Abstract
Background
Antenatal fetal heart rate (FHR) monitoring is currently limited by hospital-based accessibility as well as the availability of relevant equipment and expertise required to position device electrodes. Ambulatory FHR monitoring in the form of noninvasive fetal electrocardiography (NIFECG) is currently an area of research interest, particularly during the era of the COVID-19 pandemic, and the potential to improve maternity care and reduce hospital attendances need to be evaluated.
Objectives
To assess the feasibility, acceptability, and signal success of ambulatory NIFECG monitoring and identify research areas required to facilitate clinical utilization of this method of monitoring.
Methods
Medline, EMBASE, and PubMed databases were searched from January 2005 to April 2021 using terms relevant to antenatal ambulatory or home NIFECG. The search was compliant with PRISMA guidelines, and was registered with the PROSPERO database (CRD42020195809). All studies reporting the clinical utilization of NIFECG inclusive of its use in the ambulatory setting performed in the antenatal period, human studies, and those in the English language were included. Those reporting novel technological methods and electrophysiological algorithms, satisfaction surveys, intrapartum studies, case reports and reviews, and animal studies were excluded. Study screening and data extraction were conducted in duplicate. Risk of bias was appraised using the Modified Downs and Black tool. Due to the heterogeneity of the reported findings, a meta-analysis was not feasible.
Results
The search identified 193 citations, where 11 studies were deemed eligible for inclusion. All studies used a single NIFECG system with a duration of monitoring ranging from 5.6 to 21.4 h. Predefined signal acceptance threshold ranged from 34.0-80.0%. Signal success in the study populations was 48.6–95.0% and was not affected by maternal BMI. Good signals were achieved in the 2nd trimester, but less so in the early 3rd trimester. NIFECG was a well-accepted method of FHR monitoring, with up to 90.0% of women’s satisfaction levels when worn during outpatient induction of labor. Placement of the acquisition device needed input from healthcare staff in every report.
Conclusions
Although there is evidence for the clinical feasibility of ambulatory NIFECG, the disparity in the literature limits the ability to draw firm conclusions. Further studies to establish repeatability and device validity, whilst developing standardized FHR parameters and set evidence-based standards for signal success for NIFECG are required to ascertain the clinical benefit and potential limitations of ambulatory outpatient FHR monitoring.
Introduction
Antenatal cardiotocography (CTG), which is based on Doppler ultrasound, and its visual interpretation is currently the mainstay of fetal heart rate (FHR) monitoring in the antenatal period. Despite this, it is limited by a lack of stringent guidelines for interpretation, high intra and inter-observer variation, and does not reduce the rate of perinatal mortality compared to pregnancies that did not have CTG monitoring [Citation1]. In contrast, computerized CTGs (cCTG) have been shown to reduce the rate of perinatal mortality compared to traditional antenatal CTGs [Citation1]. Access to, and frequency of cCTG remains limited by the accessibility and availability of relevant equipment and expertise. In high-risk pregnancies that require intensive surveillance, difficulty in accessing the adequate level of healthcare prevents the women from receiving appropriate interventions, increases the chances of adverse outcomes and poses a strain on the healthcare system.
Telemedicine is a rapidly developing field, striving to provide women with the equipment that allows fetal assessments in the comfort of their own homes, simultaneously giving rise to early identification of disease deterioration whilst reducing hospital attendance and healthcare burdens [Citation2,Citation3]. In the era of the COVID-19 pandemic, such methods can potentially decrease the exposure of susceptible pregnant women to the high-risk hospital setting. Due to the difficulties of reliably monitoring the FHR using Doppler ultrasound without the expertise of a healthcare professional, home FHR monitoring obtained by another technology is a much-desired area that is yet to be developed. For instance, noninvasive fetal electrocardiography (NIFECG) offers a promising prospect into the development of this technology, where abdominal electrocardiography (ECG) can be performed to simultaneously detect both the maternal and fetal heart rates through the detection of QRS complexes and derivation of RR intervals. Nonetheless, before this application, current studies have shown the potential of NIFECG to be performed in an ambulatory setting. Existing systematic reviews have assessed the use of NIFECG in high-risk pregnancies in a hospital setting, but none has so far evaluated the feasibility or clinical utility of ambulatory NIFECG [Citation4–6].
