Abstract
Background
Oxytocin is routinely administered after delivery for prophylaxis and treatment of postpartum hemorrhage, but it is associated with considerable cardiovascular side-effects. Carbetocin, a synthetic oxytocin analogue, has a myometrial contraction effect of 60 min when given IV, compared with 16 min for oxytocin.
Objective
To investigate whether there are differences in cardiovascular effects between oxytocin and carbetocin up to 1 h after treatment.
Methods
Sixty-one healthy pregnant women undergoing elective cesarean section in spinal anesthesia were randomized to receive an IV bolus of either five units (8.3 µg) of oxytocin or 100 µg of carbetocin after delivery of the baby. Heart rate (HR), mean arterial blood pressure, ECG ST index, oxygen saturation (SaO2), and photoplethysmographic digital pulse wave analysis variables were recorded before and at 1, 5, 20, and 60 min after drug administration. Vasopressor use, uterine tonus, total bleeding, and need for additional uterotonics were also assessed. Repeated measurement ANOVA was used for statistical analyses.
Results
The drugs had equal vasodilatory and hypotensive effects. Oxytocin, but not carbetocin, caused a decrease in HR at 1 min and a sustained decrease in cardiac left ventricular ejection time. Aggregate vasopressor use was higher in the carbetocin group. Neither drug caused any change in ST index, SaO2, or subjective cardiac symptoms. Uterine tonus, need for additional uterotonics, or total bleeding did not differ significantly between the groups.
Conclusion
Single doses of oxytocin and carbetocin had similar dilatory effects on vascular tonus, where the difference in aggregate vasopressor use can be attributed to a more persistent hypotensive effect of carbetocin. A transient negative chronotropic and sustained negative inotropic effect occurred after oxytocin. Neither drug showed any alarmingly adverse effects. Differences in drug effects may be attributed to differences in oxytocin and vasopressin receptor signaling pathways.
Introduction
Oxytocin is routinely administered at cesarean section (CS) to contract the uterus and prevent postpartum hemorrhage. However, many women then experience discomfort, nausea, and chest pain. These symptoms have been attributed to the significant circulatory dose-dependent effects of oxytocin [Citation1] including ECG ST-depression, increases in heart rate (HR), stroke volume and cardiac output (CO), and decreases in systemic vascular resistance and arterial blood pressure (BP) [Citation2–8]. Detailed studies of the immediate hemodynamic response show an increase in HR and decreases in systemic vascular resistance and BP within 30–40 s after a 5 U oxytocin statim bolus, with a concomitant increase of CO, followed by a rebound decrease in HR and a slow restitution of the BP [Citation7,Citation9].
Oxytocin has a half-life of only 1–6 min, and more recently, the longer acting oxytocin analogue carbetocin was registered for PPH prevention. Carbetocin is an octapeptide that works along the same molecular pathways as oxytocin, but with a half-life of about 40 min [Citation10,Citation11]. A single IV dose of carbetocin 100 µg results in uterine contraction within 2 min, with a duration of around 60 min, in comparison with 16 min for oxytocin [Citation12–14]. In contrast to oxytocin, carbetocin is heat-stable with no need of cold storage [Citation15].
Due to its short duration, oxytocin is often given repeatedly at CS [Citation12], potentially causing receptor desensitization and deteriorating uterotonic effect, with an increased risk of hypotension and cardiovascular side-effects. Prophylactic carbetocin has in a number of meta-analyses proved superior to oxytocin in effectiveness to prevent PPH, need of additional uterotonics, blood transfusion, etc., but with a similar safety profile [Citation12,Citation14,Citation16,Citation17].
Oxytocin and carbetocin have similar physical side-effects, such as nausea, flushing, vomiting, headache, tremor, chest pain, etc., though in a large meta-analysis carbetocin was found to induce a lower rate of vomiting [Citation18]. Regarding cardiovascular side-effects, we found only three randomized controlled studies comparing hemodynamic differences as the primary endpoint [Citation9,Citation19,Citation20]. In two of these studies patients were monitored for no more than 8 min after drug administration, despite an effect time of carbetocin of 1 h [Citation9,Citation19]. In the third study, there was no clear basal measurement for drug effect comparisons [Citation20]. The longer effect time of carbetocin raises a question about more sustained cardiovascular side-effects of carbetocin in comparison with oxytocin.
Pharmacological cardiovascular effects can be studied in detail by analyzing pulse wave (PW) curve contour characteristics, determined by propagation of the forward percussion PW along the arterial vascular tree and the following reflection of the tidal PW from distal arteries. PW characteristics can be determined by digital PW analysis (DPA), which is a rapid, noninvasive, and operator-independent photoplethysmographic method. The DPA method has been validated against invasive aortic measurements and correlates well with radial pulse applanation tonometry [Citation21,Citation22]. The DPA method can assess cardiac ejection time and distinguish between tonus changes in large and small arteries [Citation22].
