Abstract
Objectives
To assess the association between allostatic load in early pregnancy and sleep-disordered breathing (SDB) during pregnancy.
Methods
High allostatic load in the first trimester was defined as ≥ 4 of 12 biomarkers (systolic blood pressure, diastolic blood pressure, body mass index, cholesterol, low-density lipoprotein, high-density lipoprotein, high sensitivity C-reactive protein, triglycerides, insulin, glucose, creatinine, and albumin) in the unfavorable quartile. SDB was objectively measured using the Embletta-Gold device and operationalized as “SDB ever” in early (6–15 weeks) or mid-pregnancy (22–31 weeks); SDB at each time point was analyzed as secondary outcomes. Multivariable logistic regression was used to test the association between high allostatic load and SDB, adjusted for confounders. Moderation and sensitivity analyses were conducted to assess the role of allostatic load in racial disparities of SDB and obesity affected the relationship between allostatic load and SDB.
Results
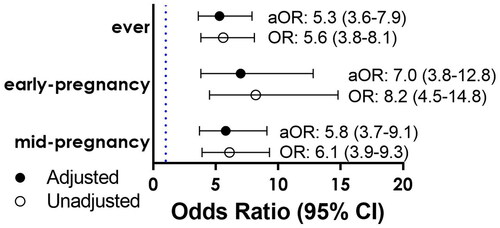
High allostatic load was present in 35.0% of the nuMoM2b cohort. The prevalence of SDB ever occurred among 8.3% during pregnancy. After adjustment, allostatic load remained significantly associated with SDB ever (aOR= 5.3; 3.6–7.9), in early-pregnancy (aOR= 7.0; 3.8–12.8), and in mid-pregnancy (aOR= 5.8; 3.7–9.1). The association between allostatic load and SDB was not significantly different for people with and without obesity. After excluding BMI from the allostatic load score, the association decreased in magnitude (aOR= 2.6; 1.8–3.9).
Conclusion
The association between allostatic load and SDB was independent of confounders including BMI. The complex and likely bidirectional relationship between chronic stress and SDB deserves further study in reducing SDB.
Introduction
Sleep-disordered breathing (SDB) is characterized by abnormal respiratory patterns including abnormal respiratory conditions (e.g. apneas, hypopneas), abnormal gas exchange (e.g. hypoxemia), obstructive sleep apneas (OSA), central sleep apneas, and OSA syndrome, all of which have been associated with poor health outcomes [Citation1,Citation2]. SDB can coexist with systemic inflammation and oxidative stress that leads to increased sympathetic nervous system activity during pregnancy [Citation1,Citation2]. Risk factors for SDB include age, socioeconomic status (SES), obesity, neck circumference, craniofacial abnormalities, chronic hypertension [Citation3], and hypothyroidism [Citation4].
Chronic stress is measured by allostatic load [Citation5], a composite index that measures the physiological response to cumulative “wear and tear” on the body that is endured by continuous response to internal and external stressors [Citation6,Citation7]. Typically, the index is comprised of multiple subclinical biomarkers from physiological systems, including (systolic blood pressure (SBP) diastolic blood pressure (DBP), body mass index (BMI), total cholesterol, high-density lipoprotein (HDL), high sensitivity C-reactive protein (hs-CRP), albumin, and creatinine) that summed into a single continuous index indicating biologic risk [Citation6]. These biomarkers exemplify organ and tissue damage within the endocrine, cardiovascular, metabolic, and immune systems that may shift to dysregulated stress responses, and potentially leading to SDB [Citation6,Citation8]. Allostatic load is deemed useful, as it captures the chronic activation of stress biomarkers as an individual attempts to maintain Allostasis state in the face of stressful challenges [Citation9]. Various definitions of allostatic load have been used, and there is no “gold standard” for its measurement and one definition is not superior to another [Citation10].
It is plausible that SDB, with its negative effects on physiological systems over time, could lead to elevated allostatic load. Limited data exist on whether social determinants of health proxies (i.e. race or ethnicity) and BMI largely or exclusively mediate the relationship between allostatic load and SDB [Citation10]. A study using the same nuMoM2b cohort, found SDB to be higher for non-Hispanic Black compared to non-Hispanic White individuals and that the severity of SDB differed across racial/ethnic groups in early pregnancy with higher apnea-hypopnea index (AHI) in non-Hispanic Black participants [Citation10]. Other studies found that OSA was more prevalent in pregnant people with preterm birth, obesity, and gestational diabetes (GDM) [Citation11–14]. A study examining the potential mediating effects of allostatic load on the association of sleep patterns found that short sleep duration and low sleep quality were associated with adverse kidney outcomes in a non-Hispanic Black cohort, mediated by allostatic load [Citation10].
