Abstract
Background
Isolated coronary artery fistula (CAF) is a rare entity in which evidence for both prognosis and need for perinatal treatment is lacking. We aim to evaluate the characteristics, evolution and perinatal outcomes of reported cases, including one from our center.
Material and methods
We performed a systematic review in Medline, Pubmed, and Embase databases for cohort studies or case series related to prenatally diagnosed isolated congenital CAF according to PRISMA guidelines. The search was restricted to articles published until January 2022, including a case report from our center. A descriptive analysis was performed, and perinatal characteristics were dichotomized by outcome (development of symptoms, as well as the need for surgery during the neonatal period). Strength of association between prenatal variables and outcome was evaluated through Odds Ratio.
Results
Only 27 cases of prenatal diagnosis of isolated CAF have been published, including our patient. Most had their origin in the right coronary artery (63%) and drained in the right ventricle (55.6%). Most cases (72%) developed progressive intrauterine dilation of the fistulous tract, which was usually associated with symptoms of cardiac overload, such as cardiomegaly (57.7%). Up to two-thirds of prenatally diagnosed patients developed heart failure symptoms in the neonatal period, and 84% required postnatal intervention. Prenatal diagnosis of both cardiomegaly and diastolic steal is associated with an OR of 52 and 41 of developing postnatal symptoms.
Conclusion
Prenatal diagnosis of isolated CAF can be achieved with adequate tools and trained sonographers. The development of cardiomegaly and diastolic steal significantly increases the risk of developing postnatal symptoms.
Introduction
Coronary artery fistulas (CAF) are heart defects in which one or more coronary arteries directly connect with a cardiac chamber (coronary-cameral fistula) or with a major thoracic vessel (coronary-arteriovenous fistula) without an interposed capillary bed [Citation1]. They can be secondary to traumatic events such as surgeries or ischemic injuries, although most have a congenital origin. They represent 48.7% of all congenital coronary anomalies and 0.08–0.4% of congenital heart defects (CHD) affecting 1 in 50,000 newborns [Citation2–5]. Although they usually are an isolated finding, up to 30% can be associated with major CHD [Citation6].
Prenatal diagnosis of CAF is usually difficult due to technical limitations in the assessment of fetal coronary arteries and their subtle echocardiographic findings. Although up to 90% of CAF are asymptomatic in childhood [Citation7], the frequency of symptoms and complications increases in the first two decades with the development of dyspnea, arrhythmias, or angina. Management may be difficult, requiring early treatment in the most severe cases, as symptoms can be present immediately after birth with congestive heart failure [Citation1,Citation8,Citation9]. Given the scarce amount of prenatally diagnosed cases, there is little data on the perinatal evolution or the echocardiographic prognostic factors to guide counseling.
We present a systematic review of the characteristics, evolution, and perinatal outcomes of all prenatally CAF cases reported to date, including a recent one from our center. Furthermore, we analyze the main echocardiographic predictors of early clinical impairment requiring treatment in the neonatal period.
Material and methods
Systematic review
A literature search was performed in Medline, PubMed, and Embase databases for cohort studies or case series related to congenital CAF using combinations of the relevant medical subject heading (MeSH) terms, keywords, and word variants for “coronary artery fistula,” “coronary vessel anomaly,” “coronary-cameral fistula,” and “prenatal diagnosis” using a comprehensive search strategy hand-searching relevant studies’ references and contacting authors of collaborating studies. The search was restricted to articles published in English until January 2022. Those cases in which CAF was diagnosed in association with another major CHD were excluded. A flowchart of the evaluated and finally included studies is presented as Supplementary Figure 1. The CHARMS-PF checklist for critical appraisal and data extraction for systematic reviews of prediction modeling studies [Citation10] was applied, and a summary of the information gathered was presented in .
Table 1. Details of the reported cases with prenatal diagnosis of isolated coronay artery fistula.
The study was conducted in line with the preferred reporting items for systematic reviews and meta-analyses (PRISMA) guidelines [Citation11] and approved by the Institutional Review Board of the Hospital Universitario 12 de Octubre Research Institute (approval number 23/118). It was conducted ethically in accordance with the World Medical Association Declaration of Helsinki, and informed consent was obtained from the patient from the included case report from our center.
