?Mathematical formulae have been encoded as MathML and are displayed in this HTML version using MathJax in order to improve their display. Uncheck the box to turn MathJax off. This feature requires Javascript. Click on a formula to zoom.
?Mathematical formulae have been encoded as MathML and are displayed in this HTML version using MathJax in order to improve their display. Uncheck the box to turn MathJax off. This feature requires Javascript. Click on a formula to zoom.Abstract
Objective
Doppler velocimetry has been widely used throughout the years as a valuable tool in the follow-up and prognosis of various pregnancy complications. Numerous Doppler indices have been introduced to qualitatively describe fetal blood flow. Currently, the Pulsatility index (PI) is the most widely used index for this purpose. In current clinical practice, middle cerebral artery (MCA) PI measurement is commonly used to assess fetal well-being, especially in late-onset fetal growth restriction (FGR). However, existing evidence suggests that MCA PI alone is inferior to the ratio between MCA and umbilical artery (UA) pulsatility indices in predicting adverse perinatal and neonatal outcomes. When comparing normal and abnormal MCA Doppler waveforms, it is evident that most changes appear in the diastolic part of the heart cycle. Therefore, the PI, which contains elements from both systole (peak systolic velocity–PSV) and diastole (end-diastolic velocity), may not be the most effective tool for quantifying fetal brain sparing (BS).
Methods
We hypothesize that another measurement modality that focuses predominantly on the diastole could be more efficient for evaluating the amount of vasodilatation. In ultrasound velocimetry of larger blood vessels, there is a well-known phenomenon called “dicrotic notch” (DN), which appears on the declining part of each Doppler waveform and can be used to precisely pinpoint the end of systole and the start of diastole. We hypothesized that the extent of cerebral vasodilation can be more accurately assessed by measuring the area between the dicrotic notch (DN) and the end-diastolic velocity (which we refer to as the “diastolic deceleration area–DDA”). In this study, we introduced a new Doppler parameter along with a rationale for DDA measurement in the fetal MCA. We also defined third-trimester nomograms and provided a preliminary assessment of the correlation between DDA and fetal oxygen deficiency.
Results
Our findings suggest that the DDA may serve as an independent instrument for identifying hypoxia during late pregnancy, either on its own or in conjunction with other Doppler and cardiotocography modalities.
Conclusion
However, before incorporating DDA into clinical practice, it is crucial to conduct further research and validation studies with larger sample sizes and more diverse populations. This would help assess the generalizability of the results and establish optimal cutoff points for DDA in various clinical settings. It is also important to prospectively study the role of DDA in early- and late-onset fetal growth restriction (FGR), Rh-isoimmunization/anemia, preeclampsia, gestational diabetes, and other pregnancy complications. In fact, we believe that the concept of measuring specific areas in arterial Doppler velocimetry indices could have significant implications not only in fetal medicine and obstetrics, but also in other areas of human and veterinary medicine.
Introduction
Doppler velocimetry has been widely used throughout the years as a valuable tool in the follow-up and prognosis of various pregnancy complications, such as fetal growth restriction (FGR), fetal anemia, and twin-to-twin transfusion syndrome (TTTS) in multiple pregnancies [Citation1].
The concept of intrauterine growth restriction (IUGR) first emerged in 1960, when Battaglia and Lubchenco began to adjust newborn birth weight according to the corresponding gestational age [Citation2].
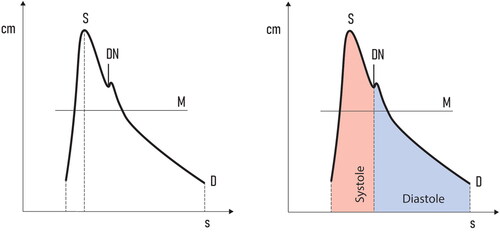
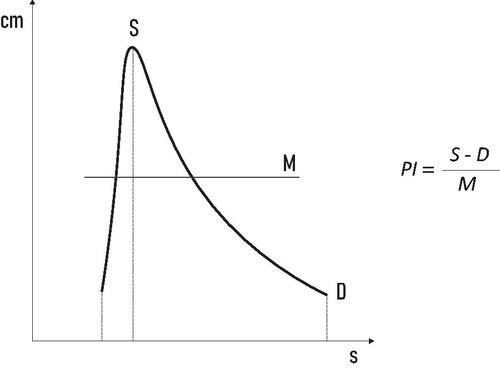
Since then, numerous Doppler indices have been introduced to qualitatively describe fetal blood flow. Currently, the Pulsatility index (PI) is the most widely used index for this purpose. It was originally defined in 1969 by Gosling and King [Citation3] and was later simplified to today’s well-known formulation ().