During elective CS we found that oxytocin causes a global arterial vasodilation and has a direct negative chronotropic effect with increased left ventricular (LV) ejection power accompanied by electrocardiographic (ECG) ST changes [Citation23]. A similar pattern with peripheral vasodilation and increased CO was seen using DPA after oxytocin during elective first trimester uterine surgery, accompanied by minor ST changes [Citation24].
Previous studies comparing hemodynamic effects of carbetocin and oxytocin have not shown any significant differences [Citation9,Citation10,Citation19,Citation20,Citation25]. In the present study, we aimed to further elucidate and compare the effects of carbetocin and oxytocin on arterial elasticity (stiffness) and hemodynamic parameters using noninvasive DPA, with a follow-up time of 1 h. We hypothesized that the cardiovascular effects of the drugs will be similar, but that the effect of carbetocin will last longer.
Material and methods
The Regional Research Ethics Committee in Lund (Dnr 2012/732) granted ethical approval. The study was pre-registered with the Swedish Medical Products Agency (EudraCT number: 2013-004224-10, Dnr 5.1-2013-95167). The study was performed at the Skåne University Hospital maternity units in Lund and Malmö, Sweden.
The inclusion criteria were healthy women 18–40 years old, scoring 1–2 according to the American Society of Anesthesiologists (ASA) physical status classification [Citation26], with singleton pregnancy planned for elective CS under spinal anesthesia at gestational week 35 or more. Exclusion criteria were significant cardiovascular disease, lung disease, hypertension, pre-eclampsia, non-gestational diabetes mellitus, medications influencing the cardiovascular system, and general anesthesia for CS. Women were invited to participate in the study at the scheduled pre-operative assessment. The women should be able to understand oral and written Swedish, giving their oral and written consent.
Randomization to either oxytocin or carbetocin was made by a web-based randomization table (www.random.org) in blocks of 10 and sealed opaque envelopes containing the name of the study drug were prepared and stored in the pharmacy storage and preparation room in the operation ward. The sort of drug administered was blinded to the patient, the anesthesiologist, the surgeon, the operating staff and the DPA measurement operators (SR, EB, HJ). The anesthesiology nurse opened the envelope and prepared the injection, but she was not involved in DPA measurements or recording of study data. The randomization key was not revealed until after the study was closed.
The physiological background to the DPA method has been described previously [Citation21,Citation27]. The DPA measurements were performed with a customized pulse oximetry probe placed on the right second or third finger and connected to a Meridian DPATM (Meridian Co. Ltd., Korea, and Salcor AB, Uppsala, Sweden) and a laptop (HP625, Hewlett Packard, Solna, Sweden). The Meridian apparatus uses both crude and second derivative photoplethysmography, known as acceleration plethysmography [Citation28], and generates several different variables reflecting cardiac performance and arterial vascular tonus. For the present study, we selected those variables that have shown best repeatability and best correlation to gold standard arterial applanation tonometry [Citation22]: pulse height (PH), cardiac LV ejection time compensated (ETc), dicrotic index (DI), cardiac ejection elasticity index (EEI), aging index (AI), and the ratios b/a and d/a (representing second derivates of the crude PW curve contour). These variables are described in . For detailed descriptions of the mathematical and physiological details of DPA, see article by von Wowern et al. [Citation22].
Table 1. Description of the digital pulse wave analysis (DPA) parameters used in the study, revised from von Wowern et al. [Citation22].
Upon arrival to the operating room, an IV-line access was established and a 1000 ml Ringer’s acetate solution (Fresenius-Kabi, Uppsala, Sweden) was started. A surveillance monitor (DASH 400, GE Medical Systems Information Technologies, Danderyd, Sweden, or Philips Intellivue MP70, Philips Healthcare, Stockholm Sweden) with a five-lead ECG, a BP cuff, and a SaO2 probe was connected. The ECG ST index was automatically derived from the ECG lead II. The BP cuff was placed on the left arm. Readings of BP, SaO2, and ST index were manually noted in a case report form (CRF).
All women received spinal anesthesia in the sitting position with 2 ml hyperbaric bupivacaine (Marcain Tung 5 mg/mL, AstraZeneca, Södertälje, Sweden) and 1 ml sufentanil (Sufenta 5 μg/mL, Janssen-Cilag, Sollentuna, Sweden). A continuous IV infusion with phenylephrine (Fenylefrin Abcur 0.1 mg/mL, Abcur AB, Helsingborg, Sweden) was given to stabilize BP. After given spinal anesthesia, the women were placed on the operation table in a supine, slightly left-tilted position. Block height was determined by pinprick and loss of cold sensation before surgery.