Statistical analysis
A descriptive study of the different variables was performed. Continuous variables were represented as mean and standard deviation (SD), and categorical variables as percentages and absolute frequency.
The main echocardiographic parameters were evaluated as possible predictors of the development of symptoms, as well as the need for surgery during the neonatal period. Univariate comparisons were performed using the chi-square or Fisher’s exact test for categorical variables. The quantification of the magnitude of the effect was expressed by calculating the odds ratio (OR) together with its 95% confidence interval. Data were analyzed using SPSS software, version 19.0 (SPSS, Chicago, IL).
Case report
A 36-year-old primigravida was referred at 20 + 6 weeks’ gestation due to suspected tetralogy of Fallot with a normal cytogenetic study.
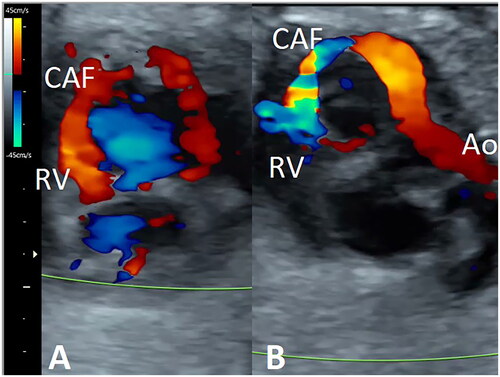
Color Doppler at fetal echocardiography showed the presence of a 2.2 mm fistulous tract with origin in a dilated right coronary artery (RCA) (Supplementary Figure 2), running through the posterior atrioventricular groove and finally draining into the right ventricle (RV) just below the septal leaflet of the tricuspid valve. Doppler study revealed bidirectional flow, antegrade during ventricular diastole, and reversed in systole (), with a peak velocity of 180 cm/s in the distal portion due to the presence of a stenotic segment previous to the drainage into the RV. An intact aortic arch was observed without narrowed portions. However, the blood flow was reversed from the ductus arteriosus during ventricular diastole, secondary to a vascular diastolic steal through CAF toward the RV (Suplementary Figure 3), without concomitant aortic regurgitation. The cardiothoracic index was not increased, and no other signs of heart failure were observed. The extracardiac study did not show any associated abnormalities.
Figure 1. Short-axis view of the fetal heart. Color Doppler shows the presence of a fistulous tract arising from the right coronary artery, running through the posterior atrio-ventricular groove and draining into the right ventricle (RV). The filling of the fistulous tract is antegrade during ventricular diastole (A) and reversed during systole (B). Ao: aorta; CAF: coronary artery fistula.
Parents were counseled regarding the presumed diagnosis and prognosis and follow-up scans were scheduled every 2–3 weeks in order to assess both the appearance of the CAF and the development of signs of heart failure. Subsequent examinations showed a progressive dilation of the CAF with a 5 mm diameter at its proximal portion and 3 mm at the distal stenotic segment, with a peak velocity of 341 cm/s in the last exam performed at 35 weeks’ gestation (Supplementary Figure 4). Intrauterine evolution was favorable, without development of cardiomegaly or any other signs of heart failure.
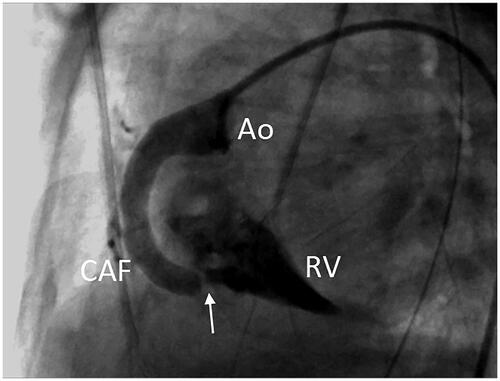
A 2920 g female was vaginally delivered at 37 weeks’ gestation with an Apgar of 10 at 5 min. The postnatal echocardiographic study confirmed the diagnosis of isolated CAF. Immediately after birth, the auscultation revealed a continuous murmur grade 2/4, but she remained hemodynamically stable. However, on day 14, diuretics were initiated due to a worsening of the respiratory status and the development of clinical and ultrasound signs of congestive heart failure with pulmonary overload. A cardiac catheterization was performed, which confirmed the presence of a dilated RCA (7.7 mm) with a distal connection of 3.3 mm draining into the RV (). In this procedure, the fistulous tract was occluded by placing a 6-mm Amplatzer AVP4 device at the drainage point by arterial antegrade way with a Headhunter 4 F catheter. Subsequent angiography of the RCA demonstrated the absence of residual flow. In the last visit, performed at 49 months, the patient was asymptomatic, with no evidence of residual shunt but with persistent dilatation of the right coronary artery.