Our aim is to systematically review all studies on NIFECG monitoring in an out-of-hospital setting with specific regard to FHR signal success, acceptability to women, need for hospitalization and frequency of monitoring. We also aim to identify aspects of further guidance and research required to allow feasible clinical application of ambulatory NIFECG monitoring.
Materials and methods
This review was performed in accordance with the prior protocol designed for systematic reviews. PRISMA guidelines were followed in the conduct of this review [Citation7], and this was registered with the PROSPERO database (Registration number: CRD42020195809). The inclusion criteria were studies assessing the clinical utilization of NIFECG inclusive of its use in the ambulatory setting performed in the antenatal period, human studies, those in the English language, and due to the novelty of this monitoring, studies which took place from January 2005 to April 2021. The exclusion criteria included research into novel engineering models or materials and electrophysiological algorithms, satisfaction surveys, intrapartum studies, case reports and reviews, and animal studies.
A systematic, electronic search by two independent reviewers (BL and AR) was performed using PubMed, MEDLINE, and EMBASE databases in April 2021. Search terms “ambulatory AND fetal ECG,” “home AND fetal ECG,” and “continuous AND fetal ECG” were used to identify the literature. Terms “foetal” and “electrocardiography” were used in place of “fetal” and “ECG” respectively. Duplicates and articles not in keeping with the inclusion criteria were excluded during the screening process ().
Figure 1. PRISMA flow diagram displaying the numbers of studies identified and screened, reasons for exclusion, the studies assessed for eligibility, and the final studies included.
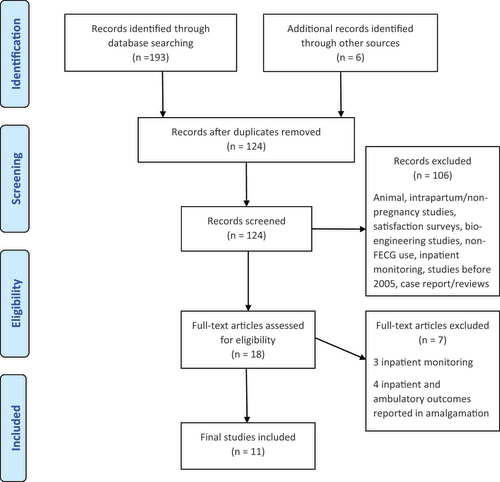
All abstracts were reviewed independently by authors BL and AR, and an agreement on full-text articles for review and inclusion was reached through a consensus. All studies where both inpatient and ambulatory monitoring signals were reported in conjunction were also excluded. Any discrepancies were discussed by the reviewers, and when required, a final decision was reached by a third reviewer (BT). Data were extracted independently by the two reviewers and compared for consistency. Studies that reported on both inpatient and ambulatory data, with the primary outcome reported separately in both groups were included, and only the data from the ambulatory groups extracted [Citation8,Citation9]. Due to the heterogeneity of data and nature of the studies available in this research field, a meta-analysis was not feasible.
The primary outcome sought for this review was the signal acceptance threshold and signal success as defined by the authors in the ambulatory setting. Secondary outcomes included the duration of monitoring required (hours), influence of maternal and fetal characteristics on signal quality (body mass index, gestational age, fetal presentation), FHR parameters (baseline, accelerations, measure of variability) in the ambulatory setting and its influential factors, acceptability of ambulatory NIFECG by women and its safety, and other limitations of the device.
Signal acceptance threshold is the minimum percentage of R waves present in a trace, or the minimum epochs containing R waves to consider the trace interpretable, and hence acceptable for analysis. Signal success was either proportion of traces that met the pre-defined signal acceptance threshold, or the proportion of each trace with an acceptable signal in the study population, depending on author definitions. Gestational ages were grouped into 2nd trimester (up to 28 weeks), early 3rd trimester (28 to 32–34 weeks), and late 3rd trimester (after 34 weeks). FHR parameters includes baseline FHR, accelerations and measure of variability - inclusive of short-term variability (STV) and root mean square of successive difference (RMSSD), as both evaluate the variability of the heart rate.
