Abstract
Objective
To evaluate the association between pulmonary hemorrhage and bronchopulmonary dysplasia (BPD) in very low birth weight infants (VLBWIs).
Methods
The study participants were all VLBW newborns admitted from January 1, 2019 to December 31, 2021. The BPD subjects finally included were VLBWIs who survived until the diagnosis was established. This study was divided into pulmonary hemorrhage group (PH group, n = 35) and non-pulmonary hemorrhage group (Non-PH group, n = 190).
Results
By univariate analysis it was found that premature rupture of membranes, tracheal intubation in the delivery room, duration of mechanical ventilation, course of invasive ventilation (≥3 courses), pulmonary surfactant (>1 dose), medically and surgically treated patent ductus arteriosus, grade III-IV RDS, early onset sepsis, BPD and moderate to severe BPD showed significant differences between groups (p < .05). By Multivariate analysis, pulmonary hemorrhage did not increase the risks of BPD and moderate to severe BPD (adjusted OR for BPD = 1.710, 95% CI 0.581–5.039; adjusted OR for moderate to severe BPD = 2.401, 95% CI 0.736–7.834).
Conclusion
It suggests that pulmonary hemorrhage is not associated with the development of BPD and moderate to severe BPD in VLBWIs.
1. Introduction
In recent years, with the increasing pregnancy rate at advanced maternal age and the improvement of assisted reproductive technology, premature infants are becoming more common. But, due to the immature lung development, neonates with smaller gestational ages are prone to respiratory distress syndrome (RDS), apnea, pulmonary hemorrhage, and other respiratory diseases, and often need mechanical ventilation [Citation1–3]. Among them, because of smaller gestational age and lower birth weight, very low birth weight infants (VLBWIs) are vulnerable to pulmonary hemorrhage, which can result in respiratory failure, death and other chronic consequences [Citation4–5].
So far, there have been few researches studying on pulmonary hemorrhage and associated respiratory morbidities in premature. And whether pulmonary hemorrhage could induce bronchopulmonary dysplasia (BPD) has been continuous controversy [Citation6–7]. In theory, pulmonary hemorrhage often requires mechanical ventilation due to ventilatory disorders. And longer mechanical ventilation has been proven associated with BPD [Citation8]. For example, Li j et al. carried out a single center retrospective study and found that moderate to severe BPD was significantly higher in the group with massive pulmonary hemorrhage (12/51 VLBWIs in the group with pulmonary hemorrhage vs 17/548 VLBWIs in the group without pulmonary hemorrhage, p < .05) [Citation7]. However, Wang TT et al. believed the opposite [8/30 extremely low birth weight infants (ELBWIs) in the pulmonary hemorrhage group vs 41/130 ELBWIs in the non-pulmonary hemorrhage group, p = .602] [Citation6]. The common point of most relevant studies is that the effects of confounding factors, such as duration of mechanical ventilation and the severity of primary pulmonary disease, are not fully considered. In this context, this retrospective study was conducted to investigate whether pulmonary hemorrhage could increase the incidence of BPD in VLBWIs.
2. Patients and methods
2.1. Preterm infants
This is a single-center, retrospective cohort study.
Inclusion criteria: The study participants were all VLBW newborns admitted to the neonatal intensive care unit (NICU) of Children’s Hospital Affiliated to Nanjing Medical University from January 1, 2019 to December 31, 2021. The BPD subjects finally included were VLBWIs who survived until the diagnosis was established.
Exclusion criteria: Infants with serious congenital malformations and hereditary metabolic diseases during hospitalization were excluded. Patients died prior to the diagnosis of BPD at 36 weeks of postmenstrual age (PMA) or occurred pulmonary hemorrhage after the diagnosis of BPD were excluded. Newborns with incomplete information in the case report form (CRF) were also excluded.
Diagnostic criteria: Pulmonary hemorrhage was defined as bright red blood secretion from the endotracheal tube that was associated with clinical deterioration, including increased ventilator support with a fraction of inspired oxygen (FiO2) increase of >0.3 from the baseline [Citation9] or an acute drop in hematocrit (>10%) [Citation10], in addition to multi-lobular infiltrates on chest radiography. RDS was defined according to the latest guideline–the 2019 European Consensus Guidelines on the Management of Respiratory Distress Syndrome (ECGMRDS) [Citation11]. Bronchopulmonary dysplasia (BPD) was defined and graded based on a requirement for oxygen at 36 weeks PMA (NHLBI/NICHD 2001) [Citation12–13]. Retinopathy of prematurity (ROP) was defined and classified according to the papers published by the International Classification Committee on retinopathy of prematurity [Citation14]. Intraventricular hemorrhage (IVH) was defined and classified according to the literature reported by Papile et al. and Volpe [Citation15–16]. Diagnostic criteria of necrotizing enterocolitis (NEC): NEC was defined and classified according to Bell’s stage [Citation17]. Sepsis was defined based on Expert consensus on the diagnosis and management of neonatal (version 2019) [Citation18].
