Abstract
Background
Regular and supervised exercise during pregnancy is worldwide recommended due to its proven benefits, but, during exercise, maternal blood flow is redirected from the viscera to the muscles and how fetal wellbeing may be affected by this redistribution is still not well known.
Objective
To analyze the longitudinal effect of a supervised moderate physical exercise program during pregnancy on uteroplacental and fetal Doppler parameters.
Methods
This is a planned secondary analysis of an randomized controlled trial (RCT), performed at Hospital Universitario de Torrejón, Madrid, Spain, including 124 women randomized from 12+0 to 15+6 weeks of gestation to exercise vs. control group. Fetal umbilical artery (UA), middle cerebral artery, and uterine artery pulsatility index (PI), were longitudinally collected by Doppler ultrasound assessment throughout gestation, and derived cerebroplacental ratio (normalized by z-score), and maternal mean PI in the uterine arteries (normalized by multiplies of the median). Obstetric appointments were scheduled at 12 (baseline, 12+0 to 13+5), 20 (19+0 to 24+2), 28 (26+3 to 31+3) and 35 weeks (32+6 to 38+6) of gestation. Generalized estimating equations were adjusted to assess longitudinal changes in the Doppler measurements according to the randomization group.
Results
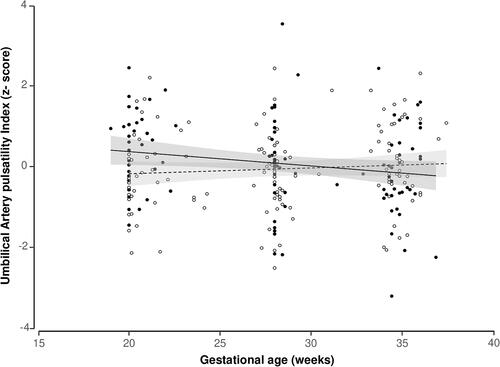
No significant differences in the fetal or maternal Doppler measurements were found at any of the different checkup time points studied. The only variable that consistently affected the Doppler standardized values was gestational age at the time of assessment. The evolution of the UA PI z-score during the pregnancy was different in the two study groups, with a higher z-score in the exercise group at 20 weeks and a subsequent decrease until delivery while in the control group it remained stable at around zero.
Conclusions
A regular supervised moderate exercise program during pregnancy does not deteriorate fetal or maternal ultrasound Doppler parameters along the pregnancy, suggesting that the fetal well-being is not compromised by the exercise intervention. Fetal UA PI z-score decreases during pregnancy to lower levels in the exercise group compared with the control group.
Introduction
Regular and supervised exercise during pregnancy is worldwide recommended due to its proven benefits [Citation1]. Certain pregnancy complications such as preeclampsia, gestational diabetes, or increased maternal weight gain could be potentially prevented or improved by the practice of exercise [Citation2–8]. However, during exercise, blood flow is redirected from the viscera to the muscles [Citation9,Citation10] and how fetal wellbeing may be affected by this redistribution is still not well known.
Two findings have been shown when practicing of physical exercise during pregnancy: a transitory fetal bradycardia, followed by a mild compensatory tachycardia, especially at higher exercise intensities [Citation11–14], and an increase in the baseline fetal heart rate [Citation15,Citation16]. Both fetal cardiac findings return to normal when the exercise is over, suggesting a fetal adaptation to a lower utero-placental perfusion [Citation11]. Additionally, few studies have reported a higher variability in the fetal heart rate trace, that could also reflect a fetal cardiac adaptation to chronic physical activity during pregnancy [Citation17–19].
On the other hand, fetuses exposed to a chronic placental under-perfusion, usually associate some changes in uteroplacental and fetal Doppler parameters that can worsen perinatal outcome and children neurologic development later in life [Citation20–23]. Fetal blood obtained by cordocentesis from small for gestational age fetuses demonstrated that a high pulsatility index (PI) in the umbilical artery (UA) and decreased PI in the fetal middle cerebral artery (MCI) are associated with fetal hypoxemia and acidemia [Citation24–27]. It was also shown that the cerebro-placental ratio (CPR), combined or not with the PI in the uterine arteries, was associated with adverse perinatal outcome not only in small for gestational age but also in normally grown fetuses [Citation28–31]. Therefore, assessing fetal and maternal Doppler measurements in pregnancies where exercise is routinely performed may help to evaluate fetal wellbeing in such cases.
