 ?Mathematical formulae have been encoded as MathML and are displayed in this HTML version using MathJax in order to improve their display. Uncheck the box to turn MathJax off. This feature requires Javascript. Click on a formula to zoom.
?Mathematical formulae have been encoded as MathML and are displayed in this HTML version using MathJax in order to improve their display. Uncheck the box to turn MathJax off. This feature requires Javascript. Click on a formula to zoom.Abstract
Background
Hypertensive disorders of pregnancy (HDP) is associated with an increased risk of adverse outcomes. The fetal middle cerebral artery (MCA) and umbilical artery (UA) blood flow detected by ultrasound are recommended to evaluate the oxygenation of the fetus. It is necessary to analyze the relationship between MCA & UA doppler indices or cerebroplacental ratio (CPR) and fetal outcomes and describe MCA and UA blood flow values across gestation.
Methods
Hospital-based retrospective case-control study during 2016 to 2020. 800 singleton pregnant women: 400 normotensive control, 219 gestational hypertension (GH), and 181 preeclampsia (PE)/eclampsia (EC). An analysis of the outcomes of mothers and neonates was performed. The fetal MCA and UA blood flow values across gestation were established, and MCA-resistance index (RI) and CPR were used to predict fetal distress and small for gestational age (SGA).
Results
In the normotensive control, GH and PE/EC groups, the mean gestational age (GA) was 38.9 ± 1.2 weeks, 39.0 ± 1.0 weeks, and 38.6 ± 1.3 weeks respectively, and the mean birth weight (BW) was 3.195 ± 0.387 kilograms, 3.198 ± 0.428 kilograms, and 2.987 ± 0.544 kilograms respectively. There were differences in GA, BW, fetal distress, SGA and intraventricular hemorrhage I-II between the hypertension group and normotensive control group (p < 0.05). The MCA-RI (sensitivity: 70.1%, specificity: 64.3%) and MCA-RI (sensitivity: 52.4%, specificity: 84.6%) were the best indices to predict fetal distress and SGA, respectively during GA of 35-40 weeks.
Conclusions
Fetal MCA blood flow values and CPR are of great benefit for obstetricians to evaluate the status of fetus evidentially in singleton pregnancy.
Introduction
Hypertensive disorders of pregnancy (HDP), including gestational hypertension (GH), preeclampsia (PE) and eclampsia (EC), is associated with an increased risk of adverse outcomes for both mothers and neonates [Citation1,Citation2]. PE/EC is the second leading cause of maternal mortality in the world, and it might result in more and worse maternal complications than GH [Citation3,Citation4], including stroke, severe headache, acute kidney injury, and uteroplacental dysfunction [Citation5].
Ultrasound is recommended for use in the detection of fetal middle cerebral artery (MCA) and umbilical artery (UA) blood flow during pregnancy [Citation6]. The ratio of peak systolic blood flow velocity to end-diastolic blood flow velocity (S/D), resistance index (RI), pulsatility index (PI) and mean flow velocity (Vm) are the most used indices to describe arterial flow velocity waveforms. According to previous studies [Citation7], the S/D has a parabolic correlation with uterine vascular resistance and RI has a logarithmic correlation with uterine vascular resistance, while PI is linearly correlated with uterine vascular resistance. Moreover, the PI, RI and S/D reflect the oxygen supply in the womb and could be used to determine the occurrence of intrauterine hypoxia. WHO had reported that preterm small-for-gestational-age (SGA) was associated with medical conditions related to PE or EC [Citation8], and studies in SGA and fetal growth restriction (FGR) had reported a relationship with abnormal umbilical and cerebral flow [Citation9]. Abnormal cerebroplacental ratio (CPR) could be used in the prediction of adverse perinatal outcomes, such as early childhood delayed neurodevelopment in the setting of FGR [Citation10]. Majority of SGA infants need more sophisticated care and treatments. Compared to appreciate for gestational age, SGA infants have more probabilities of multiple complications, such as hypoglycemia after birth [Citation11], developmental and cognitive delays in infancy [Citation12], and sudden arrhythmic death syndrome in adult [Citation13]. The severe fetal distress might eventually lead to the perinatal asphyxia and postnatal hypoxic ischemic encephalopathy. If we can make a timely and accurately evaluation on fetus status during pregnancy through MCA & UA doppler indices or CPR, it will give obstetricians more information to decide that whether ongoing pregnancy or timely cesarean section.
