Abstract
Background
The relationship between circulating miRNAs and neonatal sepsis and the mechanism of action are still unclear at this time. Therefore, the potential diagnostic role of miRNAs in neonatal sepsis (NS) was studied through meta-analysis.
Method
Web of Science, Cochrane Library, PubMed, and Embase are retrieved, supplemented by manual search, and the search was conducted to find related studies without time limit until May 2022.The quality of the literature was assessed via QUADAS criteria and meta-analyzed via Stata 11.0 software, including the assessment of specificity, sensitivity, likelihood ratio and diagnostic odds ratio. Then, sensitivity analysis and heterogeneity testing were conducted, and finally, the summary receiver operating characteristics (SROC) curve was drawn.
Result
This study included 14 articles, including 20 miRNAs and 1597 newborns(control group: 727 and case group: 870). Among them, one article was of low quality, three articles were of high quality, and the rest were of medium quality. According to the results of random effects model analysis, the pooled specificity and sensitivity of miRNA for the diagnosis of NS were 0.83 (95%CI: 0.79–0.87) and 0.76 (95%CI: 0.72–0.80), respectively. And negative likelihood ratio, positive likelihood ratio, and diagnostic odds ratio were 0.29 (95%CI: 0.24–0.34), 4.51 (95%CI: 3.52–5.78), and 15.81 (95%CI: 10.71–23.35), respectively. The area under the SROC curve was 0.86, and there was no evidence publication bias detected in the funnel plot.
Conclusion
Circulating miRNAs may be very useful in the development of early diagnostic strategies for neonatal sepsis.
1. Introduction
Sepsis is a systemic inflammatory response syndrome caused by pathogenic microorganisms including bacteria, fungi and viruses [Citation1]. Since newborns have an underdeveloped immune system, the development of neonatal immune system is not perfect, and the phagocytosis and other bactericidal activities of innate immune effector cells (such as neutrophils and macrophages) are dysfunctional. They are more susceptible to sepsis [Citation2], which may lead to serious consequences such as shock and even death [Citation3,Citation4]. Up to now, neonatal sepsis remains a significant problem threatening neonatal health worldwide. In developed countries, the incidence of neonatal sepsis is 1–8 per 1000 births, while the incidence in developing countries is about three times higher than that in developed countries [Citation5]. A report from the World Health Organization (WHO) states that 1 million deaths each year (10% of deaths in children under 5) are attributed to neonatal sepsis, 42% of which occur within the first week of life [Citation6]. In the early stages of neonatal sepsis, children generally do not show obvious clinical symptoms. When symptoms appear, however, it usually indicates that the disease is severe enough [Citation7]. At present, the detection of pathogenic bacteria in blood culture is still the gold standard for the diagnosis of sepsis [Citation8]. However, due to its long diagnostic cycle, it is difficult to get results within 24–48 h. In addition, it is susceptible to false-negative results, leading to missed opportunities for treatment [Citation9]. Therefore, timely diagnosis and targeted interventions are needed to delay disease progression. As a result, there is an urgent need for reliable biomarkers that can differentiate sepsis at an early stage. From this point of view, the exploration of new biomarkers for neonatal sepsis is of special clinical significance.
MicroRNAs (miRNAs) are short non-coding RNAs associated with regulation of gene expression that play a regulatory role in inflammation, immunity, apoptosis, and cell differentiation [Citation10]. Previous studies have shown that the expression of circulating miRNAs is regulated in the early stages of sepsis and positively correlates with disease severity and progression [Citation11], which suggests that miRNAs play a crucial role in the diagnosis, progression and prognosis of pediatric patients with sepsis. According to relevant studies, blocking pro-inflammatory effects can effectively improve related organ damage caused by sepsis [Citation12]. Compared with traditional biomarkers, miRNAs have the advantages of more reliable measurement of expression levels, more stability in human specimens, faster collection, and less invasiveness [Citation13]. Therefore, studies on circulating miRNAs for the diagnosis of neonatal sepsis have special clinical significance.
