Abstract
Background
Ultrasound is key to evaluating placental function. However, traditional ultrasound examinations cannot evaluate the changes in the biomechanical properties of the placenta in vivo. As a non-invasive technique, shear wave elastography (SWE) can be used analyze the physiological and biomechanical properties of the placenta. Moreover, it can evaluate the pathological changes in early placental insufficiency in a more direct and sensitive manner.
Objective
This study aimed to systematically introduce SWE in placental function evaluations.
Materials and methods
The terms ‘placenta’, ‘ultrasound’, and ‘elastography’ were searched on Pubmed, Embase, and CNKI databases (Apr 2023); this review was limited to results including placental sonoelastography.
Results
Twenty-six studies satisfied the inclusion criteria and were included in this review. Herein, we introduce the basic principle of SWE, analyze the factors affecting placental measurements, and summarize the prospects of clinical applications of SWE in the field of obstetrical diseases.
Conclusion
The SWE technology demonstrates excellent clinical application value and research prospects in obstetrics, particularly in placental function evaluation, owing to its objective and repeatable quantitative operation.
1. Introduction
Several indicators such as placental maturity and the Doppler parameters of umbilical and uterine artery blood flow are commonly used in clinics to evaluate the degree of chronic impairment of placental function [Citation1]. However, the aforementioned methods mostly reflect the placental function indirectly and fail to directly assess physiopathological changes in the placenta. In research studies involving animal experimentation, the use of shear wave elastography (SWE) has been demonstrated to be able to reflect the structural and biochemical changes of placentas during normal pregnancy in female dogs, and the placental stiffness of a mouse intrauterine growth restriction model under pathological conditions [Citation2,Citation3]. In recent years, it has also been used on human placentas, such as in studies on the influence of different maternal fetal factors on the value of SWE, and whether SWE can be used as a new predictor for the clinical diagnosis of intrauterine growth restriction (IUGR), preeclampsia (PE), gestational diabetes, etc. [Citation4,Citation5]. In addition to the aforementioned applications on the placenta, the clinical application of SWE in obstetrics also includes the prediction, via transvaginal ultrasound SWE, of spontaneous preterm delivery due to cervical incompetence; the risk assessment for perineal injury during delivery, via pelvic floor muscle group SWE; and the detection of fetal lung maturity [Citation6–8]. To address these problems, ultrasound elastography was developed, introducing a relatively new area of research.
2. Ultrasound elastography
Ultrasound elastography, proposed in 1991 by Ophir et al. can be used to quantify biomechanical changes in parenchymal tissues undergoing diseases, obtain elastic tissue information, evaluate tissue stiffness, and afford a new field for traditional two-dimensional and color Doppler ultrasonography. The two main elastography techniques used clinically are strain elastography (SE) and shear wave elastography (SWE). Elastography refers to the deformation of the tissue of interest under the application of an external pressure by a probe or internal tissue movements [Citation9]. However, because the external force applied by the probe is unknown, specific values for tissue stiffness quantification cannot be determined. Nevertheless, semi-quantitative evaluations can be conducted based on comparisons of the region of interest with standard tissues in the same scanned region. By contrast, SWE entails an objective, quantitative assessment of tissue stiffness; it uses focused, short-duration pulses of acoustic radiation to induce local tissue deformation and generate shear waves. The mechanical properties of these tissues can then be quantitatively estimated based on measurements of the shear wave velocity (SWV) or derivation of the Young’s modulus (YM); subsequently, the SWV or YM can be color-coded and superimposed over two-dimensional anatomical images to highlight their distributions [Citation10]. Notably, SWE is not strongly reliant on experienced operators and enables objective and reproducible quantifications of tissue elasticity, unlike SE.
SWE is widely used in examinations of the thyroid, breast, liver, kidney, skeletal muscle, lymph nodes, and other visceral tissues [Citation11]. Specifically, it is employed to assess tissue stiffness using quantitative tissue elasticity information, to aid in the differential diagnoses of benign and malignant tumors. Additionally, it can be used to evaluate liver cirrhosis. Accordingly, studies have applied the SWE technique that has been used on the liver onto the placenta in an attempt to quantify the extent of intraplacental lesions. However, ultrasound elastography of the placenta remains a relatively new area of research, and many unknown factors still need to be investigated. Some of these factors stem from the effects of maternal diseases on the placenta and also the effects of pathological changes in the placenta on fetal development.