Figure 1. The vertical axis represents blood velocity. The horizontal axis represents time. PI: Pulsatility index; S: peak systolic velocity; D: end diastolic velocity; M: mean velocity.
In current clinical practice, middle cerebral artery (MCA) PI measurement is commonly used to assess fetal well-being, especially in late-onset FGR [Citation4]. In 1986, it was shown [Citation5] that lower impedance to flow in the MCA (detectable by a decrease in the PI) is uniformly associated with a redistribution of cardiac output flow toward the central nervous system to address oxygen deficiency (brain-sparing effect). Cerebral vasodilation, and thus lowered cerebral vascular resistance, leads to increased end-diastolic flow velocity in the cerebral arteries [Citation6].
Almost 40 years after the introduction of the original concept, MCA PI measurement continues to serve as the gold standard for the assessment of fetal BS.
Limitations of current practice
However, a large number of authors have proposed that the calculation of the cerebroplacental ratio (CPR) is of additional value for diagnosing brain-sparing [Citation4,Citation7,Citation8].
Data from a recent meta-analysis showed that this ratio between MCA and UA pulsatility indices delivers higher sensitivity than MCA alone in predicting adverse perinatal and neonatal outcome [Citation9]. MCA Doppler was found to be significantly inferior to umbilical artery (UA) Doppler in predicting low neonatal Apgar scores (p = .017) and the necessity for an emergent delivery for fetal distress (p = .034). Moreover, it was considered significantly inferior to CPR in predicting composite adverse outcomes (p < .001) and the need for emergent delivery for fetal distress (p = .013).
Due to the aforementioned observations, the clinical implementation of fetal brain Doppler assessment is currently limited to monitoring late-onset FGR (≥32 weeks of gestation), where CPR and MCA Doppler may be of specific clinical value [Citation10–12].
Is there room for improvement?
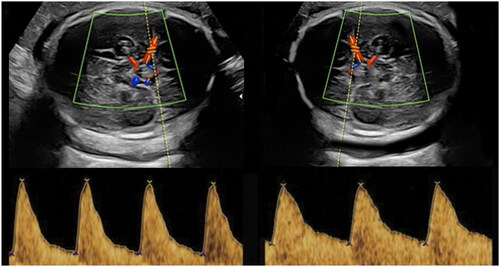
In a comparison between normal and abnormal MCA Doppler waveforms (), it becomes evident that most of the changes occur in the diastolic part of the heart cycle. Therefore, the PI, which contains elements from both systole (peak systolic velocity–S, ) and diastole (end diastolic velocity–D, ), may not be the most effective tool for quantifying fetal BS. We hypothesize that another measurement modality, focusing predominantly on diastole, needs to be introduced.
Focusing on the diastole–a possible solution
An easy to identify notching, which appears on the declining part of Doppler waveforms, can be a more accurate tool for the interpretation of brain vessel vasodilatation.
The so-called “dicrotic notch” (DN) is a small and brief increase in arterial blood pressure that appears when the aortic valve closes. This landmark has been widely referred to in the descriptive analysis of the arterial waveform (especially of the aortic and radial arteries) and is commonly used as an equivalent of end-systolic left ventricular pressure [Citation13–16].
DN is universally associated with aortic valve closure [Citation17], and possibly with changes in peripheral vascular resistance [Citation18], although to date, no physical mechanism for the existence of DN has been convincingly demonstrated.
In MCA Doppler velocimetry, dicrotic notching can be used to precisely pinpoint the end of systole and start of diastole. The visual DN representation, as it appears on pulse wave (PW) Doppler, is shown in .
With DN serving as a readily recognizable marker to indicate the beginning of diastole, it was necessary to identify a suitable measurement tool to quantify the BS effect.
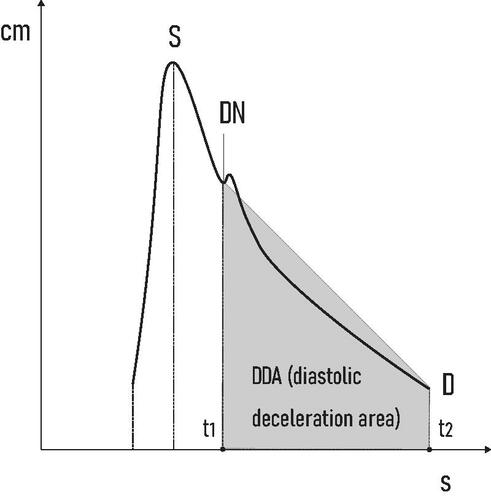
We hypothesized that the amount of vasodilatation in the fetal brain can be precisely calculated by quantifying the area of the highlighted trapezoid shape (which we refer to as “diastolic deceleration area - DDA”) ().