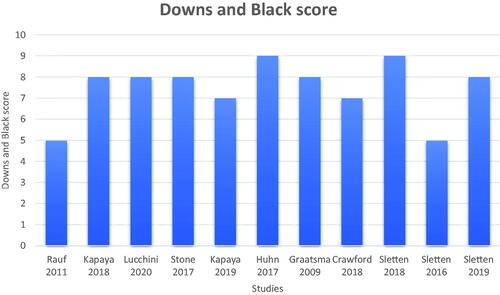
Quality assessment was performed using the Modified Downs and Black checklist [Citation10], where the quality of the observational studies was scored on a 10-point scale. These are scored on adequate reporting of the study aims, outcomes, participant characteristics, findings, and random variability estimates as well as the statistical probabilities. External and internal validities in the form of participant representation, statistical tests, and outcome measures used were also scored. Both reviewers BL and AR reached a consensus on the scoring of all papers.
Results
The literature search yielded 193 citations, and 124 records were screened following the removal of duplicates. The PRISMA diagram () demonstrates the process and reasons for exclusion. 11 full-text articles that were deemed eligible for the systematic review. summarizes the scores given by both authors in the quality assessment of the studies.
Figure 2. Downs and Black scoring for the assessment of quality in all included studies. Scores were reached through a consensus by both authors out of a total of 10 points.
The study characteristics, sample size, signal measurement, acceptance threshold, and success, together with the duration of monitoring are outlined in , which differed between author definitions in all studies. All studies used the Monica AN24 device. The time between the R-wave of consecutive fetal QRS complexes in the ECG signal is used to calculate the FHR. Each 2-s epoch is checked for a minimum of two consecutive maternal and fetal ECG complexes. If present, these complexes enable a heart rate to be calculated for that epoch, where the heart rates are averaged [Citation11]. The duration of the recording is assessed in one-minute epochs. Heart rate is reported for this epoch only if valid RR intervals can be determined for at least 30 s. The success rate is automatically calculated by the signal analysis software for each minute of recording as the sum of durations of all detected beat-to-beat intervals divided by the entire length of the reference period (60 s), expressed as a percentage [Citation9]. Studies reported either the percentage of the total duration containing valid fetal heart rate data or the proportion of the traces passing pre-defined thresholds for durations over which the fetal heart rate data were missing.
Table 1. Description of study characteristics, signal success and duration of ambulatory NIFECG monitoring in all included cohort studies.
Signal acceptance threshold ranged from 34.0–80.0% [Citation8,Citation12–19] whilst other authors did not set a threshold [Citation9,Citation20] (). Individual components of the cardiac cycle were not assessed in the studies included. The signal success ranged from 48.6–95.0%. The duration of monitoring in the studies ranged from 5.6–21.4 h [Citation8,Citation9,Citation13–20].
Few studies analyzed the impact of maternal and fetal characteristics on signal success, such as body mass index (BMI), gestation, diurnal variation, maternal activity and position, and fetal size and presentation (). Maternal BMI and fetal presentation did not affect signal success [Citation8,Citation9,Citation17,Citation20]. The signal was better acquired at night than during the day, and whilst recorded at rest [Citation8,Citation17,Citation18]. Higher signal success was obtained with the mother in the supine compared to the upright position, and fetal size did not impact the quality of the recording [Citation9]. Signal success was high in the 2nd trimester, lowest in the early 3rd trimester, with gradual improvement in the late 3rd trimester (78.9, 29.8, and 49.6% in 2nd, early 3rd and late 3rd trimesters, respectively, p < 0.001) [Citation8,Citation9,Citation18,Citation20]. Crawford et al. did not find any significant differences between the gestations (p = 0.05) [Citation17], which may have been attributed to the smaller sample size.