2. Materials
2.1. Literature retrieval
This study was prepared in strict accordance with the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) guidelines [Citation14], and it carefully retrieved literature databases (Cochrane Library, Embase, Web of Science, PubMed), including three key words “neonatal,” “sepsis,” “miRNA,” and the search was conducted to find related studies without time limit until May 2022. There are no language restrictions during the literature retrieval process. For a more comprehensive retrieval, literature tracking is performed when necessary.
2.2. Inclusion and exclusion criteria
Inclusion criteria: (1) All literatures for the purpose of evaluating or discussing the diagnostic efficacy of miRNAs for neonatal sepsis; (2) All included literature had a clear diagnosis of neonatal sepsis, including blood culture positivity or clinical diagnosis of sepsis;(3) The study subject is neonates (age <28 days); (4) The research is retrospective or prospective; (5) The diagnostic comparison data of normal neonates or non-septic neonates used as controls are included in the study; (6) The sensitivity, specificity, area under AUC curve, true positives (TP), true negatives (TN), false positives (FP), false negatives (FN) or other convertible diagnostic data of miRNA in the diagnosis of neonatal sepsis can be obtained from the original literature.
Exclusion criteria: (1) Non-diagnostic test research; (2) The sample size is too small (<20); (3) Research that cannot extract the required data and information; (4) Animal studies, reviews, case reports, and conference papers.
2.3. Literature screening
Two investigators independently reviewed and evaluated each study. When there was a disagreement, another investigator was added for evaluation. First of all, titles and abstracts were screened to identify potentially eligible studies, and full texts were then analyzed for further evaluation.
2.4. Data extraction and quality evaluation
General information such as author, publication time, country, number of cases and controls, type of miRNA, body weight, gestational age, mean age, etc., and diagnostic parameter information such as TN, TP, FN, and FP were independently extracted from the included literature by two investigators using the same standard table. Then, risk of bias in each study was assessed by two authors using the Quality Assessment of Diagnostic Accuracy Studies (QUADAS), a valid tool for assessing the quality of diagnostic studies [Citation15].
2.5. Statistical analysis
Meta-analysis was performed via STATA 11.0. The numbers of all subjects with TP,TN, FP and FN were extracted from each included study with 95% confidence intervals (95% CI). Calculations were performed using the combined SEN (sensitivity), SPE (specificity), PLR (positive likelihood ratio), NLR (negative likelihood ratio), DOR (diagnostic odds ratio). SROC curves were drawn with SEN and SPE, respectively, and the area under the SROC curve (AUC) and 95% CI was calculated. AUC not only shows the results of performance testing, but also shows the relationship between SEN and SPE. An AUC of 1.0 (100%) indicates a perfect discriminatory ability to distinguish case group from control group. The magnitude of heterogeneity in the included studies was assessed by calculating I2 by drawing a receiver operating characteristic (SROC) curve. If I2 ≥ 75%, it was considered that there was significant heterogeneity. In addition, the included studies were subjected to sensitivity analysis and publication bias test, and publication bias was assessed via Deeks’ funnel plot asymmetry test. Also, subgroup analyses were performed to explore the differences of control populations (health/unhealth) as well as miRNAs(up/down) in the studies. Statistically, p <.05 was considered a significant difference.
3. Result
3.1. Retrieved result
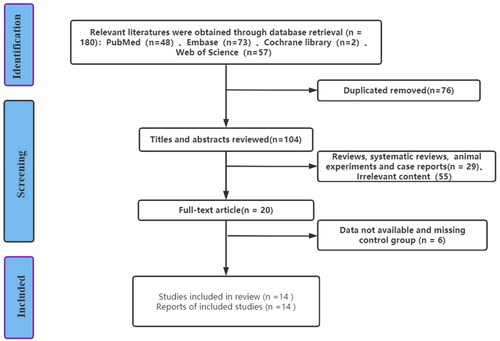
In this study, a total of 180 articles were searched in PubMed, Cochrane Library, Embase, and Web of Science, and 20 articles were preliminarily selected according to the screening process in . After further comprehensive reading of the literature, 6 literatures that could not be extracted were excluded, and a total of 14 literatures were finally included in the research. A total of 20 miRNAs were included in 14 articles, including 1597 newborns (870 in the neonatal septicemia group and 727 in the control group). The process and results of literature screening are shown in .