The aim of this study was to analyze the relationship between MCA & UA doppler indices or CPR and fetal outcomes in HDP. Moreover, we described fetal MCA and UA blood flow values across gestation for a singleton pregnancy and to determine the relationship between MCA & UA doppler indices or CPR and neonatal adverse outcomes.
Materials and methods
Participants
Hospital-based retrospective case-control study was performed from 1 April 2016 to 1 April 2020. Included singleton pregnant women were classification as follows: the normotensive control group and hypertension group (GH group and PE/EC group). The GH group was defined as pregnant women who had hypertension developing after 20 weeks of gestation and resolving within 42 days postpartum. The PE/EC group was defined as GH pregnant women who were accompanied by one of a series of syndromes [Citation5]. The normotensive control group was defined as pregnant women who had a normal level of blood pressure through whole pregnant period.
The inclusion criteria were as follows: ① the mother’s ages were between 18 and 35 years old without a series of diseases (diabetes mellitus, thyroid dysfunction, hepatic and kidney diseases, and immune system disease); ② pregnant women had negative results of routine blood and urine, blood biochemistry (liver and renal function), and coagulation function tests before 20 weeks of gestation; ③ pregnant women were at a normal pregnancy status and had a healthy embryo sac in womb confirmed by doppler ultrasound examination to determine the gestational age. ④ pregnant women had negative results of blood pressure [Citation2] measured before 20 weeks of gestation.
The exclusion criteria were as follows: ① history of alcohol, smoking, or taking drugs; ② history of chronic hypertension and HDP or family history of high blood pressure; ③ pre-gestation body mass index (BMI) < 18.5 or ≥ 25 kg/m2.
Definition was as follows: ① Fetal distress: a. Fetal heart rate was persistently lower than 110 rates per minute or higher than 180 rates per minute accompanied by turbid amniotic fluid (II or III degree). b. Turbid amniotic fluid (III degree) accompanied by oligohydramnios. c. Fetal heart rate was slowly decreased to lower than 110 rates per minute. d. Repeated late deceleration or severe variable deceleration in fetal heart rate monitoring [Citation14], and the fetal heart rate that decreased to less than 60 times per minute was lasting for more than 60 s. e. Baseline variation of fetal heart rate mapping disappeared with late deceleration. ② SGA: the infant’s birth weight (BW) is less than the 3rd percentile of the same gestational age.
Clinical characteristics
The maternal characteristics were collected: mother’s age, height (cm), the methods of delivery (Cesarean/Vaginal delivery), primipara or multipara, assisted reproductive technology (artificial impregnation or in vitro fertilization and embryo transfer), premature rupture of membranes >18 h, and turbid amniotic fluid. Albumin (ALB), alanine aminotransferase (ALT), aspartate aminotransferase (AST) and total bile acid (TBA) were collected for liver function assessment; creatinine (CREm) and a 24-h urinary protein collection were collected for renal function; prothrombin time (PT), activated partial thromboplastin time (APTT), D-dimer and fibrinogen (Fib) were collected for coagulation function; and platelet counts (PLTs) of routine blood tests were collected. All above hematologic data were collected at 20 weeks of gestation. The maternal outcomes were collected: placental abruption, and postpartum hemorrhage.
The neonatal characteristics were collected: gestational age (GA), BW, male, and Apgar 1 and 5-min. The neonatal outcomes were collected: fetal distress, SGA, congenital heart diseases (patent ductus arteriosus, atrial septal defect, ventricular septal defect, and pulmonary arterial hypertension), abnormal coagulation (increased APTT, PT and FDP, decreased Fib, and normal PLT), and intraventricular hemorrhage (IVH) I-II. Among them, IVH I-II was detected and diagnosed by brain ultrasound.