After delivery of the baby and clamping and cutting the umbilical cord, the first (baseline) measurements were performed, denoted time 0 (T0). Then a bolus dose of the study drug was administered IV, i. e., either 1 ml oxytocin (8.3 μg = 5 IU) or 1 ml carbetocin (100 μg) according to the randomization. The injection time was 1 min. A stopwatch was started and new measurements were made 1, 5, and 20 min (T1, T5, and T20) after completed bolus injection. A final measurement was performed in the recovery room, 60 min after the bolus injection (T60). Each DPA measurement takes 70 s to perform, during which surgery was halted. Additional data from the operation (CS indication, gestational week, estimated blood loss, additional uterotonics and other drugs) were registered in the CRF by the research technicians performing the measurements. Uterine tonus was assessed by the surgeon after 20 min (T20) using a Numerical Rating Scale (NRS) of 0–10, where 0 was uterine inertia and 10 was maximal contraction. Chest pain or pressure as assessed by the patient was noted at T1 and T60, also using the NRS 0–10.
Atropine 0.5 mg (Atropin 0.5 mg/mL, Mylan AB, Stockholm Sweden) or additional phenylephrine (0.1 mg/mL) was given when indicated, at the discretion of the anesthetist. The speed of the infusion pump, the amount of infused phenylephrine and the amount of infused IV fluid were noted at given times in the CRF. Decisions regarding the need of additional drugs were left at the discretion of the obstetrician or the anesthetist, but this was considered a deviation from the study protocol (dropout) and subsequent recordings were not included in the statistical analyses. A blood loss >1000 ml was considered a dropout factor and subsequent recordings were excluded.
Sample size calculation
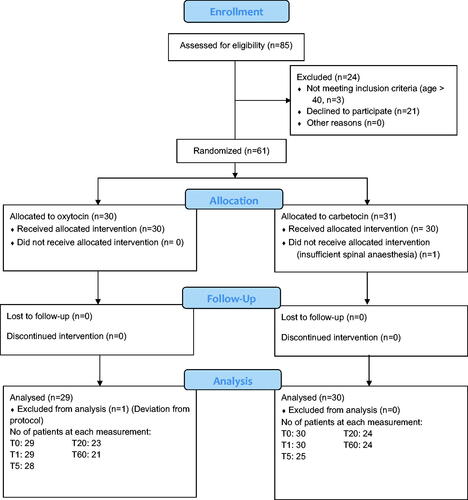
Using a single DPA variable for sample size calculation was not relevant since the pattern of change is more important than a change of a single variable. Two previous studies comparing hemodynamic effects of carbetocin and oxytocin during CS, but with other methodologies, used HR or BP to estimate sample size [Citation9,Citation19]. We considered MAP clinically most appropriate for sample size calculation. In our previous study monitoring the hemodynamic effects of oxytocin during CS, a mean MAP of 79.3 mmHg decreased to 72.0 mmHg after oxytocin with a standard deviation (SD) of 10 mmHg [Citation23]. Using these data, sample size calculation yielded that, with a type I risk of 5% and a type II risk of 20%, 29 participants would be necessary for each group (https://clincalc.com/Stats/SampleSize.aspx). In our earlier studies using DPA, we experienced a dropout rate of 10 to 25% from erroneous measurements and missing values, often due to movements or cold fingers. Therefore, we planned to randomize 40 + 40 women, but after asking 85 women for participation, the routines for planned CSs were re-organized and planned CSs were outreached from our university hospital to smaller hospitals in the region. Among the 85 women, 61 women gave their oral and written consent to participate (see ).
Statistical analyses
Statistical analyses were performed with SigmaPlot 15 computer software (Alfasoft A/S, Norway). For longitudinal drug effect changes (T0–T1–T5–T20–T60), a one-way ANOVA for repeated measurements (RM) was performed. In case of significant changes over time (two-sided p < .05), post-hoc comparisons were made using Holm-Sidak method for comparisons versus T0. In case the Shapiro–Wilk normality test failed (p < .05), Friedman’s non-parametric RM ANOVA was carried out and if significant, Dunn’s post-hoc analysis for comparisons versus T0 was used. Comparisons between the carbetocin and oxytocin groups were made by two-way RM ANOVA, using post-hoc analysis with Holm-Sidak method for comparisons at each time point. The study was randomized, but to avoid bias from possible differences in baseline values (T0) between the groups, we also compared the changes from baseline (set to null) to each time point expressed as Δ-values; For example, the change from T0 to T1, calculated as T1 minus T0, was expressed as ΔT1. Categorical data were compared with Fisher’s exact test. Grubbs outlier test (https://www.graphpad.com/quickcalcs/Grubbs1.cfm) was used to identify outliers and exclude cases (variables) with apparently erroneous values.