Exposure and grouping: The exposure factor was whether the infant had pulmonary hemorrhage during hospitalization. Pulmonary hemorrhage here referred to the occurrence before the diagnosis of BPD was established. This study was consequently divided into the pulmonary hemorrhage group (PH group) and the non-pulmonary hemorrhage group (Non-PH group).
Outcomes: The main outcome was the incidences of all BPD and moderate to severe BPD. The secondary outcomes were pneumothorax, IVH (≥grade III), NEC (≥grade II), ROP, and length of hospital stay.
2.2. Clinical data and methods
Clinical data: CRF data of all subjects were collected by two neonatologists and checked by a third person. Clinical data including maternal hypertension, maternal diabetes, amniotic fluid turbidity, prenatal glucocorticoid, birthweight, gestational age, gender, fetal distress, mode of delivery, Apgar score, history of resuscitation, invasive and noninvasive mechanical ventilation, use of caffeine and pulmonary surfactant (PS), RDS, medically and surgically treated patent ductus arteriosus (PDA), sepsis, NEC (≥stage II), IVH (≥grade III), BPD during hospitalization. The PS used in our NICU was CUROSURF®, a natural derived surfactant manufactured by the Chiesi company.
Postnatal resuscitation: Postnatal resuscitation of all VLBWIs was performed in accordance with the Chinese neonatal resuscitation guideline [Citation19]. After birth, newborns were given warm and positive pressure ventilation with a mask, and subsequent respiratory support was provided by T-piece. The need for further endotracheal intubation is determined by the neonatologist in the delivery room [Citation19]. After resuscitation, the patient was transferred to NICU for further assessment by the doctor on duty. Invasive or noninvasive ventilation was adopted, and surfactant was first supplemented according to the severity of RDS.
Therapy for respiratory diseases: During the hospitalization, the attending physician decided on the application of invasive ventilation [synchronized intermittent mandatory ventilation (SIMV), synchronized intermittent positive pressure ventilation (SIPPV), high-frequency oscillatory ventilation (HFOV)], extubation and noninvasive ventilation [nasal continuous positive airway pressure (nCPAP), nasal intermittent positive pressure ventilation (NIPPV), high flow nasal cannula (HFNC) and noninvasive high-frequency oscillatory ventilation (nHFOV)]. The indications for endotracheal intubation in NICU were evaluated by two standards [Citation20]. (1) Absolute indications: invasive ventilation is required for any of the following conditions: ① Repeated apnea; ② Partial pressure of carbon dioxide (PaCO2) >60mmHg with persistent acidosis; ③ Partial pressure of oxygen (PaO2) <50–60mmHg, inhaled oxygen concentration >60%–70%. (2) Relative indications, in which invasive ventilation can be considered for any of the following situations: ① Intermittent apnea, which is ineffective for treatment; ② Severe dyspnea; ③ Blood gas analysis deteriorated sharply, PaCO2 increased and PaO2 decreased.
The use of caffeine and repeated surfactants was also based on the 2019 ECGMRDS guidelines [Citation11].
2.3. Estimation of sample size
As previous retrospective research reported, the incidence of BPD was around 23.5% and 3.1% in VLBWIs with or without pulmonary hemorrhage [Citation7]. Based on that study, we adopted a similar design with α = 0.05 and 1 − β = 0.80. The sample size ratio of the two groups was designed as 1:5 (PH group vs. Non-PH group). We used Pearson Chi-square test by SAS software (9.4) to calculate the sample size. The sample size of PH group and non-PH group is 36 and 180, respectively.