A recent systematic review evaluating the impact of regular maternal physical activity on fetal and neonatal wellbeing in uncomplicated pregnancies, included studies assessing fetal Doppler in pregnancies where exercise was routinely carried out concluded that, although apparently safe, scientific evidence was heterogeneous and insufficient [Citation32].
In a previous randomized controlled trial (RCT), pregnant women were randomly assigned to a supervised exercise program during pregnancy or control, in order to assess maternal weight gain [Citation33]. In this planned secondary analysis, the hypothesis was that fetuses exposed to continuous maternal exercise adapt their cardiovascular system [Citation20,Citation21,Citation34,Citation35]. This adaptation could be translated in a different evolution of fetal and maternal Doppler parameters during pregnancy. Therefore, the main objective was to evaluate the fetal and maternal Doppler adaptation to maternal exercise by comparing longitudinal changes in uteroplacental and fetal Doppler measurements performed throughout the pregnancy in both study groups.
Materials and methods
Trial design and participants
This is a planned secondary analysis of an RCT performed at Hospital Universitario de Torrejón, Madrid, Spain, including 124 women randomly assigned into a supervised moderate exercise program during pregnancy or into a control group, who continued with their routine daily activity (NCT 02756143) [Citation33]. It was carried out from November 2014 to June 2015. Briefly, eligibility criteria were uncomplicated pregnancies, less than 16 weeks’ gestation who were able to complete the exercise program (to attend >70% of the exercise sessions) if allocated in this group, and who did not meet any exclusion criteria (non-availability to attend to the exercise program during pregnancy or not full filling any of the inclusion criteria) [Citation33,Citation36,Citation37]. For the present analysis, only cases where fetal and maternal Doppler ultrasound assessments were available at any hospital visit were included.
Intervention program
The intervention program was designed following the latest American College of Obstetricians and Gynecologists (ACOG) guidelines and followed the structure of previous studies [Citation5,Citation37–39]. Briefly, the program consisted of a supervised physical conditioning program [Citation40] of three-60-min-sessions per week at the hospital gymnasium from 12+3 to 15+6 weeks and during the entire duration of the pregnancy or until 39+6 weeks of gestation if delivery had not occurred before. Each session included 10 min of warming up, 25 min of cardiovascular exercise, 10 min of strengthening exercises, 5 min of coordination and balance, 5 min of pelvic floor exercises, and 5 min of stretching and relaxation. Aerobic activity was prescribed at moderate intensity, aiming for 55–60% of the age-predicted maximum heart rate reserve (HR), estimated by the Karvonen formula. All women wore an HR monitor (Polar FT7) during the training session to ensure that exercise intensity was moderate and the rating on Borg’s Rate of Perceived Exertion Scale at the end of the session should range from 12 to 14 (somewhat hard) [Citation41]. Sessions were conducted twice daily, four days per week and, per protocol, women should join at least three sessions per week.
Weekly volume of physical activity and percentage of assistance to the program were monitored all throughout the pregnancy, by a qualified exercise specialist trained in pre and postnatal exercise.
Control group
Pregnant women allocated to the control group were advised to continue with their routine activity without joining any educational exercise program which included more than 30 min per session at least three times per week. The weekly volume of physical activity was monitored by the exercise specialist at a final interview at 38+0 to 39+6 weeks of gestation.
Randomization
Randomization was carried out from 12+0 to 15+6 weeks of gestation. Epidat V.3.1 program was used to perform a simple randomization into two groups (exercise group and control group) using a computer-generated list of random numbers (n = 200) in order to create two balanced but not necessarily equal size groups, as previously described [Citation36].
Follow-up
After randomization, both groups followed the same antenatal care at the Hospital. Obstetric appointments were scheduled at 12 (baseline, range 12+0 to 13+5), 20 (range 19+0 to 24+2), 28 (range 26+3 to 31+3), and 35 weeks (range 32+6 to 38+6) of gestation. Demographic data were recorded at the 12+0 to 13+5 weeks’ appointment.