Table 2. Summary of effects of maternal and fetal characteristics on signal success and fetal heart rate parameters.
Baseline FHR was higher during the day compared to night time, higher in the 2nd trimester compared to the 3rd trimester, was lower in 1 F (quiet sleep) compared to 2 F (active sleep) fetal sleep states, and was not affected by gender or maternal activity [Citation12–14,Citation16,Citation20]. Kapaya et al. reported fewer accelerations during the day, and no gender influence [Citation12]. Conflicting findings were reported by different authors on the influence of time of the day, fetal behavioral states and gender on FHR variability [Citation12–14,Citation16]. Sletten et al. reported that FHR variability was reduced in the 2nd compared to the 3rd trimester, and was not influenced by maternal activity [Citation16].
Clinical application
Rauf et al. reported clinical feasibility of NIFECG use in the outpatient setting on low-risk women undergoing home induction of labor with high rates of signal success in the study population (89.0%) [Citation19]. Kapaya et al. achieved a 49.1% overall signal success, but this improved to 94.0% at nighttime whilst the woman was at rest [Citation18]. Graatsma et al. achieved an overall signal success of 82.0%, with a median of 95.0% epochs with available data in the ambulatory group8. It was concluded that the signal success was not dissimilar between the inpatient and outpatient monitoring groups (91.9 vs 95.0%, p = 0.15), even though one study suggested that the ambulatory group yielded superior quality traces than the inpatient group (76.1 vs 45.5%, p < 0.001) [Citation8,Citation9].
Few authors assessed women’s satisfaction levels with regard to wearing the Monica AN24 monitor [Citation8,Citation17–19]. 90.0% of women were satisfied when wearing the Monica AN24 during outpatient induction, and the awareness of being telemetrically monitored provided reassurance [Citation19]. Up to 80.0% of women found the device comfortable, but the skin irritation rates were as high as 55.0% [Citation17]. Participants also reported that the wires were cumbersome and affected daily activities and caused some sleep disturbance, and 70.0% felt that improvements could be made to this device [Citation17,Citation18].
Discussion
Our study assesses the feasibility and clinical utility of antenatal NIFECG in the ambulatory setting. Evaluation of signal success is limited by the heterogeneity of the signal acceptance threshold set by the authors, and as the device required placement and removal by healthcare professionals, the frequency of hospital attendance was not reduced. NIFECG signals were not affected by maternal BMI or fetal presentation, superior recordings were achieved when women were resting, with lower signal success in the early 3rd trimester. This method of monitoring was generally acceptable to women, except for occasional irritation from electrodes and the perceived need to allow more freedom of movement. No safety issues were reported with the use of NIFECG.
Clinical potential for NIFECG
NIFECG is currently an area of research interest as it has the potential to offer various additional benefits to the current FHR monitoring techniques using a CTG. No energy is delivered through the ECG electrodes, which allows for safe and long periods of monitoring. Signal processing techniques allow for a more precise FHR pattern derivation using RR interval calculation, whilst minimizing confusion with the MHR [Citation21]. Algorithms developed over the years have incorporated the calculation of key computerized CTG parameters such as the STV, noninvasive electrophysiological assessments (NIEA) of the cardiac morphology, such as cardiac time intervals (CTI), and the ability to assess cardiac arrhythmias, rather than FHR alone.
Existing NIFECG technology and comparison with existing literature
Fetal ECG allowed for one of the earliest forms of FHR detection, with Southern et al. describing its ability in the detection of intrauterine hypoxia through the analysis of its waveform patterns as early as 1957 [Citation22]. Due to the complexity of fetal ECG, high rate of signal interference and poor signal success, CTG became the preferred method of monitoring [Citation23]. A recent systematic scoping review published by Tamber et al. assessed various methods of antenatal continuous fetal monitoring, which required prolonged monitoring in a hospital setting, and the authors concluded that due to the inter and intra-device variability, no design is advantageous and that it cannot yet be strongly recommended as a method of routine monitoring [Citation4].