The DDA can be determined by using the trapezoid area formula:
where A is area; “a” and “b” are the bases of the trapezoid; “h” is height.
To be clinically applicable, this formula can be customized using the variables shown in , as follows:
where DDA is diastolic deceleration area; DN is the dicrotic notch (measured as velocity–m/s); D is end diastolic velocity (measured as velocity–m/s); Δt = t2–t1 (measured as deceleration time (DT) - s).
Introducing DDA as a new Doppler modality (patent pending - PCT/BG2022/050004) is an important prerequisite for future studies aimed at determining its role (alone and in relation to other Doppler indices) in the evaluation of third trimester fetal well-being.
In this study, we defined nomograms for the DDA parameter, covering an interval between 28 and 37 weeks of gestation.
Study aims
To find means to better differentiate between systole and diastole in MCA Doppler waveforms.
To precisely quantify the diastolic part of MCA Doppler waveforms.
To define nomograms for the newly introduced Doppler modality.
To make a preliminary assessment of the correlation (if any) between DDA and umbilical artery pH values, measured immediately after delivery.
Methods
Between November 2021 and February 2023, we performed a retrospective study of 300 pregnancies, divided equally into 10 categories, one for each gestational week between 28 and 37.
The inclusion criteria were:
Absence of previously documented history of placental insufficiency;
Normal fetal heart rate (FHR) monitoring;
Absence of suspected intrauterine hypoxia/anemia;
Absence of fetal growth restriction (FGR) < 10%, according to the INTERGROWTH 21st nomograms [Citation19].
Single gestation;
Absence of fetal abnormalities, either on ultrasound and/or upon delivery;
APGAR scores ≥7 at 1, 5 and 10 min
Pregnant subjects were scanned only once, thus eliminating longitudinality during the data acquisition process. All measurements were performed on a GE Voluson E8 BT21 ultrasound machine by a single operator (PNI), using a RAB6-D transabdominal convex probe. To measure the MCA blood flow characteristics (PI, PSV, and DDA), an axial section of the brain, including the thalami and cavum septi pellucidi, was obtained. The middle cerebral artery on one side was examined close to its origin, as the PSV decreased in the distal portions of the vessel [Citation20]. The angle of insonation was below 30°, and 2D pulsed wave/color Doppler was used to obtain images in the absence of marked fetal body and respiratory movements. The thermal index for bone (TIb) was set to 0.1 for all Doppler measurements.
To evaluate the correlation between DDA and fetal pH at birth, we recently initiated a large, prospective, multicenter clinical trial focused on monitoring high-risk pregnancies, divided into groups depending on the presence of complications such as early/late-onset fetal growth restriction (FGR), preeclampsia, gestational diabetes, and others.
Although this trial was in its early stages of data acquisition, we analyzed a small sample size of unselected 49 pregnancies, aiming to obtain a preliminary insight into the effectiveness of the newly introduced modality. We used ROC curve analysis to explore the correlation between DDA and umbilical artery pH. The results are discussed below.
Statistical analysis
The criteria proposed by Royston and Wright [Citation21] were used to determine the reference values for DDA.
The best-fitting model was selected on the basis of the residual SD. Z-scores (measurement − mean/SD) were calculated to assess model fit. The normal distribution of the Z-scores was further evaluated using the Shapiro–Francia W-test. The equation that best described the relationship between DDA and gestational age (GA) was a least-squares regression, which we used to estimate both mean and standard deviation:
To evaluate the sensitivity and specificity of DDA for detecting umbilical artery pH changes, we performed ROC curve and area under the curve (AUC) analysis.
As all scans were performed by a single operator (PNI), intraobserver reproducibility was based on the analysis of three DDA measurements in each of the included cases, using the intraclass correlation coefficient (ICC = 0.911), which was consistent with excellent agreement levels.
All statistical procedures were performed using SPSS (version 29.0, SPSS Inc., Chicago, IL) and MedCalc® Statistical Software, version 20.218 (MedCalc Software Ltd, Ostend, Belgium).
Results
According to the described model, we calculated the 5th, 50th and 95th percentile curves, based on the mean and adjusted SD values for each GA ( and ).
Figure 5. Scatter plot diagram showing the DDA measurements distribution (and the corresponding centiles) in the 27–37 gestational week interval.