The following maternal characteristics were recorded: maternal age, maternal weight at 12+0 to 13+5 weeks, height, body mass index, ethnicity (Caucasian vs. non-Caucasian), method of conception (natural or assisted conception), cigarette smoking during pregnancy (yes or no), parity (parous or multiparous, according to previous delivery at ≥24 weeks’ gestation), gestational age in weeks and days from the last menstrual period calculated according to the first trimester ultrasound, and level of physical activity or exercise performed before pregnancy with the Global Physical Activity Questionnaire (GPAQ) (no activity, occasional exercise but not regular, active (twice per week), very active (3–4 times per week), athlete (daily exercise)). Birthweight at delivery was recorded and transformed into centiles [Citation42].
All patients had a final appointment with the exercise specialist at 38+0 to 39+6 weeks’ gestation in other to check their final weight and assess the weekly volume of physical activity (walking minutes per week, domestic labor per week, on foot minutes per week).
Fetal ultrasound assessments were performed using a Voluson S8 (GE Healthcare, Zipf, Austria) ultrasound machine with a convex transducer (RAB6-RS) at every antenatal appointment scheduled. Fetal and maternal Doppler was performed at 20-, 28-, and 35-weeks scans.
Umbilical artery pulsatility index (UAPI), middle cerebral artery pulsatility index (MCAPI), and mean uterine arteries pulsatility index (UtPI), were recorded. The CPR was calculated as MCAPI/UAPI. Fetal Doppler measurements were normalized by z-score, and maternal Doppler uterine artery pulsatility index (UtPI) was normalized by multiples of the median (MoMs), calculated at the Fetal Medicine Foundation website [Citation43,Citation44].
Statistical analysis
For this study, a per-protocol analysis was performed, and only women attending to more than 70% of the sessions were included (being 100% assistance when attending all the sessions and 0% when not attending any session).
Descriptive analysis was performed by median (interquartile range, IQR) for continuous variables, and frequency and percentage for categorical variables.
Generalized estimating equations (GEEs) were adjusted to assess the influence of exercise in longitudinal changes of each Doppler measurement for both, the exercise and the control groups while taking into account correlation between different observations of the same patient [Citation45]. Models were also adjusted by walking minutes, on foot time and domestic work per week, removing these variables when they were not significant. To test how the exercise affected the evolution of each Doppler parameter, an interaction between the gestational age and the intervention was evaluated in the models. Coefficients of the regressions and their 95% CI were reported to quantify the association between variables. Normality of residuals hypothesis was verified for all models. A Box-Cox transformation was necessary to ensure normality of the residuals for analysis of UtPI. The number of pregnancies included in each analysis was reported wherever necessary. Level of significance was set at .05.
R software (version 4.0.2) (R Foundation for Statistical Computing, Vienna, Austria) [Citation46] was used for statistical analysis. Geepack package was used to adjust the GEE models [Citation47] and forecast and car packages were used for the Cox-Box transformation [Citation48].
Ethical approval
The study was approved by the Local Research Ethics Committee of the Hospital (CEIC Hospital Universitario Severo Ochoa) (06/07/2013; Madrid, Spain) and was in accordance with the ethical guidelines of the Declaration of Helsinki (modified in 2008). All women gave written informed consent.
Results
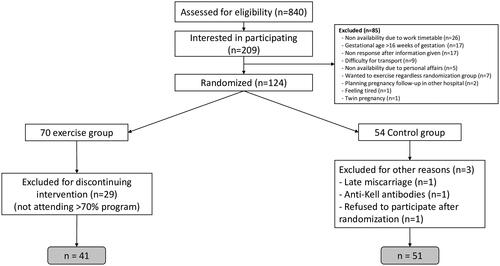
The trial finished prematurely when 124 women had been recruited and randomized into two groups; 70 women were randomized to the exercise group and 54 to the control group. After exclusions, 41 women were included in the exercise group and 51 in the control group for this study (). Baseline characteristics were similar in both groups (). Although there was a similar number of women who smoked before pregnancy in both groups, the proportion of smokers during pregnancy was lower in the exercise group. However, the reduction rate was not significantly different (90.0% in the exercise group compared to 63.6% in the control group, p value = .21).
Table 1. Maternal and pregnancy characteristics of the study population.