Ultrasound parameters
The fetal doppler ultrasound examinations were carried out in the three different periods after 20 weeks of gestation, including 20–24 weeks of gestation, 28–32 weeks of gestation and 35–40 weeks of gestation. We routinely make an ultrasound fetal malformation screening, amniotic fluid volume and early UA and MCA blood flow parameters during 20–24 weeks of gestation. The fetal parameters for determining gestational age were measured, including biparietal diameter, abdomen circumference, femur length and head circumference. We chose ultrasound placental grading to be indicative of placental maturity and function, including grade 0 to III [Citation15]. Amniotic fluid volume and UA and MCA blood flow parameters were measured by ultrasound during 28–32 weeks of gestation. The fetal doppler ultrasound was also used to evaluate the fetal position, fetal intrauterine condition, estimated fetal weight, amniotic fluid volume and late UA and MCA blood flow parameters during 35–40 weeks of gestation. Pulsed wave doppler ultrasonography was performed using a Voluson E10 machine (General Electric Health care ultrasound, Austria), and the chosen transabdominal ultrasound doppler probe frequency was set at 3–9 MHz (GA < 29 weeks) and 2–5 MHz (GA ≥ 29 weeks) to eliminate low-frequency noise from peripheral blood vessels. Color flow mapping was used to identify the circle of Willis and the proximal MCA, as shown in . Doppler measurements were reproducible and carried out by the same ultrasound physician three times. We chose the ultrasound parameters (PI, RI, S/D and Vm) to detect fetal MCA and UA blood flow during pregnancy.
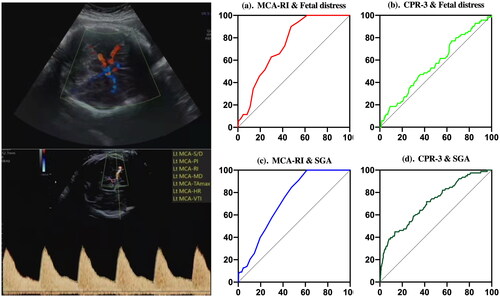
Figure 1. (a and b). Best ROC values of fetal distress predicted by the fetal middle cerebral artery resistance index (MCA-RI) and cerebroplacental ratio 3 (CPR-3); (c and d). Best ROC values of small for gestational age (SGA) predicted by MCA-RI and CPR-3. X axis refers to 100%-Specificity%; Y axis refers to Sensitivity%.
PI, was calculated using the following formula:
Vs: peak systolic velocity of blood flow; Vd: peak diastolic velocity of blood flow; Vm: mean flow velocity of blood flow.
RI, was calculated using the following formula:
S/D, indicating that the resistance of blood flow was calculated using the following formula:
CPR, was calculated using the following formula:
The study was approved by the ethics committee (Ethics approval number: 2020KY065).
Statistical analysis
The statistical analyses were performed using SPSS Statistic version 26.0 (IBM Corp, Armonk, NY, USA) and MedCalc version 19.6.4 (MedCalc Software Corp, Mariakerke, Belgium). The demographic characteristics were expressed as the mean ± SD (standard deviation) or the median (P25, P75) for continuous data. Comparisons between groups were performed using one-way analysis of variance and using the Kruskal Wallis test where appropriate. A receiver operating characteristic curve (ROC curve) was generated to compare the area under the curve (AUC), sensitivity, specificity, and cutoff values. Chi-square tests or Fisher’s exact tests were given in the analyses of categorical variables as appropriate. The results with p < 0.05 were considered statistically significant.
Results
Participants
From 2016 to 2020, a total of 2354 women with a singleton pregnancy gave birth to live neonates in our hospital. After excluded, 400 women (normotensive control group), 219 women (GH group), and 181 women (PE/EC group) were included. And a total of 400 women in the normotensive control group were included in the analysis of fetal MCA and UA blood flow values across gestation, as shown in .