Results
Patient characteristics and baseline measurements at T0 (before drug administration) were statistically not different (). The CONSORT flow-chart of women invited to the study is shown in . There was one dropout in the carbetocin group because of inadequate spinal anesthesia. There was one dropout in the oxytocin group due to glyceryl nitrate administration before delivery. A few additional cases were excluded from analyses from T5 and on because of estimated blood loss >1000 ml, additional uterotonics given, or other apparent protocol violation ().
Table 2. Patient characteristics.
The longitudinal effects of carbetocin and oxytocin, respectively, are shown in and , and presented graphically in and . For both drugs, significant changes over time were seen for all variables except for ST index, SaO2, and DI.
Figure 2. Longitudinal effects of intravenous carbetocin (100 μg) and oxytocin (8.3 μg = 5 IU), given at cesarean section at time T0 (baseline), on heart rate (HR, bpm), mean arterial blood pressure (MAP, mmHg), electrocardiogram (ECG) ST index (mm), and oxygen saturation (SaO2, %). Repeated measurements were performed after 1 min (T1), 5 min (T5), 20 min (T20), and 60 min (T60). Values denote the mean changes from baseline (set to null) to each time point expressed as Δ-values, with 95% confidence interval. Two-way repeated measurements ANOVA showed no significant differences between the drugs (p ≥ .11).
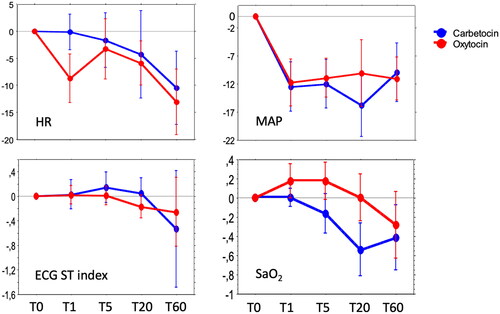
Figure 3. Longitudinal effects of intravenous carbetocin (100 μg) and oxytocin (8.3 μg = 5 IU) given at cesarean section at time T0 (baseline) on variables derived from the digital pulse wave analysis: pulse height (PH), aging index (AI), left ventricular ejection time compensated (ETc, ms), cardiac ejection elasticity index (EEI), dicrotic index (DI), and the second derivates b/a and d/a of the crude digital pulse wave curve contour. Repeated measurements were performed after 1 min (T1), 5 min (T5), 20 min (T20), and 60 min (T60). Values denote the mean changes from baseline (set to null) to each time point expressed as Δ-values, with 95% confidence interval. Two-way repeated measurements ANOVA showed a significant difference of ETc from T1 to T20.
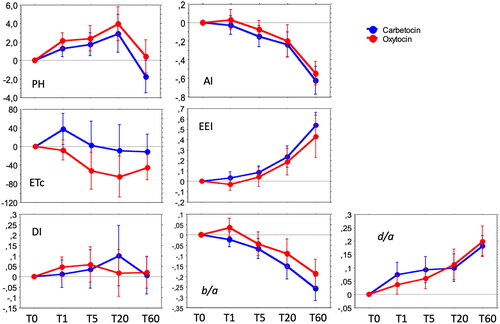
Table 3. Longitudinal effects of intravenous oxytocin (5 IU) given immediately after T0 (baseline).
Table 4. Longitudinal effects of intravenous carbetocin (100 μg) given immediately after T0 (baseline).
At T1 the HR was not affected by carbetocin (), whereas a significant decrease of about 9 bpm in mean was seen in the oxytocin group (, ). After 5 min the HR had rebounded to the same level as for carbetocin. After 60 min the HR had decreased in both groups to a level of 10–13 bpm below baseline.
The MAP decreased significantly with about 12 mmHg after 1 min in both groups and stayed significantly lower than baseline throughout the experiment ().
The global index of arterial vascular compliance/elasticity, AI, decreased onward after drug injections, indicating vasodilation, with no significant differences between the drugs. DPA variables representing large artery vascular tonus (EEI, b/a) and small artery vascular tonus (PH, d/a), also changed significantly over time, indicating vasodilation, with no significant difference between the drugs. DI, indicating peripheral resistance, did not change significantly over time in any group.
