counseling experiences in 216 patients. Am J Med Genet. 1999;89(2):96–99.
- Koberlein G, DiSantis D. The “double bubble” sign. Abdom Radiol. 2016;41(2):334–335.
- Yin C, Tong L, Ma M, et al. The application of prenatal ultrasound in the diagnosis of congenital duodenal obstruction. BMC Pregnancy Childbirth. 2020;20(1):387.
- Traubici J. The double bubble sign. Radiology. 2001;220(2):463–464.
- Morris G, Kennedy A Jr., Cochran W. Small bowel congenital anomalies: a review and update. Curr Gastroenterol Rep. 2016;18(4):16.
- Gilbertson-Dahdal DL, Dutta S, Varich LJ, et al. Neonatal malrotation with midgut volvulus mimicking duodenal atresia. Am J Roentgenol. 2009;192(5):1269–1271.
- Ohuoba E, Fruhman G, Olutoye O, et al. Perinatal survival of a fetus with intestinal volvulus and intussusception: a case report and review of the literature. AJP Rep. 2013;3(2):107–112.
- Kucińska-Chahwan A, Posiewka A, Bijok J, et al. Clinical significance of the prenatal double bubble sign – single institution experience. Prenat Diagn. 2015;35(11):1093–1096.
- Yang Y, He P, Li DZ. Clinical outcome of pregnancies with the prenatal double bubble sign – a five-year experience from one single Centre in mainland China. J Obstet Gynaecol. 2018;38(2):206–209.
- Nyberg DA, Mack LA, Patten RM, et al. Fetal bowel. Normal sonographic findings. J Ultrasound Med. 1987;6(1):3–6.
- Kepkep K, Tuncay YA, Göynümer G, et al. Nomogram of the fetal gastric size development in normal pregnancy. J Perinat Med. 2005;33(4):336–339.
- Miro J, Bard H. Congenital atresia and stenosis of the duodenum: the impact of a prenatal diagnosis. Am J Obstet Gynecol. 1988;158(3 Pt 1):555–559.
- Chen D, Tam KH, Xiao Y, et al. New sonographic feature (C-sign) to improve the prenatal accuracy of jejunal atresia. J Obstet Gynaecol Res. 2021;47(12):4196–4202.
- Kim JY, You JY, Chang KH, et al. Association between prenatal sonographic findings of duodenal obstruction and adverse outcomes. J Ultrasound Med. 2016;35(9):1931–1938.