Results
Literature review and main characteristics
A summary of the main characteristics of the identified studies and the reported cases, including our own, is presented in and . To date, 26 cases of prenatal diagnosis of isolated CAF have been reported.
Table 2. Clinical characteristics and perinatal outcome of prenatally diagnosed isolated coronary artery fistula.
Most of the CAF diagnosed prenatally (63%) had their origin in the RCA and the drained in the RV (55.6%). After excluding two patients in which parents opted for TOP, intention-to-treat analysis of cases with prenatal diagnosis showed that most of them (72.2%) developed progressive intrauterine dilation of the fistulous tract, which is usually associated with symptoms of cardiac overload such as cardiomegaly (57.7%) and mild RV dysfunction. No intrauterine fetal demise has been reported.
Most of the reported cases were delivered at term by cesarean section (62.5%). However, only two were performed electively for fetal cardiac function worsening.
Heart failure symptoms were developed in the neonatal period in 64% of cases. The severity of the situation of the newborn conditioned their death in the early neonatal period in three cases (12%), two of them before surgical or catheter-based interventions (cases 12, 21, 25).
Prognostic factor analysis
Once excluded TOP, clinical symptoms were developed by (16/25, 64%) and postnatal intervention was performed in (21/25, 84%). In most cases (14/21, 66.7%) intervention was performed in the neonatal period at a mean age of 10 days. Clinical worsening was the indication for treatment in most patients, although in six cases this was carried out in asymptomatic patients due to the progressive increase in the size of the CAF. Treatment was mainly performed by embolization of the fistulous tract using coils or placement of an Amplatzer-type device (57.1%), while surgical ligation of the CAF was indicated in 43.3%, especially in those cases with larger CAF or in older reports.
The analysis of prenatal variables for the prediction of postnatal evolution is presented in . Prenatal diagnosis of both cardiomegaly and diastolic steal is associated with a respective 52 and 41 OR of developing postnatal symptoms.
Table 3. Analysis of prenatal variables for the prediction of postnatal severity.
Discussion
Our systematic review revealed that only 26 cases of prenatal diagnosis of isolated CAF had been reported [Citation5–7,Citation12–29]. Including our currently reported case, we have found that up to 64% will develop symptoms postnatally, and 84% require treatment. Prenatal development of cardiomegaly and diastolic steal were associated with an increased risk of postnatal symptoms, but no parameters were useful for the prediction of the need for postnatal treatment.
Most cases of CAF are diagnosed postnatally, often late in life. However, prenatal diagnosis is possible if a careful and systematic cardiac examination is performed. Although it may be difficult when isolated due to the subtle distortion of cardiac anatomy, it is becoming easier thanks to technological advances, better training of sonographers, and the systematic use of the color Doppler during the fetal cardiac examination [Citation8,Citation9]. The suspicion and subsequent diagnosis of CAF is mainly based on the use of color Doppler that reveals an abnormal vascular tract that connects one or more coronary arteries, which are usually dilated, with a cardiac chamber or a thoracic vessel [Citation30,Citation31]. The flow inside this tract typically has a bidirectional pattern, retrograde toward the aortic root during systole and antegrade during diastole (although it depends on the chamber it drains into), often with stenotic segments with a turbulent and accelerated pattern in which the pulsed Doppler shows peak velocities of 200–300 cm/s. Other suggestive signs are the presence of an abnormal jet of entry into the cardiac chamber, dilation of the drainage chamber, alteration of the contractility of the ventricles due to myocardial ischemia given the coronary flow steal, insufficiency of the atrioventricular valves, or the presence of a reversed flow in the aorta during diastole without in the absence of aortic valve insufficiency revealing a vascular steal caused by the filling of the fistula [Citation14,Citation32]. Differential diagnosis should be made with ventricular septal defects, aorto-pulmonary window, aorto-ventricular tunnel, or pulmonary arteriovenous malformations [Citation32].