Considering the whole series of measurements T0–T1–T5–T20–T60, the two-way RM ANOVA showed no significant effect differences between oxytocin and carbetocin for any variable (p ≥ .14, table not shown).
A two-way RM ANOVA of ΔT-values (ΔT1–ΔT5–ΔT20–ΔT60) showed a significant difference between the drugs only for ETc, where ETc was lower in the oxytocin group from T1 to T20, but not at T60 (table not shown).
Secondary outcomes
The aggregate amount of phenylephrine infused was significantly larger in the carbetocin group. This difference was significant from 20 min onward. The total amount of Ringer’s acetate infused did not differ significantly between the groups at any time. The total estimated blood loss, uterine inertia, and extra use of uterotonics were not significantly different between the groups. Five women had an estimated blood loss >1000 ml, all in the carbetocin group. In four of them, bleeding was due to surgical complications ().
Table 5. Summary of additional measurements and side-effects.
There were no significant differences between carbetocin and oxytocin in the maternal experience of chest pressure or discomfort (five and seven women, respectively), and no associations with changes of BP, ST index, or HR were found. One woman in the carbetocin group had ST index changes of 2.3–2.9 mm from T1 onwards, but no symptoms of chest pain or discomfort. When scrutinizing her CRF, we found a decrease in BP that had not been treated properly.
Discussion
This study showed similar cardiovascular effects of carbetocin and oxytocin during the whole 1h measurement period. Both drugs caused a global vasodilation with lowering of vascular tonus in both large and small arteries, accompanied by a slight drop in BP but without any compensatory increment in HR. In contrast to carbetocin, oxytocin had a prompt but short-lived mild negative chronotropic effect and a shortening effect on LV ejection time (ETc) that lasted up to 20 min, but was equalized at 60 min.
The cardiovascular effects of uterotonics in time and magnitude depend on whether they are given statim, slowly, or as an infusion. For both carbetocin and oxytocin, when given statim IV the HR peaks after half a minute, paralleled by a nadir in BP and peripheral vascular resistance [Citation7,Citation9]. When given IV over 60 s, the peaks and nadirs seem to occur about 1 min after finishing the injections [Citation19]. This pattern has also been demonstrated for methylergometrine when given IV during 30 s [Citation6]. When oxytocin is given as an infusion over 5 min the cardiovascular effects are considerably weaker [Citation7]. For carbetocin, no difference in maximum HR occurs when given either rapidly or during 10 min IV, but as expected, the HR increase is delayed when given slowly [Citation29].
In our study the uterotonics were given as recommended, during 60 s, then there was a 60 s delay until the DPA recording started at T1, and that recording took 70 s. Transient hemodynamic changes occurring within the first 2 min after injection start could then not be captured in our study. Nevertheless, apart from a drop in HR, we found no substantial discrepancies between our findings and the findings in studies using other methodologies [Citation3,Citation6,Citation7,Citation9,Citation19]. Nota bene, a similar pattern with a drop in HR was found in our previous study of oxytocin given at CS [Citation23]. In a study by Moertl et al. comparing the hemodynamic effects of carbetocin and oxytocin [Citation9], the drugs were injected in 10 s, which could explain the slightly different cardiovascular responses compared to our study. Rapid injection of oxytocin or carbetocin is nowadays inadvisable [Citation30].
Among DPA variables indicating cardiac function, the EEI indicated an increase in LV ejection power and large artery vasodilation after 20 min in both groups, suggesting increased CO. The ETc decreased significantly in the oxytocin group but not in the carbetocin group. The differences were statistically significant from 1 to 20 min when calculated with changes from baseline, i.e. with Δ-values.
A decrease in ETc represents a shortening of the LV ejection time, which indicates negative inotropy and/or decreased preload or hypovolemia [Citation31–33]. The relation is not completely linear, since it depends on whether the patient is in a high or low preload state [Citation24]. LV ejection time can actually be shortened from both positive and negative inotropic agents, though mostly it is associated with negative inotropy [Citation34,Citation35]. The decrease of ETc in the oxytocin group is not consistent with our earlier studies on oxytocin, where ETc remained either unchanged or increased [Citation23,Citation24]. In a previous study from our group the ETc variable showed a rather poor reliability, which we believe is due to methodological difficulties in identifying the endpoint of systole [Citation22]. Thus, the findings of shortening of ETc by oxytocin, and a possible difference to carbetocin, are uncertain findings although in favor of carbetocin. The findings call for further investigations to reveal the clinical relevance.