The basic characteristics of the included literature are shown in .
Table 1. Basic characteristics of included literature.
3.2. Quality evaluation
The quality scores of the included articles were all high according to the QUADAS literature quality score. (See Supplementary Document 2.)
3.3. Meta-analysis of pooled effect sizes
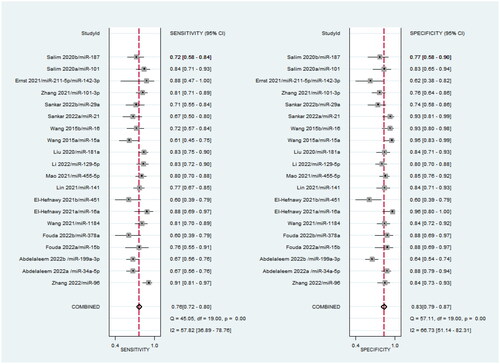
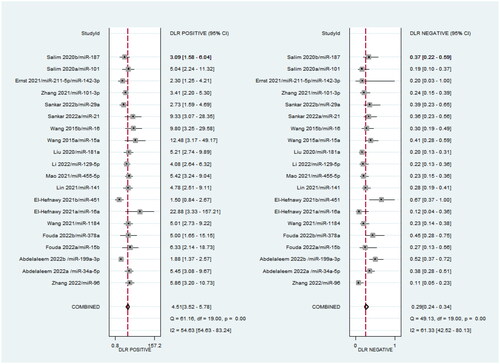
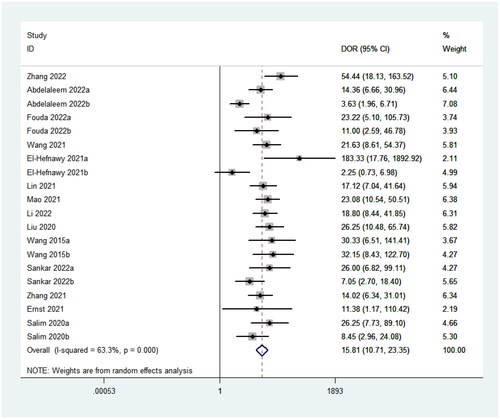
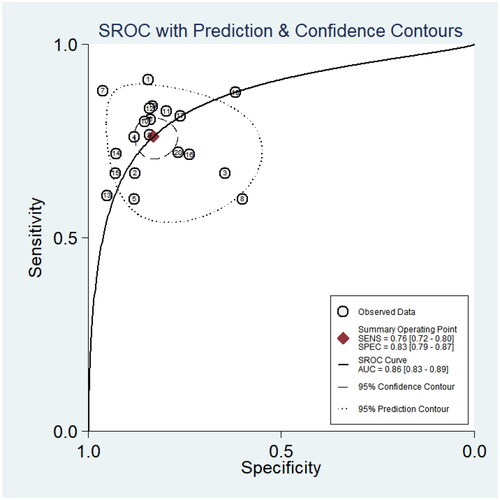
It can be clearly seen from that the sensitivity and specificity of miRNA for the diagnosis of neonatal sepsis (NS) were 0.76 and 0.83, the pooled positive likelihood ratio (PLR) was 4.51 (95% CI: 3.52–5.78), and the pooled negative likelihood ratio (NLR) was 0.29 (95% CI: 0.24–0.34), the pooled DOR was 15.81 (95%CI: 10.71–23.35). Overall, circulating miRNAs have high diagnostic potential for neonatal sepsis.