3. Elastography on normal placenta
3.1. Correlative studies on placental elastography
A comparison of ultrasound elastography studies on normal placentas by researchers from different countries revealed the following. When identical ultrasound instrument systems are used by independent teams, the values of their in-vivo placental elastography measurements can exhibit communicability [Citation12]. For example, Alan et al. [Citation13], Ohmaru et al. [Citation14], and Wu et al. [Citation15] measured approximately identical SWVs in normal placentas (1.07, 0.98 ± 0.21, and 0.983 ± 0.260 m/s, respectively) using a Siemens (2000 ultrasonic diagnostic) instrument. When Yuksel et al. [Citation16], Liu et al. [Citation17], and Chen [Citation18] used a French sonographic ultrasound diagnostic instrument to measure the YM values of normal placentas, similar to the work of Yuan et al. [Citation19], their measurements (approximately 6.29 ± 1.16 and 6.42 ± 0.63 kPa) exhibited no significant differences. Thus far, varying placental elasticity values have been recorded using different techniques and systems involving different ultrasound diagnostic instruments; however, the data obtained using identical technologies and systems from large numbers of samples have been observed to be uniform. Moreover, differences in ethnic groups of the patients seem to have no significant impact on placental elasticity.
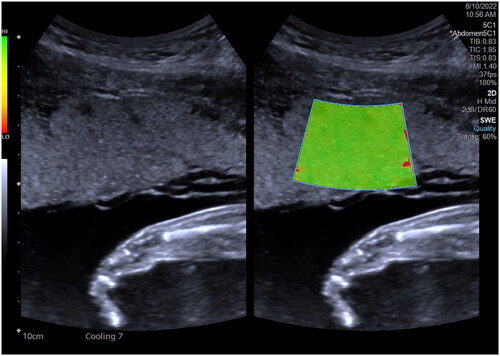
Before her examination by in vivo placental SWE, the pregnant patient is instructed to empty her bladder, breathe calmly, take the supine position, and fully expose her abdomen. The ultrasonic transducer is gently held onto the surface of her skin to avoid excessive compression and transmission of the ultrasonic beam through the fetus. At the same time, the device is kept away from the umbilical cord to be able to examine into the placenta. Then, the examiner waits for the fetus to be quiet. When the pregnant patient has no uterine contraction, she is asked to hold her breath for a few seconds. The area of interest in the placenta is measured several times (≥3 times), and the average value is obtained. This value is verified to be accurate and reliable based on a quality map. However, there is presently no consensus on measurement standards for placental SWE. By comparison, system-specific reference values have been established for chronic liver disease to quantify the severity of liver fibrosis. To quantify tissue stiffness, placental SWE can be feasibly standardized, and reference values for normal placental elasticity can be established. Such standards will help in assessing placental function ().
3.2. Factors influencing elastography of placenta
Ge et al. [Citation20] and Wu et al. [Citation15] determined that differences in the of gestational age (GA) had no significant effects on the values of placental elasticity, whereas Ohmaru et al. [Citation14] reported a slight non-significant upward trend in SWVs with an increase in GA. However, it still remains unclear whether this increasing trend is related to the degree of natural placental maturation. The limitation of the aforementioned studies on GA is that they cannot flexibly regard time as a continuous variable. The linear mixed model regression analysis used in a study by Edwards et al. modeled GA as a continuous variable and analyzed it to be a significant predictor of placental SWV, albeit with a lower impact on SWV compared with those of body mass index (BMI) and depth [Citation4].
Spiliopoulos et al. showed that maternal BMI is an important predictor of placental health based on healthy and preeclampsia pregnancy models in their SWE research, and that an increase in placental hardness is associated with higher BMI [Citation21]. Edwards et al. used a linear mixed model to determine that in normal pregnancy, pre-pregnancy obesity will significantly lead to an increase in placental stiffness, and that weight gain during pregnancy will also lead to an increase in placental stiffness [Citation22]. Therefore, the case control design will need to match the pre-pregnancy BMIs of a normal group and abnormal group to ensure accurate interpretation of the differences in placental hardness. In addition, it has been reported that the SWVs of pregnant women with deep breathing and fetal movement are significantly higher than those of women with shallow breathing and no fetal movement [Citation14]. Moreover, the amniotic fluid volume has certain intervention effects on SWVs, although these effects have been inferred to be weak [Citation4,Citation23].