Wireless models of the NIFECG such as the Monica AN24 system are no longer valid for antenatal use, but have been able to offer real-time computation of FHR, which can be accessed telemetrically on hospital databases while the woman returns home with the device [Citation18,Citation19]. The design of the Monica AN24 comprises of 5 abdominal ECG electrodes which connects to an internal processor [Citation24]. The device is fitted and removed by healthcare professionals and prolonged monitoring times were needed as the women were not able to re-apply and re-activate the device without medical assistance. In order to achieve a reduction in hospital attendance and increase the frequency of monitoring in high-risk women, a NIFECG device allowing for easy application and removal by women without assistance is required. This will enable shorter monitoring times and therefore less skin irritation and interference with daily activities should incorporate an alert system on the smartphone application to inform the mothers of when Obstetric advice needs to be sought.
NIFECG signal standards
Numerous methods of signal evaluation have been used by authors in this review. Signal acceptance thresholds were either determined through in-built algorithms or derived from FHR interpretation criteria intended for CTG use [Citation8,Citation18]. Other studies assessing signal success in inpatient cohorts that were not included in this review have used other signal processing criteria such as the FIGO guidelines (>80.0%), which was also set for CTG monitoring [Citation25,Citation26]. None of these specific thresholds have a justification. We would argue that signal success should be the ability to acquire 60 min of interpretable data (successive R-R waves to calculate instantaneous heart rate) regardless of the duration of recording, as NIFECG have the benefit of low energy emission and can therefore allow longer periods of monitoring than antenatal cCTGs. The purpose is to identify fetuses in an active state (good variability) because studies of healthy fetuses have demonstrated episodes of low variation lasting up to 50 minutes [Citation27]. Prolonged periods of low variability are increasingly likely to signify a fetus that is unwell, rather than the physiological quiescent fetal state. For the same reason, the Dawes-Redman criteria mandate a duration of 60 min to reliably identify an abnormally low variability [Citation28].
Clinical factors affecting NIFECG monitoring
Difficulties with signal success in mothers with raised BMI is frequently encountered in CTG monitoring, whilst NIFECG has shown that maternal habitus does not impact on the trace quality [Citation8, Citation9,Citation17,Citation20,Citation29]. Early 3rd trimester traces generally had a high rate of signal dropout, due to the development of the insulating vernix caseosa, which improved in the late 3rd trimester once the cardiac mass increased and the vernix to body surface ratio reduced [Citation8,Citation9,Citation18,Citation20,Citation25]. Despite this method of monitoring being feasible in the fully ambulatory state, most results have shown that recording quality is superior when the mother is at rest, which can be achieved if shorter periods of monitoring than those used in the studies in this review were feasible [Citation9,Citation17,Citation18]. Further studies assessing signal success using standardized signal criteria, together with multivariate logistic regression analysis to assess the true relationship of the maternal and fetal characteristics which may influence signal success are required.
Accuracy of NIFECG monitoring
Currently, no studies have set out to assess the repeatability and reproducibility of NIFECG. Research should assess whether the same traces can repeatedly produce the same parameters when analyzed more than once using the same processing algorithm. Following this, NIFECG parameters must be validated against simultaneous cCTG recordings (gold-standard) to assess the comparability of all FHR parameters. So far, research has only evaluated the correlation of FHR between simultaneous NIFECG and CTG measurements using a traditional CTG machine [Citation30]. Other measures of fetal autonomic state such as phase-rectified signal averaging (PRSA) may also be beneficial in the assessment of fetal hypoxemia [Citation31,Citation32]. However, further research is still required to establish its range of normality, and its use in the ambulatory setting. The reliability in the detection of deteriorating fetal states should also be established in women with pregnancies complicated by fetal hypoxemia, such as fetal growth restriction. Verified FHR indices (e.g. STV) produced by the NIFECG prior to the need for delivery as indicated by our current monitoring methods can then be used to create an alert system to indicate a need for action, thereby aiming to reduce false reassurance or unnecessary anxiety.