Based on the SROC curve drawn below (), it was finally concluded that the diagnostic value of miRNAs for neonatal sepsis was 0.86, p < .05. Thus, the accuracy of circulating miRNAs for neonatal sepsis is close to the gold standard.
3.4. Heterogeneity test and subgroup analysis
According to the heterogeneity test for sensitivity, specificity, PLR, and NLR, the I2 of the pooled results of sensitivity and specificity was 57.82% (p < .001) and 66.73% (p < .001) (), the I2 of PLR and NLR were 54.63% (p < .001) and 61.33% (p < .001) (), which showed moderate heterogeneity and it is still within the acceptable range in the meta-analysis. In addition, the results of our subgroup analysis are listed in . The results showed that there is no significant difference in the summarized diagnostic results of different control sources (healthy/non-healthy) and miRNA (up/down regulated), indicating that the results of this study are relatively reliable.
Table 2. Subgroup analysis.
3.5. Sensitivity analysis and publication bias
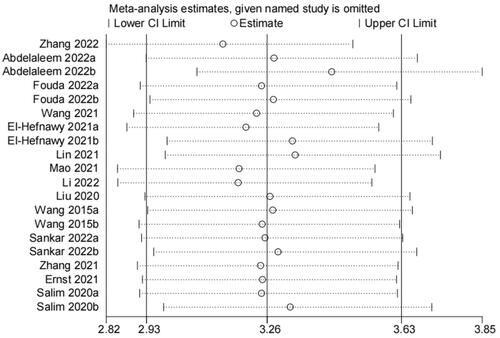
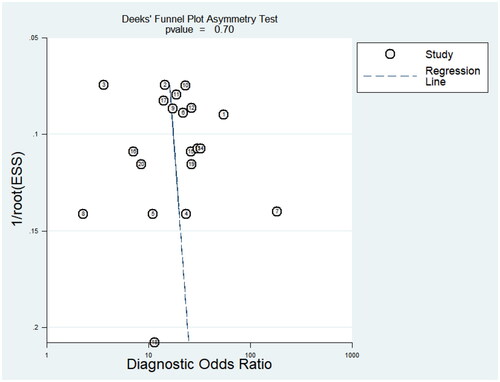
After the sensitivity analysis was carried out by gradually eliminating single studies, no single study was found to have a significant impact on the overall results, and the conclusion was relatively stable (). In addition, the results of the publication bias test showed () that Deek’s funnel plot asymmetry test did not show a surprising publication bias (p = .7), nor did the funnel plot show asymmetry. Therefore, the results of this study can be considered to be relatively reliable.
4. Discussion
A total of 20 researches were included for systematic evaluation and meta-analysis so as to evaluate the potential diagnostic performance of miRNAs in neonatal sepsis. The results suggest that miRNAs detection is beneficial for the diagnosis of neonatal sepsis. We found in this meta-analysis a pooled specificity of 0.76 (95%CI: 0.72–0.80), a pooled sensitivity of 0.83 (95%CI: 0.79–0.87), and a pooled DOR of 15.81 (95%CI: 10.71–20.35), and the overall accuracy is relatively high. Generally, LR is widely used to identify or exclude diagnostic criteria for disease. We found a pooled PLR of 4.51 (95% CI: 3.52–5.78) and a pooled NLR of 0.29 (95% CI: 0.24–0.34).
Furthermore, subgroup analysis found that the different control groups and up and down regulation of miRNAs did not had a great impact on the final results. But it is still necessary to conduct a rationally designed, high-quality, large-sample, prospective long-term follow-up study to accurately reflect the diagnostic efficacy of miRNAs. As a diagnostic test evaluation metric, DOR describes the probability of a positive result in a patient with the disease compared to the outcome in a patient without the disease. In this study, the pooled DOR was 15.81 (95%CI: 10.71–20.35), indicating that the DOR of miRNAs was close to that of traditional markers [Citation30,Citation31].