The sample depth is a clear influencing factor in measurements. Edwards et al. observed that when the sample depth was limited within a range of 2–6 cm, the maximum change in mean SWV measurement value was 0.21 m/s, which was only slightly higher than the sample difference (0.71 m/s) [Citation4]. However, in clinical application, it is difficult to ensure that the depth of the placental region of interest fulfills the standard 2–6 cm depth range. Currently, it is generally accepted that elasticity measurements at depths of 8 cm are more meaningful, and that there are no significant differences in elasticity values for different regions of the placenta [Citation14].
4. Elastography on dysfunctional placentas
A recent prospective study on the causes of stillbirth showed that placental and umbilical cord abnormalities are the main lethal factors for more than 90% of stillbirths, mainly involving placental poor perfusion and bleeding [Citation24]. Maternal diseases such as gestational hypertension, gestational diabetes, and immune system disorders can lead to the failure of trophoblast cell infiltration and spiral artery remodeling, which, in turn, can result in prolonged placental malperfusion, placental cell stress, and failure of placental cell proliferation. Severe ischemic and hemorrhagic injuries can also lead to placental tissue fibrosis [Citation25,Citation26]. These pathological alterations result in increased placental parenchymal stiffness, decrease the exchange of materials between the placenta and fetus, and impede the exchange of maternal fetal gases and nutrients.
Most intrauterine growth restriction (IUGR) cases are caused by placental defects [Citation27]. Thus, placental elasticity values are higher for IUGR fetuses; this was also demonstrated by Habibi et al. [Citation28] and Alfirevic et al. [Citation29], and by Deeba et al. [Citation30] in their in vitro placenta study using three-dimensional multifrequency SWE. Akbas et al. [Citation31] confirmed that placental elasticity values are significantly and positively correlated with the Doppler indices of uterine and umbilical arteries and with adverse clinical outcomes. Therefore, SWE could serve as a new technique for the effective diagnosis and prediction of IUGR, while increased placental stiffness could also serve as a useful predictor for worsened perinatal outcomes. Anuk et al. [Citation32] believed that SWE could enhance the effectiveness of Doppler parameters in predicting adverse perinatal outcomes in high-risk pregnancies. A recent study by Akbas et al. [Citation33] showed that pregnant women with hard placentas in the second trimester of pregnancy are more likely to experience adverse obstetric outcomes.
Hypertensive disorders of pregnancy (HDP) are conditions specific to pregnancy, whereas preeclampsia (PE) is a severe form of HDP that can lead to fine atherosclerosis, when PE occurs, the uterine artery spasm in the mother causes narrowing of the vascular lumen, increased resistance, decreased blood volume, and decreased placental blood supply. Additionally, placental microvessels are prone to thrombosis due to the hypercoagulable state of the mother, leading to villous embolism or necrosis. On the cytological level, PE changes include trophoblast cell infiltration and spiral artery reconstruction failure, which lead to long-term poor placental perfusion, cell stress, and placental cell proliferation failure, thus leading to microscopic atherosclerosis, fibrin deposition, and calcification. Under the continuous aggravation of ischemia and bleeding injury, placental tissue fibrosis and infarction can be induced. The progression of PE involves the deposition of fibrin and calcification in placental tissue, leading to tissue fibrosis. Therefore, placental hardness increases with the occurrence of PE, placental insufficiency and fetal growth restriction [Citation34,Citation35]. The results of studies by Ge et al. [Citation36] and Cimsit et al. [Citation37] revealed correlations with respect to increases in the placental modulus values of their PE groups; there were no statistical differences in the elastic modulus values between different regions. Fujita et al. [Citation34] and Yuan et al. [Citation19] showed that, before the onset of PE, the placental elastic value (YM) increases, whereas the umbilical artery blood flow parameters remain unchanged. Kiliç et al. [Citation38] showed that the maximum area under the curve (AUC) for diagnosing preeclampsia is the central placental area of the fetus, with the highest diagnostic accuracy threshold of 7.35 kPa (AUC value of 0.895), sensitivity of 90%, specificity of 86%, and diagnostic accuracy of 88%. Fujita et al. [Citation34] and Sirinoglu et al. [Citation39] believed that the optimal cutoff values for predicting PE were 1.188 m/s (AUC of 0.912) and 7.43 kPa (AUC of 0.924), suggesting that placental elasticity had already changed before the onset of PE. Therefore, SWE could serve as a more sensitive technique for detecting abnormal early placental changes in patients with gestational hypertension, thereby facilitating predictions of the occurrence of PE.