2.4. Statistical methods
Statistical analysis was performed using SPSS 13.0 software. For univariate analysis, quantitative data which obey normal distribution were showed as mean and standard deviation. Comparisons between the two groups were performed using the t or t’ test. For skew distribution data, the median and interquartile range are used. Mann–Whitney test was used for comparison. In terms of qualitative data, Pearson Chi-square test or Fisher’s exact test was performed. For multivariate analysis, binary logistic regression analysis was used (Enter method). We assessed the potential confounding variables in our statistical modeling if the p value of univariate analysis was less than .01. Adjusted odds ratio (aOR) with 95% confidence interval (CI) were then collected. p < .05 was considered statistically significant.
3. Results
3.1. Comparison of perinatal history between pulmonary hemorrhage group and non-pulmonary hemorrhage group
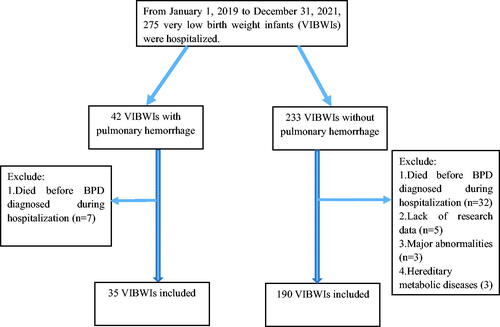
From January 1, 2019 to December 31, 2021, 275 VLBWIs were hospitalized in our NICU, including 42 cases with pulmonary hemorrhage and 233 cases with non-pulmonary hemorrhage. After screening by inclusion and exclusion criteria, 225 VLBWIs were subsequently included in the study, containing 35 cases in the pulmonary hemorrhage group and 190 cases in the non-pulmonary hemorrhage group (). The sample size finally included is similar to that estimated in “Method-2.3”. The comparison between the groups showed that there were significant differences in premature rupture of membranes (PROM) and tracheal intubation in the delivery room (p < .05). .
Table 1. Comparison of perinatal history between pulmonary hemorrhage group and non-pulmonary hemorrhage group.
3.2. Comparison of main diagnosis and therapy for respiratory diseases between pulmonary hemorrhage group and non-pulmonary hemorrhage group
There were significant differences between the two groups in the main diagnosis, including medically and surgically treated PDA as well as grade III-IV RDS (p < .05). There were also significant differences in the early onset sepsis, times of using surfactant and the duration and course of invasive ventilation (p < .05) .
Table 2. Comparison of main diagnoses and therapies between pulmonary hemorrhage group and non-pulmonary hemorrhage group.
3.3. Comparison of main outcomes between pulmonary hemorrhage group and non-pulmonary hemorrhage group
There were significant differences in BPD, moderate to severe BPD, IVH (≥grade III), and length of hospital stay between groups (p < .05) .
Table 3. Comparison of main outcomes between pulmonary hemorrhage group and non-pulmonary hemorrhage group.
3.4. Multivariate logistic regression analysis of pulmonary hemorrhage
Variables with p < .01 in and (PROM, tracheal intubation in the delivery room, use of surfactant more than once, grade III-IV RDS, medically and surgically treated PDA, early onset sepsis, and duration of invasive ventilation) were included in the multivariable analysis as confounding factors. It was found that pulmonary hemorrhage did not increase the risks of BPD and moderate to severe BPD (aOR for BPD = 1.710, 95% CI 0.581–5.039; aOR for moderate to severe BPD = 2.401, 95%CI 0.736–7.834) .
Table 4. Binary logistic regression analysis between pulmonary hemorrhage and BPD.
4. Discussion
Due to the smaller gestational age, lower birth weight, and immature lung development, very preterm infants are vulnerable to pulmonary hemorrhage. According to reports from different centers, the incidence in VLBWI vary from 0.5% to 11.0%, while the mortality is as high as 50–82% [Citation21–23]. Our data showed that the incidence was 15.3% (42/275), and the mortality rate was 21.4% (9/42) in VLBWIs. Although the survival rate seems improved, longer mechanical ventilation is often needed after pulmonary hemorrhage.