Early markers for diagnosing neonatal sepsis include PCT, CRP, etc., but these traditional markers lack specificity. The gold standard for diagnosis of this disease is still blood culture. However, the time for culturing bacteria in clinic is often long, and the probability of false negative results is high, leading to delayed diagnosis [Citation32]. Therefore, there is an urgent need for a biomarker in the early diagnosis of neonatal sepsis. It happens that miRNAs have strong specificity and high sensitivity. It is a biomarker for the diagnosis of neonatal sepsis and can be used as a minimally invasive early detection. In addition, some miRNAs can be used for the diagnosis of myocardial damage in children with sepsis. Relevant studies have shown that the diagnostic value of miRNA-497 in plasma for neonatal septicemia myocardial damage is comparable to that of cTnI, which is expected to be a potential marker for early diagnosis of myocardial damage [Citation33]. Yang et al. found that plasma miRNA-1, miRNA-208a, and miRNA-499 can be used as biomarkers for the diagnosis of myocardial damage, providing information for diagnosis other than cTnI [Citation34]. At present, however, the research on miRNA remains limited, and it is believed that miRNA will provide considerable value for the diagnosis of more diseases in the future.
Currently, it is not possible to conduct specific studies on the subtypes of pathogens. However, Abdelaleem et al. have found that the expression of miRNA-34a-5p is significantly downregulated in neonates with sepsis infected with gram negative cocci (Klebsiella, Enterobacter, Acinetobacter). When infected with gram positive cocci, the expression of miRNA-199a-3p was significantly downregulated [Citation17]. Ernst et al. found that miRNA-211-5p/miRNA-142-3p was significantly upregulated in neonates with sepsis when the pathogenic bacteria were Escherichia coli, Group B streptococci, Listeria monocytogenes, and Haemophilus influenzae [Citation28].
Research shows that male infants are more susceptible to infectious diseases than female infants [Citation35]. Rafi’s study showed that males are an independent risk factor for late onset neonatal sepsis [Citation36]. A recent study has also shown that genetic variations associated with the risk of late onset neonatal sepsis are also related to gender [Citation37]. The underlying mechanism of this sexual dimorphism may be related to a variety of factors, including endocrine and genetic effects on the immune system and physiology, as well as gender related behavioral differences [Citation38]. However, in the literature we have included, there is no statistical difference between the sexes of newborns, and there is no gender division when studying miRNA in the article. Therefore, we did not separately analyze the gender of newborns.
This meta-analysis has several limitations. First, in literature retrieval, although we included relevant miRNAs as comprehensively as possible, there are still many miRNAs lacking diagnostic studies, resulting in miRNAs that have not been studied fail to be effectively assessed. Second, this meta-analysis included only Asians, Egyptians and Americans. As a result, the relationship between miRNAs expression and neonatal sepsis in other ethnic groups and regions may require further study and validation. Furthermore, our study included 20 miRNAs, all of which were not reused in individual publications, so we did not analyze the same miRNAs in the study. In the future, a large amount of research is still needed to further explore the diagnostic effect of a single miRNA on diseases.
5. Conclusion
To sum up, our research results suggest that miRNAs have great potential as biomarkers for the diagnosis of neonatal sepsis, which have better application prospects than existing diagnostic methods. However, a large number of clinical studies are required before practical application.
Author contributions
WXH and SBL co-conceptualized and designed the study, performed the initial screening of the articles, performed initial analyses including risk of bias assessment, drafted the manuscript, and provided methodological and statistical expertise in data analyses and interpretation. NW double checked the initial screening of articles and double checked all the data extraction from included studies. XQC devised the search strategy for the systematic review based on study protocol. JJM and JJL assisted in the conduct of the systematic review, provided logistical support, reviewed the study protocol, and helped revise the manuscript. LH and CJR co-conceptualized and designed the study, provided guidance in the conduct of the review and meta-analysis, oversaw the conduct of all meta-analyses, revised the initial manuscript draft, and approved the final version.