In this systematic review, we have observed that similarly to the descriptions in the postnatal series, most of the CAF diagnosed prenatally had their origin in the RCA, (52–66.7%) [Citation5,Citation9,Citation32] and most frequently drain in the RV (14–55%), right atrium (19–24%), left ventricle (2–19%) and in the coronary sinus (1–7%) [Citation1] A possible explanation for the higher frequency of the connection of CAF with the right heart may be the presence of a more abundant trabecular pattern during embryological development of the right heart [Citation16].
Intention-to-treat analysis of cases with prenatal diagnosis shows that most of them (72.2%) develop a progressive intrauterine dilation of the fistulous tract, which is usually associated with symptoms of cardiac overload such as cardiomegaly and mild RV dysfunction. Therefore, close surveillance of this finding with serial follow-up scans every 2–4 weeks until delivery is warranted. Most of the reported cases were delivered at term by cesarean section, although only in 2 was it performed electively due to fetal cardiac function deterioration. Therefore, in absence of sonographic signs of cardiac failure, modification of the timing or route of delivery is not recommended.
Overall after birth, most patients with CAF (90%) remain asymptomatic during childhood, and diagnosis is incidental or after the development of symptoms like dyspnea or arrhythmia (60%) in the second decade of life [Citation33]. Other complications such as CAF rupture, angina, thrombosis, hemoptysis, or pulmonary hypertension are more infrequent (8, 9, and 29). However, up to two-thirds of prenatally diagnosed patients develop heart failure in the neonatal period, secondary to ventricular overload due to left-to-right shunt [Citation1,Citation9] or myocardial ischemia secondary to coronary steal [Citation22,Citation23]. Clinical discordance between pre and postnatal series can probably be explained by the fact that cases diagnosed before birth comprise the most severe cases [Citation27]. Therefore, it is highly recommended that delivery and perinatal care always take place in a tertiary center. In addition, we have found that prenatal diagnosis of both cardiomegaly and diastolic steal is associated with a 41 and 52 OR of developing postnatal symptoms. This had been described as well by Sharland et al. [Citation23] in the largest published series.
The treatment of CAF after birth remains controversial. Although some authors argue for surgical treatment in all cases, spontaneous closure of the fistulous tract has been reported in up to 23%, mainly in small-sized CAFs [Citation5,Citation34]. This is the reason why expectant management is preferred, and surgery is indicated in severely symptomatic cases, those with large fistulous tracts, or those with persistent CAFs after 3–5 years in order to avoid clinical worsening with development of complications [Citation1]. An intervention was performed in 84% of prenatally diagnosed cases, most of them in the neonatal period. Clinical worsening was the indication for treatment in most, although in some, this was carried out in asymptomatic patients due to the progressive increase in the size of the coronary artery.
Regarding the type of treatment, although percutaneous closure of the CAF is often preferred [Citation1,Citation27,Citation33], surgical ligation of the CAF was indicated in 43.3%, especially in those cases with larger CAF or in older reports. Percutaneous technique has shown a successful closure of 87–97%, with a recurrence rate of 9–19% [Citation1,Citation35]. Complications such as of arrhythmias, myocardial ischemia, thrombosis, or sudden death are not common (1–2%) [Citation1]. In this review, two deaths were observed after the intervention, with an 84% survival rate in those intention-to-treat cases and a postoperative mortality of 9.5%.
We acknowledge some limitations. Firstly, the retrospective nature of the analysis and secondly, the heterogeneity of the studies, most of them with a very limited number of cases. These aspects make the analysis of the results necessarily conditioned by the differences regarding both intrauterine and postnatal management of each center, as well as the improvement of technology throughout the period of study concerning the diagnosis and treatment. However, we consider that our review shows that prenatal diagnosis of this entity is possible when fetal cardiac examination is performed in detail.