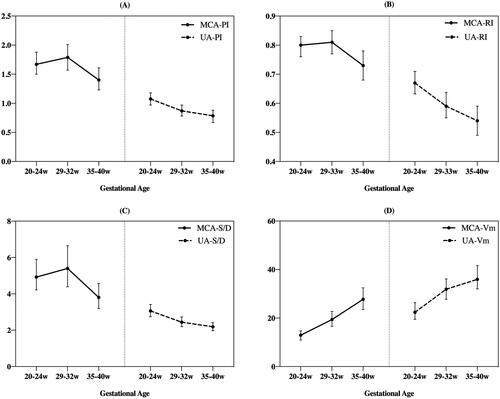
Figure 2. Mean and standard deviation were used to describe the fetal middle cerebral artery (MCA) and umbilical artery (UA) blood flow in the three different periods (20–24 weeks, 29–32 weeks and 35-40 weeks. (A). MCA-PI and UA-PI; (B). MCA-RI and UA-RI; (C). MCA-S/D and UA-S/D; (D). MCA-Vm and UA-Vm.
Outcomes of mothers and neonates
Both proportion of primipara and cesarean delivery rates of women in the normotensive control group were found to be significantly lower than those in the hypertension group (p < 0.001). A series of perinatal outcomes, including placental abruption, and postpartum hemorrhage, and counts of 24-h urine protein collections in the hypertension group were found to be higher than in the normotensive control group (p < 0.05). Although the data of CREm were significant higher in the hypertension group in comparison with that in the normotensive control group (p = 0.016), both were at normal ranges, as shown in sTable 1.
There were significant differences in GA and BW between the hypertension group and normotensive control group (p < 0.05). After post hoc subgroup comparisons, we found that there were significant differences in GA between the group of GH and PE/EC, and neonates in the PE/EC group had the lower GA than the other one, as shown in sTable 1. Moreover, the neonates’ BW of GH and normotensive control group were both higher than that of PE/EC group (p < 0.001). Obviously, the incidence of SGA in both the GH group (10.0%) and PE/EC group (20.4%) were higher than the normotensive control group (4.8%), p < 0.001. There were significant differences of fetal distress between the normotensive control group and hypertension group (p = 0.011). Meanwhile, there were significant differences in postnatal IVH I-II between hypertension group (GH:1.4%; PE/EC:5.5%) and normotensive control group (0.75%), p = 0.002. No differences in male, Apgar 1-min, Apgar 5-min, congenital heart diseases, and abnormal coagulation among the three groups were found (p > 0.05), as shown in sTable 1.
Relationship between doppler indices and fetal outcomes in HDP
The MCA-PI and MCA-RI in both the GH and PE/EC groups were significantly decreased compared to the normotensive control group (p < 0.05), and no differences were found between the GH group and PE/EC group (p > 0.05). The MCA-S/D and MCA-Vm were not different among the three groups (p > 0.05). The UA-PI, UA-RI, UA-S/D and UA-Vm were found differences in the GA of 35-40 weeks among three groups (p < 0.05). The CPR-1 of hypertension group was higher than normotensive control group (p = 0.043), while both CPR-2 and CPR-3 of hypertension group were lower than normotensive control group (p < 0.005), as shown in .
Table 1. The ultrasound detection of the fetal middle cerebral artery, umbilical artery blood flow of the normotensive controls group, GH group and PE/EC group.
The MCA-RI and CPR-3 had significant value in the prediction of fetal distress during 35-40 weeks of gestation (p < 0.05). The MCA-RI showed the largest AUC (0.740) and Youden index (0.34), and its specificality was 0.643. The MCA-PI or RI (GA of 35–40 weeks), CPR-2 and CPR-3 could be used as a predictor for SGA (p < 0.05). The MCA-RI showed the largest AUC (0.728) and Youden index (0.37), and its specificality was 0.846. When MCA-RI (GA of 35-40 weeks) decreased to 0.70, the probability incidence of fetal distress could reach up to 70.1%. Additionally, if MCA-RI (GA of 35–40 weeks) decreased to 0.73, the accurate incidence of SGA could reach up to 84.6%, shown in and . Moreover, in the prediction of SGA, the CPR-2 (AUC:0.648, cutoff:2.21) and CPR-3 (AUC:0.712, cutoff:1.77) could be used to improve the specificity (92.3%) and sensitivity (58.2%), respectively.