Supplementary Materials
Download Zip (103.5 KB)Disclosure statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Data availability statement
The original contributions presented in the study are included in the article/Supplementary Material, further inquiries can be directed to the corresponding authors.
Additional information
Funding
References
- Shane AL, Sánchez PJ, Stoll BJ. Neonatal sepsis. Lancet. 2017;390(10104):1770–1780.
- Cuenca AG, Wynn JL, Moldawer LL, et al. Role of innate immunity in neonatal infection. Am J Perinatol. 2013;30(2):105–112.
- Futata EA, Fusaro AE, de Brito CA, et al. The neonatal immune system: immunomodulation of infections in early life. Expert Rev anti Infect Ther. 2012;10(3):289–298.
- Hansen LW, Jacob A, Yang WL, et al. Deficiency of receptor-interacting protein kinase 3 (RIPK3) attenuates inflammation and organ injury in neonatal sepsis. J Pediatr Surg. 2018;53(9):1699–1705.
- Thaver D, Zaidi AK. Burden of neonatal infections in developing countries: a review of evidence from community-based studies. Pediatr Infect Dis J. 2009;28(1 Suppl):S3–S9.
- Edmond K, Zaidi A. New approaches to preventing, diagnosing, and treating neonatal sepsis. PLoS Med. 2010;7(3):e1000213.
- Cerra FB. The systemic septic response: multiple systems organ failure. Crit Care Clin. 1985;1(3):591–607.
- Glaser MA, Hughes LM, Jnah A, et al. Neonatal sepsis: a review of pathophysiology and current management strategies. Adv Neonatal Care. 2021;21(1):49–60.
- Qiu X, Zhang L, Tong Y, et al. Interleukin-6 for early diagnosis of neonatal sepsis with premature rupture of the membranes: a meta-analysis. Medicine. 2018;97(47):e13146.
- Benz F, Roy S, Trautwein C, et al. Circulating microRNAs as biomarkers for sepsis. Int J Mol Sci. 2016;17(1):78.
- Cui S, Liu L, Wan T, et al. MiR-520b inhibits the development of glioma by directly targeting MBD2. Am J Cancer Res. 2017;7(7):1528–1539.
- Chen M, Wang F, Xia H, et al. MicroRNA-155: regulation of immune cells in sepsis. Mediators Inflamm. 2021;2021:8874854.
- Chen X, Ba Y, Ma L, et al. Characterization of microRNAs in serum: a novel class of biomarkers for diagnosis of cancer and other diseases. Cell Res. 2008;18(10):997–1006.
- Moher D, Liberati A, Tetzlaff J, et al. Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. PLoS Med. 2009;6(7):e1000097.
- Whiting P, Rutjes AW, Reitsma JB, et al. The development of QUADAS: a tool for the quality assessment of studies of diagnostic accuracy included in systematic reviews. BMC Med Res Methodol. 2003;3:25.
- Zhang C, Li X, Liu N, et al. MicroRNA-96 is downregulated in sepsis neonates and attenuates LPS induced inflammatory response by inhibiting IL-16 in monocytes. Comb Chem High Throughput Screen. 2022;25(1):90–96.
- Abdelaleem OO, Mohammed SR, El Sayed HS, et al. Serum miR-34a-5p and miR-199a-3p as new biomarkers of neonatal sepsis. PLoS One. 2022;17(1):e0262339.
- Fouda E, Elrazek Midan DA, Ellaban R, et al. The diagnostic and prognostic role of MiRNA 15b and MiRNA 378a in neonatal sepsis. Biochem Biophys Rep. 2021;26:100988.
- Wang D, Han L. Downregulation of miR-1184 serves as a diagnostic biomarker in neonatal sepsis and regulates LPS-induced inflammatory response by inhibiting IL-16 in monocytes. Exp Ther Med. 2021;21(4):350.