No significant differences in the fetal or maternal Doppler measurements were found at any of the different checkup time points ( and eFigures S1–S4).
Table 2. Doppler parameters in the two study groups at the 20-, 28-, and 35-weeks’ gestation assessments.
In the GEE models performed to evaluate repeated measurements, the only variable that consistently affected the Doppler standardized values was gestational age at the time of assessment (eTables S1–S4). The evolution of the UAPI z-score during the pregnancy was different in the two study groups (p value of the interaction = .01), with a higher z-score in the exercise group at 20 weeks and a subsequent decrease until delivery while in the control group it remained stable at around zero ( Table S1).
Discussion
Principal findings
Maternal regular supervised moderate exercise during pregnancy does not deteriorate fetal or maternal Doppler parameters suggesting that fetal wellbeing is not compromised. Fetal UAPI z-score decreases during pregnancy, probably reflecting an improved placental perfusion and fetal oxygenation.
Strengths of the study
The main strength of our study resides in being an RCT with several checkups at different timepoints and with prospectively collected data. Additionally, we followed the international recommendations for exercise during pregnancy when designing the training program, which allows comparisons with other studies and future combination of data.
Limitations of the data
The main limitation of this study relates to the small sample size which might be the reason for the lack of differences found between groups when evaluating the ultrasound assessment performed at each gestational age independently. Although almost 50% of the women were excluded in the intervention group, this is a per protocol analysis and therefore it is unlikely that the results are biased for this reason. Moreover, this small sample size has prevented us to perform any subgroup analyses which could have been of interest. We also acknowledge that there is an increased proportion of nulliparous women as compared to a routine population, which may represent a selection bias. The most likely explanation for the lower uptake in parous women is that they find more difficult to schedule time for exercising. However, we have not found evidence that exercise affects the pregnancy differently according to parity [Citation43]. Another limitation relates to the fact that physical activity in the control group was evaluated at the end of the study, so we cannot ensure that these women did not join any exercise program during pregnancy. However, Doppler patterns encountered in this group are similar to those reported in routine populations [Citation43,Citation49,Citation50]. Although the exclusion of patients without Doppler measurements may represent a selection bias, we do not believe those cases corresponded to patients of any specific characteristics, but they were just random women for whom the researcher forgot to measure any of the Doppler parameters. Finally, only low-risk pregnancy women were included, and therefore the results might not be valid for a higher risk population.
Interpretation
Several studies have evaluated adverse pregnancy outcome on the basis of cord pH or Apgar scores at birth [Citation51,Citation52]. However, such outcome measures are highly influenced by the events occurring during labor and therefore, unlikely to accurately reflect subtle interventions performed during pregnancy. The only outcome of interest would be the long-term follow up of these babies. Until these long-term studies are available, evaluation of fetal Doppler seems to be the best approach to assess fetal wellbeing.
In this study, we have shown that first, basal Doppler parameters were not significantly different between groups at any gestational age, suggesting that fetal wellbeing is not compromised by the exercise; and second, the evolution of the UAPI z-score was different between groups, toward an increased placental blood flow from the second to the third trimester in the exercise group as compared to the control group. Since the fetal Doppler indexes are presumably reflecting placental perfusion and oxygenation, it is possible that, during the early stages of exercise, blood is diverted away from the placenta toward other vascular trees, supplying the muscles involved in the exercise. With continuing exercise, as it happens with non-pregnant individuals, an improvement in the maternal cardiovascular system is expected and therefore, there may well be better placental perfusion and fetal oxygenation, reflected as a decrease in the UAPI.