Table 2. ROC values of fetal distress and small for gestational age predicted by the ultrasound detection of the fetal middle cerebral artery blood flow and CPR.
Fetal MCA and UA blood flow values across gestation
It showed an upward trend of MCA blood flow (PI, RI, and S/D) in the early period and a downward trend in the late period during pregnancy. It showed a continuous upward trend of MCA-Vm throughout pregnancy. It showed a downward trend of UA (PI, RI, and S/D) and an upward trend of UA-Vm throughout pregnancy ().
Discussion
Adverse maternal outcomes [Citation16] were found in the PE/EC and GH groups. Primipara was found to be a risk factor for hypertension pregnancy, which was consistent with a previous report [Citation17]. HDP might lead to uterine arteriole spasm or sclerosis, capillary degeneration, necrosis, and even rupture and bleeding. After blood forms a hematoma between the uterus and placenta, separation of the placenta and uterine wall will occur, and the risk of placental abruption and postpartum hemorrhage will rise [Citation18,Citation19].
Acute arteriosclerosis occurs in some parts of the blood vessels of the myometrium and decidua, which reduces the diameter of the blood lumen by half in the HDP, and it could result in insufficient blood supply to the placenta and affect the growth and development of the fetus. If intravascular embolization occurs on this basis, it could greatly increase the risk of fetal distress and even asphyxia [Citation20]. In animal models with reported fetal hypoxemia, reduced thyroid hormones triiodothyronine and thyroxine, elevated catecholamines and reduced plasma insulin-like growth factor-1 could do damage to the development of cardiomyocytes in early gestation [Citation21]. Botting et al. mentioned that even if the fetus was hypoxemic at late gestation, the fetal heart was not hypoxic, nor did it have a greater percentage of apoptotic cardiomyocytes or a diminished percentage of cardiomyocytes in the cell cycle [Citation22]. The mother diagnosed as HDP terminated pregnancy suddenly to avoid the risk of bad outcomes. The process of brain development was forced to be interrupted and IVH was caused by insufficient periventricular blood flow [Citation23]. We also found the higher incidence of IVH I-II in hypertension group.
UA blood flow ultrasound indices (PI, RI, S/D) went down with advancing gestation () because of decreased placental vascular resistance, which physiologically occurred with advancing gestation [Citation24]. Studies have reported that antenatal changes in UA flow velocity waveforms are accompanied by a specific microvascular lesion in the placenta characterized by obliteration of small muscular arteries in the tertiary stem villi [Citation25]. To prevent the fetal brain from IVH via inhibition of angiogenesis, the UA blood mean flow velocity slows down. This may explain the reason for the significant decrease in UA-Vm in the PE/EC group at late stage of pregnancy compared to the other two groups, as shown in .
Although previous studies have found that the MCA-PI and MCA-RI have specific value in the prediction of fetal distress [Citation26], research on women with pregnancy-induced hypertension has not been involved in. Despite abnormal umbilical and cerebral flow had been mentioned in SGA and FGR [Citation9], no proper fetal ultrasound values of MCA or CPR was put forward to predict the SGA. We found that MCA-RI (GA of 35–40 weeks) had the best diagnostic value (AUC:0.74) in the prediction of fetal distress, and CPR-2 had the best specificality value (0.923) in the prediction of SGA. Due to a weak response to the short duration of hypoxia and the effect of brain sparing, the decrease in MCA blood flow resistance was not obvious in early pregnancy, and a significant decreasing trend was only found in the late period of pregnancy. Before 34 weeks of gestation, when the systolic blood flow resistance of UA significantly increases and the end diastolic blood flow reverses, it is recommended by obstetricians to terminate pregnancy at 32 weeks of gestation to avoid adverse fetal outcomes [Citation27]. However, if only the systolic blood flow resistance increased and the end diastolic blood flow decreased without or reversed end diastolic flow velocity in the fetal UA, terminating pregnancy could be delayed until after 37 weeks of gestation. We found that the lower CPR-3, the higher risk factor for fetal distress or SGA in the late pregnancy, as shown in . The reasons account for these phenomena were that CPR reflect the oxygen supply in the womb. If these indices decreased, the oxygen supply in the womb could decrease, and the incidence of fetal distress or SGA could increase in the late pregnancy.