- El-Hefnawy SM, Mostafa RG, El Zayat RS, et al. Biochemical and molecular study on serum miRNA-16a and miRNA- 451 as neonatal sepsis biomarkers. Biochem Biophys Rep. 2021;25:100915.
- Lin X, Wang Y. miR-141 is negatively correlated with TLR4 in neonatal sepsis and regulates LPS-induced inflammatory responses in monocytes. Braz J Med Biol Res. 2021;54(7):e10603.
- Mao YY, Su C, Fang CC, et al. Clinical significance of the serum miR-455-5p expression in patients with neonatal sepsis. Bioengineered. 2021;12(1):4174–4182.
- Li M, Huang X, Zhuo Q, et al. Clinical significance of miR-129-5p in patients with neonatal sepsis and its regulatory role in the LPS-induced inflammatory response. Bosn J Basic Med Sci. 2021;22(2):185–190.
- Liu G, Liu W, Guo J. Clinical significance of miR-181a in patients with neonatal sepsis and its regulatory role in the lipopolysaccharide-induced inflammatory response. Exp Ther Med. 2020;19(3):1977–1983.
- Wang X, Wang X, Liu X, et al. miR-15a/16 are upregulated in the serum of neonatal sepsis patients and inhibit the LPS-induced inflammatory pathway. Int J Clin Exp Med. 2015;8(4):5683–5690.
- Sankar S, Maruthai K, Bobby Z, et al. MicroRNA expression in neonates with late-onset sepsis - a cross-sectional comparative study. Immunol Invest. 2022;14:1–13.
- Zhang J, Xu X, Wang M. Clinical significance of serum miR-101-3p expression in patients with neonatal sepsis. Per Med. 2021;18(6):541–550.
- Ernst LM, Mithal LB, Mestan K, et al. Umbilical cord miRNAs to predict neonatal early onset sepsis. PLoS One. 2021;16(5):e0249548.
- Salim RF, Sobeih AA, Abd El Kareem HM. Evaluation of the clinical value of circulating miR-101, miR-187 and miR-21 in neonatal sepsis diagnosis and prognosis. Egypt J Med Hum Genet. 2020;21(1):12.
- Shabuj KH, Hossain J, Moni SC, et al. C-reactive protein (CRP) as a single biomarker for diagnosis of neonatal sepsis: a comprehensive meta-analysis. Mymensingh Med J. 2017;26(2):364–371.
- Liu Y, Zhao L, Wu Z. Accuracy of C-reactive protein test for neonatal septicemia: a diagnostic meta-analysis. Med Sci Monit. 2019;25:4076–4081.
- Dima M, Iacob D, Marginean O, et al. New emerging biological markers of neonatal sepsis. J Res Med Sci. 2017;22:65.
- Wu ZJ, Chen YF, Wang HD, et al. Expression of plasma miRNA-497 in children with sepsis-induced myocardial injury and its clinical significance. Zhongguo Dang Dai Er Ke Za Zhi. 2018;20(1):32–36.
- Yang W, Shao J, Bai X, et al. Expression of plasma microRNA-1/21/208a/499 in myocardial ischemic reperfusion injury. Cardiology. 2015;130(4):237–241.
- Fish EN. The X-files in immunity: sex-based differences predispose immune responses. Nat Rev Immunol. 2008;8(9):737–744.
- Rafi MA, Miah MMZ, Wadood MA, et al. Risk factors and etiology of neonatal sepsis after hospital delivery: a case-control study in a tertiary care hospital of Rajshahi, Bangladesh. PLoS One. 2020;15(11):e0242275.
- Ciesielski TH, Zhang X, Tacconelli A, et al. Late-onset neonatal sepsis: genetic differences by sex and involvement of the NOTCH pathway. Pediatr Res. 2023;93(4):1085–1095.
- Muenchhoff M, Goulder PJ. Sex differences in pediatric infectious diseases. J Infect Dis. 2014;209(Suppl 3):S120–S126.