Considering the lung injury caused by pulmonary hemorrhage and subsequent long-term invasive ventilation, it seems that pulmonary hemorrhage can increase the incidence of BPD. A retrospective study which included infants aged 25–32 gestational weeks between January 2014 and January 2018 found that moderate to severe BPD was significantly higher in the group with massive pulmonary hemorrhage (15/28 vs. 10/56, p < .001) [Citation24]. In contrast, another study showed that no differences in moderate to severe BPD between pulmonary hemorrhage group and non-pulmonary hemorrhage group (7/17 and 40/107 ELBWIs respectively, p > .05) [Citation6]. Tomaszewska et al. further performed a retrospective case-control study and found infants with oxygen dependence at 28 d in the pulmonary hemorrhage group is not significantly more than infants in non-pulmonary hemorrhage group (24/29 and 17/29 VLBWIs respectively, p > .05) [Citation9]. However, almost all the reports studying on the association between BPD and pulmonary hemorrhage only used univariate analysis which limits the reliability of the results.Previous study has definitely shown that severe RDS and hemodynamically significant PDA (hsPDA) are triggers of pulmonary hemorrhage [Citation25]. And the mechanism of pulmonary hemorrhage is mainly related to pulmonary congestion, pulmonary edema and pulmonary small vessel embolism [Citation26]. Moreover, newborns with severe RDS and hsPDA often need longer ventilation, which may increase the possibility of BPD. Our univariate analysis showed that in the comparison of VLBWIs, pulmonary hemorrhage increased the risks of BPD (pulmonary hemorrhage group 28/35 vs non-pulmonary hemorrhage group 107/190, p = .009) and moderate to severe BPD (pulmonary hemorrhage group 18/35 vs non-pulmonary hemorrhage group 43/190, p = .023). In fact, the correlation between invasive ventilation and BPD has already been widely reported [Citation27]. Pulmonary hemorrhage also could prolong and increase the duration and frequency of invasive ventilation (). However, when considering the potential confounding factors, pulmonary hemorrhage did not increase the risks of BPD (aOR for BPD = 1.710, 95% CI 0.581–5.039) and moderate to severe BPD (aOR for moderate to severe BPD = 2.401, 95% CI 0.736–7.834) through further multivariate analysis.
Limitations
We should note that the sample size of this study is not large enough. In addition, some data including coagulation function, cardiac hemodynamics are not available because of the respective study design. Consequently, a better-designed prospective multicenter study is still necessary.
Conclusion
In conclusion, by univariate analysis, we found that PROM, tracheal intubation in the delivery room, duration of mechanical ventilation, course of invasive ventilation (≥3 courses), PS (>1 dose), medically and surgically treated PDA, grade III-IV RDS, early-onset sepsis, BPD and moderate to severe BPD showed significant differences between pulmonary hemorrhage group and non-pulmonary hemorrhage group. By multivariate analysis, pulmonary hemorrhage did not increase the risks of BPD and moderate to severe BPD.
Ethics
This study was approved by the ethics committee of the Children’s Hospital of Nanjing Medical University (Number: NJCH202004037-1). The study was exempt from informed consent by the institutional review board committee due to its retrospective nature. All data were fully anonymized before further statistical analysis. All the procedures were followed in accordance with the Declaration of Helsinki.
Authors contributions
Jing-jing Pan and Yun-su Zou wrote the manuscript. Jing Wang and Xiao-yu Zhou revised this paper. Mei-ling Tong and Yun-su Zou collected the clinical data. Yang Yang analyzed the data. Rui Cheng and Yang Yang designed this study.
Consent form
All authors listed have read the complete manuscript and have approved submission of the paper.
Acknowledgements
The authors would like to thank the parents of the patients for their understanding.
Disclosure statement
No potential conflict of interest was reported by the author(s).
Data availability statement
The dataset used during this study are available from the corresponding author on reasonable request.
Additional information
Funding
References
- Donda K, Vijayakanthi N, Dapaah-Siakwan F, et al. Trends in epidemiology and outcomes of respiratory distress syndrome in the United States. Pediatr Pulmonol. 2019;54(4):405–414.
- Fauroux B, Hascoët JM, Jarreau PH, et al. Risk factors for bronchiolitis hospitalization in infants: a French nationwide retrospective cohort study over four consecutive seasons (2009–2013). PLOS One. 2020;15(3):e0229766.
- Niesłuchowska-Hoxha A, Cnota W, Czuba B, et al. A retrospective study on the risk of respiratory distress syndrome in singleton pregnancies with preterm premature rupture of membranes between 24 + 0 and 36 + 6 weeks, using regression analysis for various factors. Biomed Res Int. 2018;2018:7162478.
- Hirata K, Kimura T, Hirano S, et al. Outcomes of outborn very-low-birth-weight infants in Japan. Arch Dis Child Fetal Neonatal Ed. 2021;106(2):131–136.
- McEvoy CT, Schilling D, Go MD, et al. Pulmonary function in extremely low birth weight infants with bronchopulmonary dysplasia before hospital discharge. J Perinatol. 2021;41(1):77–83.