One woman in the carbetocin group showed already after 1 min an ST index increase of 2.3–2.9 mm but had no subjective symptoms. We scrutinized her CRF and found poorly managed hypotension. The importance of proper treatment of hypotension associated with spinal anesthesia, drugs, and hypovolemia must be emphasized. For all other women ST index remained stable during the whole 1-h period. This is important since a growing number of pregnant women have concomitant heart disease [Citation36]. Since oxytocin may cause a dose-dependent ST-depression, troponin release, prolongation of QT-time, and arrhythmia, it is essential to investigate the myocardial effects also of carbetocin [Citation37].
In agreement with previous studies [Citation10,Citation12,Citation25], there was no difference between the groups in blood loss. These studies, as well as meta-analyses [Citation14,Citation38,Citation39], show a reduced need for extra uterotonics when using carbetocin compared to oxytocin, in both elective and non-elective CS, although in our study, this difference did not reach significance. Repeated doses of oxytocin might increase the risk of hypotension and cardiovascular side-effects [Citation3]. Like other studies [Citation9,Citation19], we found no difference in the frequency of chest pain and discomfort.
Oxytocin receptors (OXTR) are present in the uterus, mammary glands, heart, brain, and blood vessels [Citation40]. The biology of OXTR is intricate, with varied, context-dependent cellular processing, similarities to vasopressin receptors, and widespread peripheral and central expression. OXTR belongs to a large family of G protein-coupled cell surface receptors, which are activated by various signaling pathways [Citation40]. Oxytocin and vasopressin differ in only two amino acid sequences, which explains why oxytocin also activates vasopressor receptors V1a (V1aR) and V1b (V1bR). Passoni et al. found that carbetocin activates the OXTR but not V1aR and V1bR, and that carbetocin can even act as a competitive antagonist on vasopressin receptors. [Citation41]. Carbetocin selectively activates only the OXTR/Gq pathway, whereas oxytocin activates OXTR coupling to G-protein subtypes Gq, Gi, and Go. The Passoni study thus indicates important differences in key molecular pharmacological properties between carbetocin and oxytocin, where carbetocin exerts a “weaker” action on OXTR. The unique functional selective OXTR/Gq coupling of carbetocin might explain the small but significant differences in vascular activity between carbetocin and oxytocin in our study.
Strengths and limitations: A few cases were lost to full statistical analyses due to additional uterotonics and blood loss above 1000 ml, and occasional variable analyses were lost due to technical problems or finger movements. The dropouts constrained mainly the T20 and T60 measurements, thus reducing the power of the study. We minimized selection and operator bias by using a double-blinded randomized design. As the main outcome was the effects on large and small artery elasticity, we only included healthy (ASA 1–2) women without cardiovascular and systemic disorders. The photoplethysmographic DPA method is sensitive to movements and cold fingers, but when that is mastered, it is one of very few simple and painless noninvasive methods to measure hemodynamics. When interpreting the results, one must realize that CS is a complex procedure from a hemodynamic viewpoint, with effects caused not only by uterotonics but also by interference of spinal anesthesia, phenylephrine and IV fluid infusions, bleeding, emptying of uterus, relief of aortocaval compression, and maternal emotions.
Conclusion
This double-blinded randomized study showed only minor differences between single doses of oxytocin and carbetocin when comparing cardiac performance and large and small artery elasticity. Neither drug showed any alarmingly adverse effects on the cardiovascular system. For both drugs equally, within the first minutes a peripheral vasodilation occurred with a drop in BP. The higher accumulated vasopressor dose after 20 min can be attributed to a more sustained hypotensive effect of carbetocin, conveniently treated with phenylephrine. Despite vasodilation and a relative hypotension after both carbetocin and oxytocin, the HR did not increase in any group. In fact, oxytocin had a transient negative chronotropic effect. In addition, oxytocin had a sustained negative inotropic effect, as reflected by a shortening of the LV ejection time. Differences in drug effects may be attributed to differences in oxytocin and vasopressin receptor signaling pathways. Bleeding, use of extra uterotonics and other side-effects did not differ between the drugs. It should be noted that our conclusion is valid only for single doses of 5 U oxytocin and 100 μg carbetocin; With those doses given at elective CS additional uterotonics are needed three times as often after oxytocin (9% vs 3%) [Citation12], where repeated oxytocin doses might increase the risk of hypotension and cardiovascular side-effects.
Author contributions
SR: conceptualization, planning, recruiting, performing, statistical analyses, analyzing results, literature search, writing the manuscript. HJ: recruiting, performing, statistical analyses, analyzing results, literature search, reviewing manuscript. EJ: recruiting, performing, statistical analyses, analyzing results, literature search, reviewing manuscript. PO: conceptualization, planning, ethics approval, medical agency approval, statistical analyses, analyzing results, literature search, writing the manuscript.