The limitation of our study is lacking in each point of gestational data to calculate detailed reference ranges. Additionally, the long-term follow-up and neonatal outcomes of the hypertension group should be analyzed in the future.
Fetal MCA blood flow values or CPR are of great benefit for obstetricians to evaluate the status of fetus evidentially in singleton pregnancy.
Ethics approval and consent to participate
The study was approved by the ethics committee of Fujian Maternity and Child Health Hospital (Ethics approval number: 2020KY065). Informed written and verbal consent was obtained from the infants’ parents or guardians. The trial was performed in accordance with the approved guidelines and regulations of the participating institutions.
Author contributions
X, L proposed the idea of this work, analyzed the data, prepared tables, and and , and wrote the main manuscript text. L-l, L collected the clinical data and took part in the analysis of data. L-j, Z collected the clinical data and prepared Figure 1. C-y, Y amended the manuscript text. All authors reviewed the manuscript.
Supplemental Material
Download TIFF Image (618.5 KB)Acknowledgments
The authors want to thank the hospitals of Fujian Maternity and Child Health Hospital (the department of neonatology, electrophysiology, and ultrasonic diagnosis Department) for their permission and the nurses and medical officers who were hard working during the study for their support. This study was carried out without grant. The name of “The School of Medical Technology and Engineering” was changed to “The School of Medical Imaging” to fit with the development of school in 2023. Thus, the unit of Li-Juan Zheng has to be changed into “The School of Medical Imaging, Fujian Medical University, Fujian Province, P.R. China”.
Disclosure statement
No potential conflict of interest was reported by the author(s).
Correction Statement
This article has been republished with minor changes. These changes do not impact the academic content of the article.
Additional information
Funding
References
- Regitz-Zagrosek V, Roos-Hesselink JW, Bauersachs J, et al. 2018 ESC guidelines for the management of cardiovascular diseases during pregnancy. Eur Heart J. 2018;39(34):3165–3241.
- ACOG Practice Bulletin No 202: gestational hypertension and preeclampsia. Obstet Gynecol. 2019; 133(1):1.
- Huppertz B. Placental origins of preeclampsia: challenging the current hypothesis. Hypertension. 2008;51(4):970–975.
- Ghulmiyyah L, Sibai B. Maternal mortality from preeclampsia/eclampsia. Semin Perinatol. 2012;36(1):56–59.
- Agrawal A, Wenger NK. Hypertension during pregnancy. Curr Hypertens Rep. 2020;22(9):64.
- Bhide A, Acharya G, Bilardo CM, et al. ISUOG practice guidelines: use of Doppler ultrasonography in obstetrics. Ultrasound Obstet Gynecol. 2013;41(2):233–239.
- Ochi H, Suginami H, Matsubara K, et al. Micro-bead embolization of uterine spiral arteries and changes in uterine arterial flow velocity waveforms in the pregnant ewe. Ultrasound Obstet Gynecol. 1995;6(4):272–276.
- Ota E, Ganchimeg T, Morisaki N, et al. Risk factors and adverse perinatal outcomes among term and preterm infants born small-for-gestational-age: secondary analyses of the WHO Multi-Country survey on maternal and newborn health. Plos One. 2014;9(8):e105155.
- Morales-Roselló J, Buongiorno S, Loscalzo G, et al. Mathematical simulation of Doppler changes in late-onset smallness; progression patterns of cerebral and umbilical anomalies define two types of late-onset fetal growth restriction. J Matern Fetal Neonatal Med. 2021;34(17):2869–2879.