Several small RCTs have studied the effect of chronic exercise [Citation53–56]. However, most of these studies evaluated fetal heart rate, neonatal Apgar scores and birth cord gases to assess fetal wellbeing. There are only three RCTs evaluating the effect of chronic exercise during pregnancy in uteroplacental and fetal Doppler parameters [Citation56–58]. The largest one, compared 54 women who started exercising at 13 weeks’ and 60 women who started at 20 weeks’ with 57 women who remained sedentary [Citation56] and they did not find any differences. The second study [Citation57] compared 26 women who followed a pelvic floor muscle training from 20 to 36 weeks with 33 women without any intervention, and no differences were found. However, in these two studies, the degree of physical activity was substantially lower than in our study, and results may not be comparable. The third RCT, involved 26 women who underwent a similar exercise program as in the present study, and 26 women who remained sedentary [Citation58]. In this study, women were exposed to a cycle-ergometer test at 34 weeks and fetal and maternal Doppler were assessed before and after the test. No significant differences between groups were found in the UAPI, MCAPI, CPR nor UtPI before the intervention, but UAPI was significantly lower in the exercise group after the cycle-ergometer test. These findings might reflect a better feto-placental adaptation to stressful situations (like acute exercise) in the exercise group, while basal conditions remain unchanged. In contrast, Szymanski and Satin [Citation13] found no differences before and after a peak treadmill test to volitional fatigue according to a modified Balke protocol, in women who did not routinely perform any exercise nor in women normally active, but they did find an increase in the UAPI at high-intensity physical activity. Therefore, they concluded that only high-intensity physical activity may compromise fetal wellbeing. Other studies evaluating acute fetal response to exercise have shown that Doppler modifications due to placental under perfusion are more likely to occur at higher exercise intensities [Citation13,Citation14,Citation59]. In a recent systematic review [Citation32], no adverse effect of chronic maternal physical activity on fetal nor maternal Doppler was found, concluding that maternal exercise during low-risk pregnancy is safe for fetal and neonatal well-being when practiced according to current recommendations. Nevertheless, this meta-analysis included very heterogeneous studies, most of them not randomized and of very small sample size, with different interventions and outcome measures. Therefore, the authors also recommended bigger randomized trials, with similar exercise programs, to better clarify this research question [Citation32].
The general benefits of exercise both in cardiovascular profile and quality of life are well known. Similarly, exercise during pregnancy has shown to prevent the typical pregnancy associated musculoskeletal pain [Citation60,Citation61], to provide a better sleep and to lower the stress and anxiety during pregnancy while decreasing the risk of depression [Citation37,Citation62–64] and adverse perinatal outcome [Citation65–67]. The findings of this study may help encourage expectant mothers to join a specifically designed program, reassuring them about the safeness of exercise during pregnancy. However, more data are needed to further evaluate this hypothesis.
Conclusions
In low-risk pregnancy women, a regular supervised moderate exercise program during pregnancy does not deteriorate fetal or maternal Doppler parameters along pregnancy, reflecting that the exercise practice during pregnancy is safe for the fetus since placental perfusion and fetal oxygenation is conserved. However, further studies are needed to confirm these findings.
Supplemental Material
Download Zip (483.3 KB)Acknowledgements
The authors thank the women who participate in the study and the Obstetrics Team at Hospital Universitario de Torrejón for their collaboration in the project. We also acknowledge Hospital Universitario de Torrejón, Universidad Politécnica de Madrid, Sanitas Healthcare and the iMaterna Foundation for the financial support.
Disclosure statement
No potential conflict of interest was reported by the author(s).
Additional information
Funding
References
- Exercise during pregnancy and the postpartum period – UpToDate; 2019 [Internet] [cited 2019 Dec 16]. Available from: https://www-uptodate-com.m-htj.a17.csinet.es/contents/exercise-during-pregnancy-and-the-postpartum-period?search=Exercise%20during%20pregnancy%20and%20the%20postpartum%20period&source=search_result&selectedTitle=1∼150&usage_type=default&display_rank=1
- Aune D, Saugstad OD, Henriksen T, et al. Physical activity and the risk of preeclampsia: a systematic review and meta-analysis. Epidemiology. 2014;25(3):331–343.
- Haakstad LA, Bø K. Effect of regular exercise on prevention of excessive weight gain in pregnancy: a randomised controlled trial. Eur J Contracept Reprod Health Care. 2011;16(2):116–125.
- Amezcua-Prieto C, Lardelli-Claret P, Olmedo-Requena R, et al. Compliance with leisure-time physical activity recommendations in pregnant women. Acta Obstet Gynecol Scand. 2011;90(3):245–252.
- ACOG Committee opinion no. 650: physical activity and exercise during pregnancy and the postpartum period. Obstet Gynecol. 2015;126(6):e135–e142.