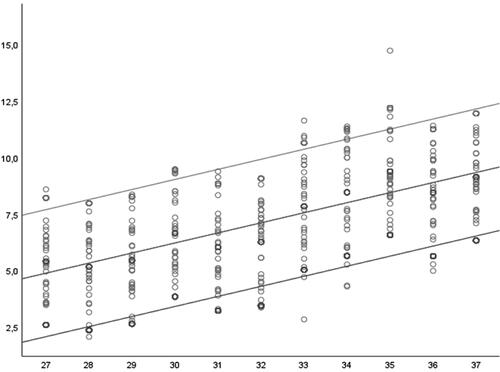
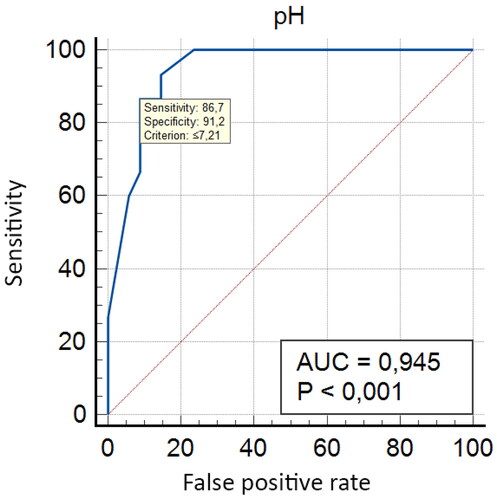
Figure 6. Area under the ROC curve (AUC) = 0.945; Standard Error–0.0292; 95% Confidence interval–0.840–0.990; z statistic–15.224; Significance level p (Area = .5)–<.0001.
The presented DDA nomogram was then used to explore a possible correlation between DDA values and pH fetal blood samples, taken at birth ().
Table 1. 5th, 50th and 95th centiles for the measured DDA values in relation to the corresponding gestational weeks.
indicates the existence of an important threshold (pH ≤ 7.24) that provides clinically acceptable sensitivity/specificity ratios (93.33%/85.29%). In this unselected population of high-risk pregnancies, further data analysis demonstrated an even stronger correlation (AUC = 0.945, p < .001) between DDA and umbilical artery pH ≤ 7.21. This observation has several implications. First, if this high specificity is confirmed in larger sample sizes, the diastolic deceleration area could have a significant role in improving current fetal well-being monitoring protocols during the third trimester of pregnancy. Furthermore, integrating DDA with other commonly used parameters such as the cerebroplacental ratio (CPR) and ductus venosus PIV, could potentially enhance the accuracy and reliability of detecting fetal distress. This would enable healthcare providers to make better-informed decisions and, when necessary, intervene in a timely manner to minimize the risk of adverse neonatal outcomes. Second, and more importantly, DDA could potentially be used as a single screening tool for fetal oxygen deficiency. This hypothesis needs to be tested in larger number of patients and evaluated in different pathological conditions.
Table 2. Criterion values and coordinates of the ROC curve.
Given the early stages of ongoing research, there are some important limitations. Notably, the observed a decline in sensitivity below pH 7.15. This can be attributed to the fact that there was a limited number of fetuses (three cases, or 6.22% of all newborns) with umbilical artery pH below 7.15. Also, at this early stage of preliminary studies, it would be premature to make conclusive assumptions about optimal pH thresholds, as we expect to observe changes in the sensitivity/specificity ratios with the accumulation of a larger number of patients.
It can be reasonably concluded that a correlation between DDA and key fetal pH cutoffs does indeed exist, suggesting that further investigation of this parameter is warranted. Nonetheless, since pH is a parameter often affected by labor and intrapartum events, in future research, it is important to examine other indicators of adverse perinatal outcomes as well. These include low Apgar scores at 1st, 5th, and 10th min, admission to a neonatal special care unit, and the incidence of emergency operative deliveries.
Conclusion
In this study, we introduce a novel Doppler modality, the diastolic deceleration area (DDA), and propose nomograms for its application.
However, before incorporating DDA into clinical practice, it is crucial to conduct further research and validation studies with larger sample sizes and more diverse populations. This would help assess the generalizability of the results and establish optimal cutoff points for DDA in various clinical settings.
Additionally, it would be beneficial to evaluate the potential advantages of using DDA as a standalone tool for preventing adverse outcomes in late pregnancy and in combination with other Doppler/cardiotocography modalities.
It is also important to prospectively study the role of DDA in early- and late-onset fetal growth restriction (FGR), Rh-isoimmunization/anemia, preeclampsia, gestational diabetes, and other pregnancy complications.