Disclosure statement
PO was previously Consultant Medical Adviser of Ferring Pharmaceuticals AB, Malmö, Sweden, the manufacturer of carbetocin (Pabal©), but resigned in 2016. The company has not been involved in planning, performance or supporting the study. SR, HJ and EB declare no conflicts of interest.
Additional information
Funding
References
- Hendricks CH, Brenner WE. Cardiovascular effects of oxytocic drugs used post partum. Am J Obstet Gynecol. 1970;108(5):751–760.
- Carvalho JCA, Balki M, Kingdom J, et al. Oxytocin requirements at elective cesarean delivery: a dose-finding study. Obstet Gynecol. 2004;104(5 Pt 1):1005–1010.
- Langesaeter E, Rosseland LA, Stubhaug A. Haemodynamic effects of repeated doses of oxytocin during caesarean delivery in healthy parturients. Br J Anaesth. 2009;103(2):260–262.
- Mukaddam-Daher S, Yin Y-L, Roy J, et al. Negative inotropic and chronotropic effects of oxytocin. Hypertension. 2001;38:292–296.
- Pinder AJ, Dresner M, Calow C, et al. Haemodynamic changes caused by oxytocin during caesarean section under spinal anaesthesia. Int J Obstet Anesth. 2002;11(3):156–159.
- Svanström MC, Biber B, Hanes M, et al. Signs of myocardial ischaemia after injection of oxytocin: a randomized double-blind comparison of oxytocin and methylergometrine during caesarean section. Br J Anaesth. 2008;100(5):683–689.
- Thomas JS, Koh SH, Cooper GM. Haemodynamic effects of oxytocin given as i.v. bolus or infusion on women undergoing caesarean section. Br J Anaesth. 2007;98(1):116–119.
- Jonsson M, Hanson U, Lidell C, et al. ST depression at caesarean section and the relation to oxytocin dose. A randomised controlled trial. BJOG. 2010;117(1):76–83.
- Moertl M, Friedrich S, Kraschl J, et al. Haemodynamic effects of carbetocin and oxytocin given as intravenous bolus on women undergoing caesarean delivery: a randomised trial. BJOG. 2011;118(11):1349–1356.
- Larciprete G, Montagnoli C, Frigo M, et al. Carbetocin versus oxytocin in caesarean section with high risk of post-partum haemorrhage. J Prenat Med. 2013;7:12–18.
- Rath W. Prevention of postpartum haemorrhage with the oxytocin analogue carbetocin. Eur J Obstet Gynecol Reprod Biol. 2009;147(1):15–20.
- Holleboom CAG, Van Eyck J, Koenen SV, et al. Carbetocin in comparison with oxytocin in several dosing regimens for the prevention of uterine atony after elective caesarean section in the Netherlands. Arch Gynecol Obstet. 2013;287(6):1111–1117.
- Hunter DJS, Schulz P, Wassenaar W. Effect of carbetocin, a long-acting oxytocin analog on the postpartum uterus. Clin Pharmacol Ther. 1992;52(1):60–67.
- Su L-L, Chong Y-S, Samuel M. Carbetocin for preventing postpartum haemorrhage. In: Su L-L, editor. Cochrane database of systematic review [Internet]. Chichester: John Wiley & Sons, Ltd; 2012. Available from: https://onlinelibrary.wiley.com/10.1002/14651858.CD005457.pub4
- Widmer M, Piaggio G, Nguyen TMH, et al. Heat-Stable carbetocin versus oxytocin to prevent hemorrhage after vaginal birth. N Engl J Med. 2018;379(8):743–752.
- Voon HY, Shafie AA, Bujang MA, et al. Cost effectiveness analysis of carbetocin during cesarean section in a high volume maternity unit. J Obstet Gynaecol Res. 2018;44(1):109–116.
- Sun H, Xu L, Li Y, et al. Effectiveness and safety of carboxytocin versus oxytocin in preventing postpartum hemorrhage: a systematic review and meta-analysis. J Obstet Gynaecol. 2022;48(4):889–901.
- Ai W, Zeng Y, Ma Y, et al. Side-effects of carbetocin to prevent postpartum hemorrhage: a systematic review and meta-analysis of randomized controlled trials. Pharmacol Res Perspect. 2021;9:11.
- Rosseland LA, Hauge TH, Grindheim G, et al. Changes in blood pressure and cardiac output during cesarean delivery the effects of oxytocin and carbetocin compared with placebo. Anesthesiology. 2013;119(3):541–551.