- Olson CM, Strawderman MS. Modifiable behavioral factors in a biopsychosocial model predict inadequate and excessive gestational weight gain. J Am Diet Assoc. 2003;103(1):48–54.
- Clapp JF. Morphometric and neurodevelopmental outcome at age five years of the offspring of women who continued to exercise regularly throughout pregnancy. J Pediatr. 1996;129(6):856–863.
- Ruchat SM, Mottola MF. The important role of physical activity in the prevention and management of gestational diabetes mellitus. Diabetes Metab Res Rev. 2013;29(5):334–346.
- Flamm SD, Taki J, Moore R, et al. Redistribution of regional and organ blood volume and effect on cardiac function in relation to upright exercise intensity in healthy human subjects. Circulation. 1990;81(5):1550–1559.
- Froelich JW, Strauss HW, Moore RH, et al. Redistribution of visceral blood volume in upright exercise in healthy volunteers. J Nucl Med. 1988;29(10):1714–1718.
- Carpenter MW, Sady SP, Hoegsberg B, et al. Fetal heart rate response to maternal exertion. JAMA. 1988;259(20):3006–3009.
- Artal R, Paul R, Romem Y, et al. Fetal bradycardia induced by maternal exercise. Lancet. 1984;2(8397):258–260.
- Szymanski LM, Satin AJ. Strenuous exercise during pregnancy: is there a limit? Am J Obstet Gynecol. 2012;207(3):179.e1–179.e6.
- Salvesen KÅ, Hem E, Sundgot-Borgen J. Fetal wellbeing may be compromised during strenuous exercise among pregnant elite athletes. Br J Sports Med. 2012;46(4):279–283.
- Morrow RJ, Ritchie JW, Bull SB. Fetal and maternal hemodynamic responses to exercise in pregnancy assessed by Doppler ultrasonography. Am J Obstet Gynecol. 1989;160(1):138–140.
- Callaway LK, Prins JB, Chang AM, et al. The prevalence and impact of overweight and obesity in an Australian obstetric population. Med J Aust. 2006;184(2):56–59.
- Dietz P, Watson ED, Sattler MC, et al. The influence of physical activity during pregnancy on maternal, fetal or infant heart rate variability: a systematic review. BMC Pregnancy Childbirth. 2016;16(1):326.
- Monga M. Fetal heart rate response to maternal exercise. Clin Obstet Gynecol. 2016;59(3):568–575.
- Szymanski LM, Kogutt BK. Uterine artery Doppler velocimetry during individually prescribed exercise in pregnancy. Obstet Gynecol. 2018;132(4):1026–1032.
- Crispi F, Figueras F, Cruz-Lemini M, et al. Cardiovascular programming in children born small for gestational age and relationship with prenatal signs of severity. Am J Obstet Gynecol. 2012;207(2):121.e1–121.e9.
- Crispi F, Hernandez-Andrade E, Pelsers MM, et al. Cardiac dysfunction and cell damage across clinical stages of severity in growth-restricted fetuses. Am J Obstet Gynecol. 2008;199(3):254.e1–254.e8.
- Abeysekera JB, Gyenes DL, Atallah J, et al. Fetal umbilical arterial pulsatility correlates with 2-year growth and neurodevelopmental outcomes in congenital heart disease. Can J Cardiol. 2020;37(3):425–432.
- Paules C, Youssef L, Rovira C, et al. Distinctive patterns of placental lesions in pre-eclampsia vs small-for-gestational age and their association with fetoplacental Doppler. Ultrasound Obstet Gynecol. 2019;54(5):609–616.
- Nicolaides KH, Soothill PW, Rodeck CH, et al. Ultrasound-guided sampling of umbilical cord and placental blood to assess fetal wellbeing. Lancet. 1986;1(8489):1065–1067.
- Soothill PW, Nicolaides KH, Campbell S. Prenatal asphyxia, hyperlacticaemia, hypoglycaemia, and erythroblastosis in growth retarded fetuses. Br Med J. 1987;294(6579):1051–1053.
- Nicolaides KH, Bilardo CM, Soothill PW, et al. Absence of end diastolic frequencies in umbilical artery: a sign of fetal hypoxia and acidosis. BMJ. 1988;297(6655):1026–1027.