In fact, we believe that the concept of measuring specific areas in arterial Doppler velocimetry indices could have significant implications not only in fetal medicine and obstetrics, but also in other areas of human and veterinary medicine.
Disclosure statement
No potential conflict of interest was reported by the author(s).
Additional information
Funding
References
- Mone F, McAuliffe FM, Ong S. The clinical application of Doppler ultrasound in obstetrics. Obstet Gynecol. 2015;17(1):13–19.
- Battaglia FC, Lubchenco LO. A practical classification of newborn infants by weight and gestational age. J Pediatr. 1967;71(2):159–163.
- Gosling RG, King DH, Newman DL, et al. Transcutaneous measurement of arterial blood velocity ultrasound. In: Ultrasonics for industry conference papers. Guildford: IPC; 1969. p. 16–32.
- Lees CC, Stampalija T, Baschat A, et al. ISUOG practice guidelines: diagnosis and management of small-for-gestational-age fetus and fetal growth restriction. Ultrasound Obstet Gynecol. 2020;56(2):298–312.
- Wladimiroff JW, Tonge HM, Stewart PA. Doppler ultrasound assessment of cerebral blood flow in the human fetus. Br J Obstet Gynaecol. 1986;93(5):471–475.
- Cohen E, Baerts W, van Bel F. Brain-sparing in intrauterine growth restriction: considerations for the neonatologist. Neonatology. 2015;108(4):269–276.
- Baschat AA, Gembruch U. The cerebroplacental doppler ratio revisited. Ultrasound Obstet Gynecol. 2003;21(2):124–127. PMID: 12601831.
- DeVore GR. The importance of the cerebroplacental ratio in the evaluation of fetal well-being in SGA and AGA fetuses. Am J Obstet Gynecol. 2015;213(1):5–15. PMID: 26113227.
- Vollgraff Heidweiller-Schreurs CA, de Boer MA, Heymans MW, et al. Prognostic accuracy of cerebroplacental ratio and middle cerebral artery Doppler for adverse perinatal outcome: systematic review and meta-analysis. Ultrasound Obstet Gynecol. 2018;51(3):313–322.
- Dunn L, Sherrell H, Kumar S. Review: systematic review of the utility of the fetal CPR measured at term for the prediction of adverse perinatal outcome. Placenta. 2017;54:68–75.
- Meher S, Hernandez-Andrade E, Basheer SN, et al. Impact of cerebral redistribution on neurodevelopmental outcome in small-for-gestational-age or growth-restricted babies: a systematic review. Ultrasound Obstet Gynecol. 2015;46(4):398–404.
- Gordijn SJ, Beune IM, Thilaganathan B, et al. Consensus definition for placental fetal growth restriction: a Delphi procedure. Ultrasound Obstet Gynecol. 2016;48(3):333–339.
- Dahlgren G, Veintemilla F, Settergren G, et al. Left ventricular end-systolicpressure estimated from measurements in a peripheral artery. J Cardiothorac Vasc Anesth. 1991;5(6):551–553.
- Falsetti HL, Mates RE, Carroll RJ, et al. Analysis and correction of pressure wave distortion influid-filled catheter systems. Circulation. 1974;49(1):165–172.
- Guyton AC, Hall JE. Textbook of medical physiology. 11th ed. Philadelphia (PA): Elsevier Inc.; 2006. p. 109.
- Hebert JL, Lecarpentier Y, Zamani K, et al. Relation between aortic dicrotic notch pressure andmean aortic pressure in adults. Am J Cardiol. 1995;76(4):301–306.
- Politi MT, Ghigo A, Fernández JM, et al. The dicrotic notch analyzed by a numerical model. Comput Biol Med. 2016;72:54–64.)
- Gamrah MA, Xu J, el Sawy A, et al. Mechanics of the dicrotic notch: an acceleration hypothesis. Proc Inst Mech Eng H. 2020;234(11):1253–1259.
- Papageorghiou AT, Ohuma EO, Altman DG, et al. International standards for fetal growth based on serial ultrasound measurements: the fetal growth longitudinal study of the INTERGROWTH-21st project. Lancet. 2014;384(9946):869–879.
- Mari G, Deter RL, Carpenter RL, et al. Noninvasive diagnosis by Doppler ultrasonography of fetal anemia due to maternal Red-Cell alloimmunization. N Engl J Med. 2000;342(1):9–14.
- Royston P, Wright EM. How to construct “normal ranges” for fetal variables. Ultrasound Obstet Gynecol. 1998;11(1):30–38.