- Monteith C, Flood K, Pinnamaneni R, et al. An abnormal cerebroplacental ratio (CPR) is predictive of early childhood delayed neurodevelopment in the setting of fetal growth restriction. Am J Obstet Gynecol. 2019;221(3):273.e1–273.e9.
- Lewandowski KC, Biesiada L, Grzesiak M, et al. C-Peptide and leptin system in dichorionic, small and appropriate for gestational age twins-possible link to metabolic programming? Nutr Diabetes. 2020;10(1):29. DOI:10.1038/s41387-020-00131-2
- Feldman R, Eidelman AI. Neonatal state organization, neuromaturation, mother-infant interaction, and cognitive development in small-for-gestational-age premature infants. PEDIATRICS. 2006;118(3):e869–e878. 118 PEDIATRICS.
- Waaler Loland V, Ågesen FN, Lynge TH, et al. Low birth weight increases the risk of sudden cardiac death in the young: a nationwide study of 2.2 million people. J Am Heart Assoc. 2021;10(7):e018314. DOI:10.1161/JAHA.120.018314
- Macones GA, Hankins GD, Spong CY, et al. The 2008 national institute of child health and human development workshop report on electronic fetal monitoring: update on definitions, interpretation, and research guidelines. J Obstet Gynecol Neonatal Nurs. 2008;37(5):510–515.
- Delle Donne RD, Araujo Júnior E, Rolo LC, et al. Reproducibility of placental maturity grade classification using a dynamic ultrasonography. J Matern-Fetal Neo M. 2017;30(8):987–989.
- Kamp JC, von Kaisenberg C, Greve S, et al. Pregnancy in pulmonary arterial hypertension: midterm outcomes of mothers and offspring. J Heart Lung Transplant. 2021;40(3):229–233.
- Jasovic-Siveska E, Jasovic V, Stoilova S. Previous pregnancy history, parity, maternal age and risk of pregnancy induced hypertension. Bratisl Lek Listy. 2011;112(4):188–191.
- Ruiter L, Ravelli AC, de Graaf IM, et al. Incidence and recurrence rate of placental abruption: a longitudinal linked national cohort study in The Netherlands. Am J Obstet Gynecol. 2015;213(4):573.e1–8.
- Oyelese Y, Ananth CV. Placental abruption. Obstet Gynecol. 2006;108(4):1005–1016.
- Podymow T, August P. Hypertension in pregnancy. Adv Chronic Kidney Dis. 2007;14(2):178–190.
- Camm EJ, Botting KJ, Sferruzzi-Perri AN. Near to one’s heart: the intimate relationship between the placenta and fetal heart. Front Physiol. 2018;9:629.
- Botting KJ, McMillen IC, Forbes H, et al. Chronic hypoxemia in late gestation decreases cardiomyocyte number but does not change expression of hypoxia-responsive genes. J Am Heart Assoc. 2014;3(4):e000531.
- Kuoqin X, Yali W, Wenzhi L, et al. The effect of hypertensive disorder complicating pregnancy on neonatal brain injury and brain development. Chinese J Prac Nervous Dis. 2017;20(13):66–68.
- Mari G, Hanif F. Fetal Doppler: umbilical artery, Middle cerebral artery, and venous system. Semin Perinatol. 2008;32(4):253–257.
- Giles WB, Trudinger BJ, Baird PJ. Fetal umbilical artery flow velocity waveforms and placental resistance: pathological correlation. Br J Obstet Gynaecol. 1985;92(1):31–38.
- Liu Q, Li B. The diagnostic value of ultrasound detection of the fetal Middle cerebral artery, umbilical artery blood flow and fetal movement reduction in fetal distress. Am J Transl Res. 2021;13(4):3529–3535.
- Gairabekova D, van Rosmalen J, Duvekot JJ. Outcome of early-onset fetal growth restriction with or without abnormal umbilical artery Doppler flow. Acta Obstet Gynecol Scand. 2021;100(8):1430–1438.