- Vyas S, Nicolaides KH, Bower S, et al. Middle cerebral artery flow velocity waveforms in fetal hypoxaemia. Br J Obstet Gynaecol. 1990;97(9):797–803.
- Khalil AA, Morales-Rosello J, Morlando M, et al. Is fetal cerebroplacental ratio an independent predictor of intrapartum fetal compromise and neonatal unit admission? Am J Obstet Gynecol. 2015;213(1):54.e1–54.e10.
- Khalil AA, Morales-Rosello J, Elsaddig M, et al. The association between fetal Doppler and admission to neonatal unit at term. Am J Obstet Gynecol. 2015;213(1):57.e1–57.e7.
- Khalil A, Morales-Rosello J, Khan N, et al. Is cerebroplacental ratio a marker of impaired fetal growth velocity and adverse pregnancy outcome? Am J Obstet Gynecol. 2017;216(6):606.e1–606.e10.
- Khalil A, Morales-Roselló J, Townsend R, et al. Value of third-trimester cerebroplacental ratio and uterine artery Doppler indices as predictors of stillbirth and perinatal loss. Ultrasound Obstet Gynecol. 2016;47(1):74–80.
- Michalek IM, Comte C, Desseauve D. Impact of maternal physical activity during an uncomplicated pregnancy on fetal and neonatal well-being parameters: a systematic review of the literature. Eur J Obstet Gynecol Reprod Biol. 2020;252:265–272.
- Brik M, Fernández-Buhigas I, Martin-Arias A, et al. Does exercise during pregnancy impact on maternal weight gain and fetal cardiac function? A randomized controlled trial. Ultrasound Obstet Gynecol. 2019;53(5):583–589.
- Godfrey KM, Sheppard A, Gluckman PD, et al. Epigenetic gene promoter methylation at birth is associated with child’s later adiposity. Diabetes. 2011;60(5):1528–1534.
- Van Mieghem T, Gucciardo L, Doné E, et al. Left ventricular cardiac function in fetuses with congenital diaphragmatic hernia and the effect of fetal endoscopic tracheal occlusion. Ultrasound Obstet Gynecol. 2009;34(4):424–429.
- Fernández-Buhigas I, Brik M, Martin-Arias A, et al. Maternal physiological changes at rest induced by exercise during pregnancy: a randomized controlled trial. Physiol Behav. 2020;220:112863.
- Perales M, Refoyo I, Coteron J, et al. Exercise during pregnancy attenuates prenatal depression: a randomized controlled trial. Eval Health Prof. 2015;38(1):59–72.
- Barakat R, Pelaez M, Montejo R, et al. Exercise during pregnancy improves maternal health perception: a randomized controlled trial. Am J Obstet Gynecol. 2011;204(5):402.e1–402.e7.
- Barakat R, Pelaez M, Cordero Y, et al. Exercise during pregnancy protects against hypertension and macrosomia: randomized clinical trial. Am J Obstet Gynecol. 2016;214(5):649.e1–649.e8.
- Barakat R. An exercise program throughout pregnancy: Barakat model. Birth Defects Res. 2021;113(3):218–226.
- O'Neill ME, Cooper KA, Mills CM, et al. Accuracy of Borg’s ratings of perceived exertion in the prediction of heart rates during pregnancy. Br J Sports Med. 1992;26(2):121–124.
- Nicolaides KH, Wright D, Syngelaki A, et al. Fetal Medicine Foundation fetal and neonatal population weight charts. Ultrasound Obstet Gynecol. 2018;52(1):44–51.
- Ciobanu A, Wright A, Syngelaki A, et al. Fetal Medicine Foundation reference ranges for umbilical artery and middle cerebral artery pulsatility index and cerebroplacental ratio. Ultrasound Obstet Gynecol. 2019;53(4):465–472.
- The Fetal Medicine Foundation; 2020 [Internet] [cited 2020 Feb 26]. Available from: https://fetalmedicine.org/research/doppler
- Prentice RL, Zhao LP. Estimating equations for parameters in means and covariances of multivariate discrete and continuous responses. Biometrics. 1991;47(3):825–839.