Strengths and limitations
Although few of the studies have been included in a recent review [Citation4], our paper specifically focuses on the feasibility of NIFECG in the remote setting. It is limited by the heterogeneity of signal assessment and signal acceptance threshold across the studies, and therefore, a concrete conclusion cannot be drawn from the available data. Different outcomes were assessed in each study, which limits the numbers of studies which reported on the outcomes assessed in this review, and is further restricted by a lack of standardized reporting criteria. Furthermore, the current design of the NIFECG monitors does not allow for frequent home measurements, nor a reduction in hospital attendance. The observational nature and design of some of the studies render the evidence quality at moderate to high risk of bias. Despite these limitations, we have been able to identify the specific areas where further research is required, and the developments needed to facilitate the clinical utilization of ambulatory NIFECG.
Conclusions and implications
Current studies in ambulatory NIFECG have shown promising results for clinical utility, but the disparity of the current evidence limits the ability to establish clinical feasibility. We suggest a yardstick for defining an interpretable fetal monitoring trace using NIFECG. Repeatability and validation of the device, as well as standardization of the FHR parameters and success criteria, needs to be established. New NIFECG design which allows women to apply and remove them on their own can overcome the long monitoring times and hospital attendance. Irrespective of these limitations, we demonstrate steps made toward an exciting and novel area of telemedicine, which can have the potential to significantly improve the delivery of maternity care.
Disclosure statement
No potential conflict of interest was reported by the author(s).
Additional information
Funding
References
- Grivell RM, Alfirevic Z, Gyte GML, et al. Antenatal cardiotocography for fetal assessment. Cochrane Database Syst Rev. 2015;2015. (9):CD007863.
- Perry H, Sheehan E, Thilaganathan B, et al. Home blood-pressure monitoring in a hypertensive pregnant population. Ultrasound Obstet Gynecol. 2018;51(4):524–530.
- Xydopoulos G, Perry H, Sheehan E, et al. Home blood-pressure monitoring in a hypertensive pregnant population: cost-minimization study. Ultrasound Obstet Gynecol. 2019;53(4):496–502.
- Tamber KK, Hayes DJL, Carey SJ, et al. A systematic scoping review to identify the design and assess the performance of devices for antenatal continuous fetal monitoring. PLoS One. 2020;15(12):e0242983.
- Smith V, Nair A, Warty R, et al. A systematic review on the utility of non-invasive electrophysiological assessment in evaluating for intra uterine growth restriction. BMC Pregnancy Childbirth. 2019;19(1):230.
- Verdurmen KMJ, Eijsvoogel NB, Lempersz C, et al. A systematic review of prenatal screening for congenital heart disease by fetal electrocardiography. Int J Gynaecol Obstet. 2016;135(2):129–134.
- Moher D, Liberati A, Tetzlaff J, et al. Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. PLOS Med. 2009;6(7):e1000097.
- Graatsma EM, Jacod BC, Van Egmond LAJ, et al. Fetal electrocardiography: feasibility of long-term fetal heart rate recordings. BJOG. 2009;116(2):334–337.
- Huhn EA, Müller MI, Meyer AH, et al. Quality predictors of abdominal fetal electrocardiography recording in antenatal ambulatory and bedside settings. Fetal Diagn Ther. 2017;41(4):283–292.
- Downs SH, Black N. The feasibility of creating a checklist for the assessment of the methodological quality both of randomised and non-randomised studies of health care interventions. J Epidemiol Community Health. 1998;52(6):377–384.
- Monica Healthcare Ltd. Monica AN24 Reference operator manual. 2009. 100-TF-006:1–40.
- Kapaya H, Jacques R, Anumba D. Comparison of diurnal variations, gestational age and gender related differences in fetal heart rate (FHR) parameters between appropriate-for-gestational-age (AGA) and small-for-gestational-age (SGA) fetuses in the home environment. PLoS ONE. 2018;13(3):e0193908.
- Lucchini M, Wapner RJ, Chia-Ling NC, et al. Effects of maternal sleep position on fetal and maternal heart rate patterns using overnight home fetal ECG recordings. Int J Gynaecol Obstet. 2020;149(1):82–87.