Conclusion
Prenatal diagnosis of CAF is essential for providing parents with the most accurate prognostic information and tailoring prenatal follow-up that may help detect fetal heart failure or sonographic findings related to the more severe clinical forms in the immediate neonatal period and, therefore, subsidiary of early surgical/interventional treatment.
Authors’ contributions
All authors have accepted responsibility for the entire content of this manuscript and approved its submission. All authors read and approved the final manuscript.
Ethical approval
The study was conducted in line with the preferred reporting items for systematic reviews and meta-analyses (PRISMA) guidelines (11) and approved by the Institutional Review Board of the Hospital Universitario 12 de Octubre Research Institute (approval number 23/118). It was conducted ethically in accordance with the World Medical Association Declaration of Helsinki, and informed consent was obtained from the patient from the included case report from our center.
Supplemental Material
Download Zip (760.3 KB)Acknowledgments
We sincerely acknowledge all the co-authors who have collaborated in the development of this study and we are grateful to the mother who has selflessly contributed to the study.
Disclosure statement
No potential conflict of interest was reported by the author(s).
Data availability statement
The datasets used and/or analyses performed during the study are available from the corresponding author upon reasonable request.
Additional information
Funding
References
- Challoumas D, Pericleous A, Dimitrakaki IA, et al. Coronary arteriovenous fistulae: a review. Int J Angiol. 2014;23(1):1–10.
- Fernandes ED, Kadivar H, Hallman GL, et al. Congenital malformations of the coronary arteries: the Texas Heart Institute experience. Ann Thorac Surg. 1992;54(4):732–740.
- Dodge-Khatami A, Mavroudis C, Backer CL. Congenital Heart Surgery Nomenclature and Database Project: anomalies of the coronary arteries. Ann Thorac Surg. 2000;69(4 Suppl):S270–S297.
- Gowda RM, Vasavada BC, Khan IA. Coronary artery fistulas: clinical and therapeutic considerations. Int J Cardiol. 2006;107(1):7–10.
- Zeng S, Zhou Q, Tian L, et al. Isolated coronary artery fistula in fetal heart: case reports and literature review. Fetal Pediatr Pathol. 2016;35(5):348–352.
- Zhao L, Wang Y, Wang M, et al. Prenatal diagnosis of fetal isolated right coronary artery to left ventricle fistula. Echocardiography. 2019;36(5):1009–1013.
- Hayashi T, Inuzuka R, Ono H, et al. Prenatal diagnosis of coronary artery fistula: a case report and review of literature. Pediatr Int. 2012;54(2):299–302.
- Qureshi SA. Coronary arterial fistulas. Orphanet J Rare Dis. 2006;1:51.
- Loukas M, Germain AS, Gabriel A, et al. Coronary artery fistula: a review. Cardiovasc Pathol. 2015;24(3):141–148.
- Moons KG, de Groot JA, Bouwmeester W, et al. Critical appraisal and data extraction for systematic reviews of prediction modelling studies: the CHARMS checklist. PLoS Med. 2014;11(10):e1001744.
- Page MJ, McKenzie JE, Bossuyt PM, et al. The PRISMA 2020 statement: an updated guideline for reporting systematic reviews. BMJ. 2021;372:n71.
- Sharland GK, Tynan M, Qureshi SA. Prenatal detection and progression of right coronary artery to right ventricle fistula. Heart. 1996;76(1):79–81.
- Cotton JL. Diagnosis of a left coronary artery to right ventricular fistula with progression to spontaneous closure. J Am Soc Echocardiogr. 2000;13(3):225–228.
- Mielke G, Sieverding L, Borth-Bruns T, et al. Prenatal diagnosis and perinatal management of left coronary artery to right atrium fistula. Ultrasound Obstet Gynecol. 2002;19(6):612–615.
- Khan MD, Qureshi SA, Rosenthal E, et al. Neonatal transcatheter occlusion of a large coronary artery fistula with Amplatzer duct occluder. Catheter Cardiovasc Interv. 2003;60(2):282–286.