- Pisani I, Tiralongo GM, Gagliardi G, et al. The maternal cardiovascular effect of carbetocin compared to oxytocin in women undergoing caesarean section. Pregnancy Hypertens. 2012;2(2):139–142.
- Millasseau SC, Ritter JM, Takazawa K, et al. Contour analysis of the photoplethysmographic pulse measured at the finger. J Hypertens. 2006;24(8):1449–1456.
- von Wowern E, Östling G, Nilsson PM, et al. Digital photoplethysmography for assessment of arterial stiffness: repeatability and comparison with applanation tonometry. West J, editor. PLoS One. 2015;10(8):e0135659.
- Rabow S, Olofsson P. Pulse wave analysis by digital photoplethysmography to record maternal hemodynamic effects of spinal anesthesia, delivery of the baby, and intravenous oxytocin during cesarean section. J Matern Neonatal Med. 2017;30(7):759–766.
- Rabow S, Hjorth U, Schönbeck S, et al. Effects of oxytocin and anaesthesia on vascular tone in pregnant women: a randomised double-blind placebo-controlled study using non-invasive pulse wave analysis. BMC Pregnancy Childbirth. 2018;18(1):453.
- Attilakos G, Psaroudakis D, Ash J, et al. Carbetocin versus oxytocin for the prevention of postpartum haemorrhage following caesarean section: the results of a double-blind randomised trial. BJOG. 2010;117(8):929–936.
- American Society of Anesthesiologists - ASA Physical Status Classification System [Internet]; [cited 2017 Sep 21]. Available from: https://www.asahq.org/resources/clinical-information/asa-physical-status-classification-system
- Elgendi M. On the analysis of fingertip photoplethysmogram signals. Curr Cardiol Rev. 2012;8(1):14–25.
- Elgendi M. Standard terminologies for photoplethysmogram signals. Curr Cardiol Rev. 2012;8(3):215–219.
- Boisselle MÈ, Zaphiratos VV, Fortier A, et al. Comparison of carbetocin as a bolus or an infusion with prophylactic phenylephrine on maternal heart rate during cesarean delivery under spinal anesthesia: a double-blinded randomized controlled trial. Can J Anaesth. 2022;69(6):715–725.
- Heesen M, Carvalho B, Carvalho JCA, et al. International consensus statement on the use of uterotonic agents during caesarean section. Anaesthesia. 2019;74(10):1305–1319.
- Geeraerts T, Albaladejo P, Declère AD, et al. Decrease in left ventricular ejection time on digital arterial waveform during simulated hypovolemia in normal humans. J Trauma. 2004;56(4):845–849.
- Tavakolian K. Systolic time intervals and new measurement methods. Cardiovasc Eng Tech. 2016;7(2):118–125.
- Alhakak AS, Teerlink JR, Lindenfeld J, et al. The significance of left ventricular ejection time in heart failure with reduced ejection fraction. Eur J Heart Fail. 2021;23(4):541–551.
- Boudoulas H. Systolic time intervals. Eur Heart J. 1990;11(suppl I):93–104.
- Akc GDL, Chamberlain BM, Rgn EJP, et al. Oesophageal doppler monitor (ODM) guided individualised goal directed fluid management (iGDFM) in surgery - a technical review. Deltex Med. 2010;9051–3014:1–12.
- Parsonage WA, Zentner D, Lust K, et al. Heart disease and pregnancy: the need for a twenty-first century approach to care…. hear. Lung Circ. 2021;30(1):45–51.
- Bekkenes M, Jørgensen MM, Flem Jacobsen A, et al. A study protocol for the cardiac effects of a single dose of either oxytocin 2.5 IU or carbetocin 100 µg after caesarean delivery: a prospective randomized controlled multi-centre trial in Norway. F1000Res. 2021;10:973.
- Onwochei DN, Owolabi A, Singh PM, et al. Carbetocin compared with oxytocin in non-elective cesarean delivery: a systematic review, meta-analysis, and trial sequential analysis of randomized-controlled trials. Can J Anaesth. 2020;67(11):1524–1534.
- Onwochei DN, Van Ross J, Singh PM, et al. Carbetocin reduces the need for additional uterotonics in elective caesarean delivery: a systematic review, meta-analysis and trial sequential analysis of randomised controlled trials. Int J Obstet Anesth. 2019;40:14–23.
- McKay EC, Counts SE. Oxytocin receptor signaling in vascular function and stroke. Front. Neurosci. 2020;14:1–18.
- Passoni I, Leonzino M, Gigliucci V, et al. Carbetocin is a functional selective Gq agonist that does not promote oxytocin receptor recycling after inducing β‐arrestin‐independent internalisation. J Neuroendocrinol. 2016;28(4):12363.