Further, SWE is useful for the prediction of gestational diabetes mellitus (GDM), Rh alloimmunization pregnancy, pregnancy with structural abnormalities, abnormal placental infiltration (API), and in the short-term prediction of threatened preterm labor (TPL) [Citation16,Citation17,Citation40–44].
5. Safety of shear wave elastography in pregnancy
At present, there has been no report that acoustic radiation force impulse (ARFI) imaging poses a threat to the safety of pregnancy [Citation4]. Although elastic imaging based on radiation force uses a high thermal index (TI), the TI and mechanical index (MI) of the equipment used in the procedure are within the limits set by the American Institute of Ultrasound Medicine (AIUM) [Citation45,Citation46] (TI ≤ 0.7, MI ≤ 1.9). Ge et al. [Citation20] believed that the shear wave would not propagate in amniotic fluid, and that SWE would have little impact on the fetus. Moreover, SWE ensures the time interval between two measurements by intermittently emitting low-density acoustic radiation, avoiding the risk of long-term irradiation of the placenta. On the other hand, the British Medical Ultrasound Society (BMUS) recommends that the duration of SWE be limited to 15 min [Citation4]. The World Federation of Medical and Biological Ultrasound (WFUMB) especially reported that commercially available ultrasound equipment using radiation force “can diagnose within the radiation safety range.” To obtain more comprehensive data related to the safety of ultrasonic radiation force, SWE and its use in obstetrics should continue to be explored in future scientific research.
6. Limitations and prospects
Currently, direct predictors of clinical placental function are scarce. As a solution, SWE can help quantify biomechanical alterations in placental tissues and subsequently assess placental function. Thus, SWE could serve as a new method for clinical assessments of placental function. However, the current use of placental elastography in obstetrics suffers from certain limitations and inconsistencies: lack of uniform, normative measurement standards; vulnerability to operator techniques; and limitations due to posteriorly placed placentas. In addition, the influence of maternal fetal biological factors on the value of SWE in relation to changes in the placental structure is not limited to placental dysfunction (preeclampsia and growth restriction), but also related to maternal obesity. These shortcomings warrant further investigations. With the continuous development of elastic imaging technologies, further research involving multiple centers and larger samples, and continuous standardization of diagnostic criteria, elastic imaging will play a more important role in clinical applications in obstetrics. Nevertheless, to summarize, SWE shows significant potential for clinical applications in obstetrics, in addition to acceptable research prospects ().
Table 1. Elastography in placental function evaluations.
7. Conclusions
Shear wave elastography technology has good clinical application value and research prospects in obstetrics due to its objective and repeatable quantitative operation. In recent years, research on SWE in the placenta have shown that this technique is helpful in providing information on placental dysfunction such as in PE and IUGR and predicting adverse perinatal outcomes.
Disclosure statement
No potential conflict of interest was reported by the author(s).
Additional information
Funding
References
- Xinyao L, Yuan Y, Shengli L, et al. Research progress of medical ultrasound assessment of placental function. Sheng wu yi Xue Gong Cheng Xue za Zhi = J Biomed Engineer = Shengwu Yixue Gongchengxue Zazhi. 2015;32(4):914–918,923.
- Simoes APR, Maronezi MC, Uscategui RAR, et al. Placental ARFI elastography and biometry evaluation in bitches. Anim Reprod Sci. 2020;214:9.
- Quibel T, Deloison B, Chammings F, et al. Placental elastography in a murine intrauterine growth restriction model. Prenat Diagn. 2015;35(11):1106–1111.
- Edwards C, Cavanagh E, Kumar S, et al. Changes in placental elastography in the third trimester - Analysis using a linear mixed effect model. Placenta. 2021;114:83–89.
- Edwards C, Cavanagh E, Kumar S, et al. The use of elastography in placental research - A literature review. Placenta. 2020;99:78–88.