- Core Team. R: a language and environment for statistical computing; 2020. Available from: https://www.R-project.org/
- Højsgaard S, Halekoh U, Yan J. The R package geepack for generalized estimating. Eq J Stat Softw. 2006;15(2):1–11.
- R: the R project for statistical computing; 2020 [Internet] [cited 2020 Nov 6]. Available from: https://www.r-project.org/
- Khalil A, Nicolaides KH. How to record uterine artery Doppler in the first trimester. Ultrasound Obstet Gynecol. 2013;42(4):478–479.
- Papageorghiou AT, Yu CKH, Cicero S, et al. Second-trimester uterine artery Doppler screening in unselected populations: a review. J Matern Fetal Neonatal Med. 2002;12(2):78–88.
- Rizzo G, Mappa I, Bitsadze V, et al. Role of Doppler ultrasound at time of diagnosis of late-onset fetal growth restriction in predicting adverse perinatal outcome: prospective cohort study. Ultrasound Obstet Gynecol. 2020;55(6):793–798.
- Akolekar R, Ciobanu A, Zingler E, et al. Routine assessment of cerebroplacental ratio at 35–37 weeks’ gestation in the prediction of adverse perinatal outcome. Am J Obstet Gynecol. 2019;221(1):65.e1–65.e18.
- Szymanski LM, Satin AJ. Exercise during pregnancy: fetal responses to current public health guidelines. Obstet Gynecol. 2012;119(3):603–610.
- Nguyen NC, Evenson KR, Savitz DA, et al. Physical activity and maternal–fetal circulation measured by Doppler ultrasound. J Perinatol. 2013;33(2):87–93.
- Pijpers L, Wladimiroff JW, McGhie J. Effect of short-term maternal exercise on maternal and fetal cardiovascular dynamics. Br J Obstet Gynaecol. 1984;91(11):1081–1086.
- de Oliveria Melo AS, Silva JLP, Tavares JS, et al. Effect of a physical exercise program during pregnancy on uteroplacental and fetal blood flow and fetal growth: a randomized controlled trial. Obstet Gynecol. 2012;120(2 Pt 1):302–310.
- Okido MM, Valeri FL, Martins WP, et al. Assessment of foetal wellbeing in pregnant women subjected to pelvic floor muscle training: a controlled randomised study. Int Urogynecol J. 2015;26(10):1475–1481.
- Barakat R, Ruiz JR, Rodríguez-Romo G, et al. Does exercise training during pregnancy influence fetal cardiovascular responses to an exercise stimulus? Insights from a randomised, controlled trial. Br J Sports Med. 2010;44(10):762–764.
- Brenner I, Wolfe L, Monga M, et al. Physical conditioning effects on fetal heart rate responses to graded maternal exercise. Med Sci Sports Exerc. 1999;31(6):792–799.
- Liddle SD, Pennick V. Interventions for preventing and treating low‐back and pelvic pain during pregnancy. Cochrane Database Syst Rev. 2015;2015(9):CD001139.
- Owe KM, Bjelland EK, Stuge B, et al. Exercise level before pregnancy and engaging in high-impact sports reduce the risk of pelvic girdle pain: a population-based cohort study of 39 184 women. Br J Sports Med. 2016;50(13):817–822.
- Piercy KL, Troiano RP, Ballard RM, et al. The physical activity guidelines for Americans. JAMA. 2018;320(19):2020–2028.
- Schuch FB, Vancampfort D, Firth J, et al. Physical activity and incident depression: a meta-analysis of prospective cohort studies. Am J Psychiatry. 2018;175(7):631–648.
- Vargas-Terrones M, Barakat R, Santacruz B, et al. Physical exercise programme during pregnancy decreases perinatal depression risk: a randomised controlled trial. Br J Sports Med. 2018;53(6):348–353.
- Field T. Prenatal depression effects on early development: a review. Infant Behav Dev. 2011;34(1):1–14.
- Diego MA, Field T, Hernandez-Reif M, et al. Prenatal depression restricts fetal growth. Early Hum Dev. 2009;85(1):65–70.
- Ertel KA, Koenen KC, Rich-Edwards JW, et al. Antenatal and postpartum depressive symptoms are differentially associated with early childhood weight and adiposity. Paediatr Perinat Epidemiol. 2010;24(2):179–189.