- Wang TT, Zhou M, Hu XF, et al. Perinatal risk factors for pulmonary hemorrhage in extremely low-birth-weight infants. World J Pediatr. 2020;16(3):299–304.
- Li J, Xia H, Ye L, et al. Exploring prediction model and survival strategies for pulmonary hemorrhage in premature infants: a single-center, retrospective study. Transl Pediatr. 2021;10(5):1324–1332.
- Jensen EA, DeMauro SB, Kornhauser M, et al. Effects of multiple ventilation courses and duration of mechanical ventilation on respiratory outcomes in extremely low-birth-weight infants. JAMA Pediatr. 2015;169(11):1011–1017.
- Tomaszewska M, Stork E, Minich NM, et al. Pulmonary hemorrhage: clinical course and outcomes among very low-birth-weight infants. Arch Pediatr Adolesc Med. 1999;153(7):715–721.
- Yen TA, Wang CC, Hsieh WS, et al. Short-term outcome of pulmonary hemorrhage in very-low-birth-weight preterm infants. Pediatr Neonatol. 2013;54(5):330–334.
- Sweet DG, Carnielli V, Greisen G, et al. European consensus guidelines on the management of respiratory distress syndrome–2019 update. Neonatology. 2019;115(4):432–450.
- Jobe AH, Bancalari E. Bronchopulmonary dysplasia. Am J Respir Crit Care Med. 2001;163(7):1723–1729.
- Finer NN, Carlo WA, Walsh MC, et al. Early CPAP versus surfactant in extremely preterm infants. N Engl J Med. 2010;362(21):1970–1979.
- International Committee for the Classification of Retinopathy of Prematurity. The international classification of retinopathy of prematurity revisited. Arch Ophthalmol. 2005;123(7):991–999.
- Papile LA, Burstein J, Burstein R, et al. Incidence and evolution of subependymal and intraventricular hemorrhage: a study of infants with birth weights less than 1500 gm. J Pediatr. 1978;92(4):529–534.
- Volpe JJ. Neurology of the newborn. 5th ed. Philadelphia: Saunders; 2008. p. 541.
- Bell MJ, Ternberg JL, Feigin RD, et al. Neonatal necrotizing enterocolitis. Therapeutic decisions based upon clinical staging. Ann Surg. 1978;187(1):1–7.
- Yu J-L, Yu H-M, Yu H. Neonatology group of scientific branch of Chinese medical association, infection committee of neonatology branch of Chinese medical association. Expert consensus on diagnosis and treatment of neonatal sepsis (2019 edition). Chin J Pediatr. 2019;57(4):252–257.
- Expert Group of Chinese Neonatal Resuscitation Project. Guidelines for neonatal resuscitation in China (revised in Beijing in 2016). Chin J Perinat Med. 2016;19(7):481–486.
- Xiao-Mei S, Hong-Mao Y, Xiao-Shan Q. Practical neonatology. 5th ed. Beijing: People’s Health Publishing House; 2019.
- Bhandari V, Gagnon C, Rosenkrantz T, et al. Pulmonary hemorrhage in neonates of early and late gestation. J Perinat Med. 1999;27(5):369–375.
- Garland J, Buck R, Weinberg M. Pulmonary hemorrhage risk in infants with a clinically diagnosed patent ductus arteriosus: a retrospective cohort study. Pediatrics. 1994;94(5):719–723.
- Dufourq N, Thomson M, Adhikari M, et al. Massive pulmonary haemorrhage as a cause of death in the neonate e a retrospective review. S Afr Med J. 2004;94:299–e302.
- Bozkaya A, Yurttutan S, Özkars MY, et al. Respiratory problems in preterm infants with pulmonary hemorrhage. J Matern Fetal Neonatal Med. 2022;35(25):7505–7510.
- Chen YY, Wang HP, Lin SM, et al. Pulmonary hemorrhage in very low-birthweight infants: risk factors and management. Pediatr Int. 2012;54(6):743–747.
- Welde MA, Sanford CB, Mangum M, et al. Pulmonary hemorrhage in the neonate. Neonatal Netw. 2021;40(5):295–304.
- Kalikkot Thekkeveedu R, Guaman MC, Shivanna B. Bronchopulmonary dysplasia: a review of pathogenesis and pathophysiology. Respir Med. 2017;132:170–177.