- Woo J, Ge WR, Mancheri J, et al. Shear wave elastography: the relationship of the cervical stiffness with gestational age and cervical length- a feasibility study. J Matern-Fetal Neonatal Med. 10:9684–9693.
- Gachon B, Fritel X, Pierre F, et al. In vivo assessment of the elastic properties of women’s pelvic floor during pregnancy using shear wave elastography: design and protocol of the ELASTOPELV study. BMC Musculoskelet Disord. 2020;21(1):12.
- Mottet N, Cochet C, Vidal C, et al. Feasibility of two-dimensional ultrasound shear wave elastography of human fetal lungs and liver: a pilot study. Diagn Interv Imaging. 2020;101(2):69–78.
- Cotoi L, Amzar D, Sporea I, et al. Shear wave elastography versus strain elastography in diagnosing parathyroid adenomas. Int J Endocrinol. 2020;2020:3801902.
- Bian JY, Zhang JL, Hou XK. Diagnostic accuracy of ultrasound shear wave elastography combined with superb microvascular imaging for breast tumors a protocol for systematic review and meta-analysis. Medicine. 2021;100(25):e26262.
- Sigrist RMS, Liau J, El Kaffas A, et al. Ultrasound elastography: review of techniques and clinical applications. Theranostics. 2017;7(5):1303–1329.
- Ferraioli G, Barr RG, Farrokh A, et al. How to perform shear wave elastography. Part I. Med Ultrason. 2022;24(1):95–106.
- Alan B, Tunc S, Agacayak E, et al. Diagnosis of pre-eclampsia and assessment of severity through examination of the placenta with acoustic radiation force impulse elastography Int J Gynaecol Obstet. 2016;135(1):43–46.
- Ohmaru T, Fujita Y, Sugitani M, et al. Placental elasticity evaluation using virtual touch tissue quantification during pregnancy. placenta. Placenta. 2015;36(8):915–920.
- Wu S, Nan R, Li Y, et al. Measurement of elasticity of normal placenta using the virtual touch quantification technique. Ultrasonography. 2016;35(3):253–257.
- Yuksel MA, Kilic F, Kayadibi Y, et al. Shear wave elastography of the placenta in patients with gestational diabetes mellitus [article. J Obstet Gynaecol. 2016;36(5):585–588.
- Liu Yunli WD, Zhiwei GUO, Weiqing YANG, et al. Study on the clinical value of real-time SWE in detecting placenta elasticity of patients with GDM. China Med Equipment. 2021;18(07):103–107.
- Lu C. Application values of real-time shear wave elastography evaluated placental elasticity of pre-eclampsia in the third trimester of pregnancy [master], Nan Chang University. 2019.
- Yuan Shengmei LY, Xuemei WANG, Kun H. The application research of shear wave elasticity imaging placenta of gestational hypertension disease. Chinese Ultrasound Me. 2017;33(01):34–41. (in Chinese).
- Ge Chengxia GJ. Evaluation of the placental elastic modulus in the normal second or third trimester of pregnancy and its influencing factors. Chin Clin Med Imaging. 2019;30(10):726–729.
- Spiliopoulos M, Kuo CY, Eranki A, et al. Characterizing placental stiffness using ultrasound shear-wave elastography in healthy and preeclamptic pregnancies. Arch Gynecol Obstet. 2020;302(5):1103–1112.
- Edwards C, Cavanagh E, Kumar S, et al. Relationship between placental elastography, maternal pre-pregnancy body mass index and gestational weight gain. Placenta. 2022;121:1–6.
- Altunkeser A, Alkan E, Günenç O, et al. Evaluation of a healthy pregnant placenta with shear wave elastography. Iran J Radiol. 2018;16:e68280.
- McClure EM, Saleem S, Goudar SS, et al. The causes of stillbirths in South asia: results from a prospective study in India and Pakistan (PURPOSe) [article. Lancet Glob Health. 2022;10(7):E970–E977.
- Booker W, Moroz L. Abnormal placentation [review]. Semin Perinatol. 2019;43(1):51–59.
- Burton GJ, Jauniaux E. Pathophysiology of placental-derived fetal growth restriction [review. Am J Obstet Gynecol. 2018;218(2S):S745–S761.