- Hung J-H, Lu J-H, Hung J, et al. Prenatal diagnosis of a right coronary-cameral fistula. J Ultrasound Med. 2006;25(8):1075–1078.
- Karagöz T, Ozkutlu S, Celiker A. Percutaneous closure of a prenatally diagnosed large coronary artery fistula with an Amplatzer vascular plug immediately after delivery. Acta Cardiol. 2008;63:405–408.
- Daniel M, Mavroudis C, Preminger T, et al. Prenatal diagnosis and neonatal surgical management of a giant proximal right coronary artery to right ventricular fistula. World J Pediatr Congenit Heart Surg. 2010;1(2):243–248.
- Zhao X, Yang Y, Li R. A large hemodynamically significant right coronary artery fistula to right ventricle: prenatal detection and progression. Echocardiography. 2012;29(7):E173–E175.
- Matsumoto Y, Hoashi T, Kagisaki K, et al. Successful surgical treatment of a gigantic congenital coronary artery fistula immediately after birth. Interact Cardiovasc Thorac Surg. 2012;15(3):520–522.
- Nagiub M, Mahadin D, Gowda S, et al. Prenatal diagnosis of coronary artery fistula: a case report and review of literature. AJP Rep. 2014;4(2):e83–e86.
- Oztunc F, Gokalp S, Yuksel MA, et al. Prenatal diagnosis of left coronary artery to right ventricle fistula. J Clin Ultrasound. 2015;43(2):129–131.
- Sharland GK, Konta L, Qureshi SA. Prenatal diagnosis of isolated coronary artery fistulas: progression and outcome in five cases. Cardiol Young. 2016;26(5):915–920.
- Kaldararova M, Tittel P, Zahorec M. Giant coronary artery fistula: prenatal diagnosis, newborn manifestation. Rev Espanola Cardiol. 2016;69(11):1100.
- Wacker-Gussmann A, Esser T, Lobmaier SM, et al. Prenatally diagnosed isolated coronary arterial fistula leading to severe complications at birth. Case Rep Cardiol. 2018;2018:2509502.
- Tekesin I, Uhlemann F. Prenatal diagnosis of coronary artery fistula using 2D and 3D/4D ultrasound. Ultrasound Obstet Gynecol. 2018;51(2):274–275.
- Chae U, Lee M-Y, Kim H, et al. Prenatal diagnosis of isolated coronary arteriovenous fistula. Obstet Gynecol Sci. 2018;61(1):161–164.
- Cui C, Liang W, Fan T, et al. Prenatal diagnosis of a right coronary artery to right atrial fistula with a giant coronary artery aneurysm: a case report. J Clin Ultrasound. 2020;48(8):489–492.
- Li TG, Ma B, Nie F, et al. An unusual case of prenatal diagnosis of right coronary artery to right ventricle fistula with HD-flow render mode and spatiotemporal image correlation (STIC). Echocardiography. 2020;37(7):1105–1108.
- Baschat AA, Gembruch U. Evaluation of the fetal coronary circulation. Ultrasound Obstet Gynecol. 2002;20(4):405–412.
- Mielke G, Wallwiener D. Picture of the month. Visualization of fetal arterial and venous coronary blood flow. Ultrasound Obstet Gynecol. 2001;18(4):407.
- Lee M-L, Chen M. Diagnosis and management of congenital coronary arteriovenous fistula in the pediatric patients presenting congestive heart failure and myocardial ischemia. Yonsei Med J. 2009;50(1):95–104.
- Zamani H, Meragi M, Moghadam A, et al. Clinical presentation of coronary arteriovenous fistula according to age and anatomic orientation. Casp J Intern Med. 2015;6:108–112.
- Gembruch U, Baschat AA. Demonstration of fetal coronary blood flow by color-coded and pulsed wave Doppler sonography: a possible indicator of severe compromise and impending demise in intrauterine growth retardation. Ultrasound Obstet Gynecol. 1996;7(1):10–16.
- Harikrishnan S, Bimal F, Ajithkumar V, et al. Percutaneous treatment of congenital coronary arteriovenous fistulas. J Interv Cardiol. 2011;24(3):208–215.