- Stone PR, Burgess W, McIntyre J, et al. An investigation of fetal behavioural states during maternal sleep in healthy late gestation pregnancy: an observational study. J Physiol. 2017;595(24):7441–7450.
- Sletten J, Lund A, Ebbing C, et al. The fetal circadian rhythm in pregnancies complicated by pregestational diabetes is altered by maternal glycemic control and the morning cortisol concentration. Chronobiol Int. 2019;36(4):481–492.
- Sletten J, Cornelissen G, Assmus J, et al. Maternal exercise, season and sex modify the daily fetal heart rate rhythm. Acta Physiol. 2018;224(2):e13093.
- Crawford A, Anyadi P, Stephens L, et al. A mixed-methods evaluation of continuous electronic fetal monitoring for an extended period. Acta Obstet Gynecol Scand. 2018;97(12):1515–1523.
- Kapaya H, Dimelow ER, Anumba D. Is portable foetal electrocardiogram monitor feasible for foetal heart rate monitoring of small for gestational age foetuses in the home environment. J Obstet Gynaecol. 2019;39(8):1081–1086.
- Rauf Z, O'Brien E, Stampalija T, et al. Home labour induction with retrievable prostaglandin pessary and continuous telemetric trans-abdominal fetal ECG monitoring. PLOS One. 2011;6(11):e28129.
- Sletten J, Kiserud T, Kessler J. Effect of uterine contractions on fetal heart rate in pregnancy: a prospective observational study. Acta Obstet Gynecol Scand. 2016;95(10):1129–1135.
- Smith V, Arunthavanathan S, Nair A, et al. A systematic review of cardiac time intervals utilising non-invasive fetal electrocardiogram in normal fetuses. BMC Pregnancy Childbirth. 2018;18(1):370.
- Southern EM. Fetal anoxia and its possible relation to changes in the prenatal fetal electrocardiogram. Am J Obstet Gynecol. 1957;73(2):233–247.
- Huntingford PJ, Pendleton HJ. The clinical application of cardiotocography. J Obstet Gynaecol Br Commonw. 1969;76(7):586–595.
- Arya B, Govindan R, Krishnan A, et al. Feasibility of noninvasive fetal electrocardiographic monitoring in a clinical setting. Pediatr Cardiol. 2015;36(5):1042–1049.
- Fuchs T, Pomorski M, Grobelak K, et al. Signal loss during fetal heart rate monitoring using maternal abdominal surface electrodes between 28 and 42 weeks of pregnancy. Adv Clin Exp Med. 2014;23(5):813–819.
- Rooth G, Huch A. HR. Guidelines for the use of fetal monitoring. Int J Gynecol Obstet. 1987;25(2):159–167.
- Ayres-de-Campos D, Spong CY, Chandraharan E, FIGO consensus guidelines on intrapartum fetal monitoring: Cardiotocography. Int J Gynaecol Obstet. 2015;131(1):13–24.
- Dawes GS, Moulden M, Redman CWG. Criteria for the design of fetal heart rate analysis systems. Int J Biomed Comput. 1990;25(4):287–294.
- Graatsma EM, Miller J, Mulder EJH, et al. Maternal body mass index does not affect performance of fetal electrocardiography. Am J Perinatol. 2010;27(7):573–577.
- Kisilevsky BS, Brown CA. Comparison of fetal and maternal heart rate measures using electrocardiographic and cardiotocographic methods. Infant Behav Dev. 2016;42:142–151.
- Lobmaier SM, Mensing van Charante N, Ferrazzi E, et al. Phase-rectified signal averaging method to predict perinatal outcome in infants with very preterm fetal growth restriction- a secondary analysis of TRUFFLE-trial. Am J Obstet Gynecol. 2016;215(5):630.e1-630–e7.
- Stampalija T, Casati D, Montico M, et al. Parameters influence on acceleration and deceleration capacity based on trans-abdominal ECG in early fetal growth restriction at different gestational age epochs. Eur J Obstet Gynecol Reprod Biol. 2015;188:104–112.