- Gao Jin HB. Progresses of shear wave elastography evaluation of placental function. Chin J Med Imaging Techno. 2020;36(05):780–783. (in Chinese).
- Habibi HA, Davutoglu EA, Kandemirli SG, et al. In vivo assessment of placental elasticity in intrauterine growth restriction by shear-wave elastography. Eur J Radiol. 2017;97:16–20.
- Alfirevic Z, Stampalija T, Dowswell T. Fetal and umbilical doppler ultrasound in high-risk pregnancies. [Cochrane Database Syst Rev. 2017;6(6):CD007529.
- Deeba F, Hu R, Lessoway V, et al. SWAVE 2.0 imaging of placental elasticity and viscosity: potential biomarkers for Placenta-Mediated disease detection. Ultrasound Med Biol. 2022;48(12):2486–2501.
- Akbas M, Koyuncu FM, Artunc-Ulkumen B. Placental elasticity assessment by point shear wave elastography in pregnancies with intrauterine growth restriction. J Perinat Med. 2019;47(8):841–846.
- Anuk AT, Tanacan A, Erol Sa , et al. Value of shear-wave elastography and cerebral-placental-uterine ratio in women diagnosed with preeclampsia and fetal growth restriction in prediction of adverse perinatal outcomes. J Matern-Fetal Neonatal Med. 35(25):10001–10009.
- Akbas M, Koyuncu FM, Artunc-Ulkumen B, et al. The relation between second-trimester placental elasticity and poor obstetric outcomes in low-risk pregnancies. J Perinat Med. 2021;49(4):468–473.
- Fujita Y, Nakanishi TO, Sugitani M, et al. Placental elasticity as a new non-invasive predictive marker of pre-eclampsia. Ultrasound Med Biol. 2019;45(1):93–97.
- Phipps EA, Thadhani R, Benzing T, et al. Pre-eclampsia: pathogenesis, novel diagnostics and therapies [review]. Nat Rev Nephrol. 2019;15(5):275–289.
- Ge Chengxia GJ. Preliminary study of shear wave elastography in evaluation of placental elasticity in preeclampsia. Clin Ultrasound in Med. 2019;21(11):855–857.
- Cimsit C, Yoldemir T, Akpinar IN. Strain elastography in placental dysfunction: placental elasticity differences in normal and preeclamptic pregnancies in the second trimester. Arch Gynecol Obstet. 2015;291(4):811–817.
- Kılıç F, Kayadibi Y, Yüksel MA, et al. Shear wave elastography of placenta: in vivo quantitation of placental elasticity in preeclampsia. Diagn Interv Radiol. 2015;21(3):202–207.
- Sirinoglu HA, Uysal G, Nazik H, et al. Efficacy of shear wave elastography in predicting preeclampsia in the first trimester. Rev Assoc Med Bras (1992). 2021;67(11):1558–1563.
- Arslan H, Tolunay HE, Cim N, et al. Shear-wave elastography - virtual touch tissue quantification of fetal placentas with a single umbilical artery. J Matern Fetal Neonatal Med. 2019;32(15):2481–2485.
- Cetin O, Karaman E, Arslan H, et al. Acoustic radiation force impulse elastosonography of placenta in maternal red blood cell alloimmunization: a preliminary and descriptive study. Med Ultrason. 2017;19(1):73–78.
- Tolunay HE, Eroğlu H, Çelik ÖY, et al. Can placental elasticity predict the time of delivery in cases of threatened preterm labor?. J Obstet Gynaecol Res. 2021;47(2):606–612.
- Tepe NB, Yilmaz FG, Bozdag Z, et al. Subgroup analysis of accreta, increta and percreta cases using acoustic radiation force impulse elastography. J Obstet Gynaecol Res. 2020;46(5):699–706.
- Dokumaci DS, Uyanikoglu H. Shear-wave elastography for detection of placenta percreta: a case-controlled study. Acta Radiol. 2022;63(3):424–430.
- Simon EG, Calle S. Safety of elastography applied to the placenta: be careful with ultrasound radiation force. J Obstet Gynaecol Res. 2017;43(9):1509–1509.
- Karaman E. Response to 'safety of elastography applied to the placenta: be careful with ultrasound radiation force’. J Obstet Gynaecol Res. 2017;43(9):1510–1510.