The primary purpose of this study was to determine whether allostatic load is associated with early- or mid-pregnancy SDB. We propose that a cumulative pathway model addresses the influence of other risk factors and the additive effects of stress. We hypothesize that there may be an effect modification by obesity, race, and or ethnicity in the association between allostatic load and SDB.
Methods
Study population
The Nulliparous Pregnancy Outcomes Study: Monitoring mothers-to-be (nuMoM2b) was a prospective cohort study in which 10,038 nulliparous participants with singleton pregnancies were enrolled between October 2010 and September 2013. Details of the parent nuMoM2b study have been described elsewhere [Citation15]. Only participants enrolled in NuMoM2b-Heart Health (HHS) had biomarker assessment and were included in this study. Further details on HHS have been described elsewhere [Citation16].
A subset of individuals in the parent study participated in the nuMoM2b-SDB ancillary sub-study. Women were excluded from the SDB sub-study for the following conditions: current continuous positive airway pressure (CPAP) treatment for SDB; severe asthma requiring continuous oral steroid therapy for more than 14 days; and conditions requiring oxygen supplementation. Participants participated in a home sleep apnea study test to obtain objective measure of SDB after the first and third study visits. Trained scorers were blinded to all other data while scoring sleep data at a central sleep reading center. The assessment of SDB was performed using the Embletta-Gold device (Embla, Broomfield, CO), a self-administered level 3 in-home sleep apnea test. The sleep monitor assessed SDB by recording nasal airflow wave patterns, respiratory efforts from abdominal and thoracic inductance plethysmography, the level and duration of oxygen desaturation by pulse oximetry, heart rate, and body position. Study participants were instructed to wear the monitor overnight for one night between 6 and 15 weeks of gestation and again for one night between 22 and 31 weeks of pregnancy. Details of the study have been described elsewhere [Citation7,Citation11,Citation15,Citation16].
Study design
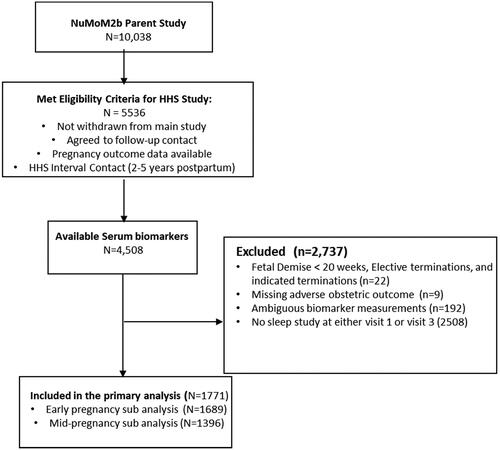
This study was a secondary analysis of the nuMoM2b, nuMoM2b-HHS and nuMoM2b-SDB cohorts and excluded participants who experienced fetal demise < 20 weeks, termination of pregnancy, not enrolled in the SDB study, not enrolled in nuMoM2b-HHS, had missing adverse outcomes or delivery details, or did not have early pregnancy biomarker assessment from parent study samples ().
Allostatic load definition
Allostatic load biomarkers were assayed based on available data from nuMoM2b-HHS from stored urine or serum samples collected during the nuMoM2b parent study in the first trimester [Citation15]. Samples were stored at −80 °C at a central core biorepository. Assays were completed at the HHS core laboratory (Lundquist Institute, Torrance, CA) using standard protocols on a Beckman AU480.
We defined allostatic load using clinically measured biomarkers or indicators from the following physiological pathways: cardiovascular, inflammatory, metabolic, and immunologic [Citation17,Citation18]: BMI (kg/m2); serum-measured (SBP, DBP, cholesterol (mg/dL), LDL (mg/dL), HDL (mg/dL), hs-CRP (mg/dL)); and urine-measured (creatinine (mg/dL) and albumin (mg/dL)). This study’s definition of allostatic load modified the National Health and Nutrition Survey (NHANES) [Citation7] defined set of biomarkers focused on health disparities by adding serum-measured (triglycerides (mg/dL), insulin (ulU/mL), and fasting glucose (mg/dL)) [Citation8].
High allostatic load was defined as four or more out of 12 biomarkers in the “unfavorable” quartile; the “unfavorable” quartile represented those with the lowest values for albumin and HDL and the highest values for the rest of the biomarkers. [Citation19] For each biomarker, values in the “unfavorable” quartile (high risk), received a score of “1.” Values not in the unfavorable quartile (low risk) and received a score of “0.” The total allostatic load index score ranged from 0 to 12. We dichotomized the score into two groups, as previous studies [Citation19] found similar thresholds discriminatory of 3 or greater and 4 or greater [Citation20], and as such low allostatic load was defined as an index score of < 4, and high allostatic load was defined an index score of ≥ 4.
Sleep-disordered breathing
SDB was characterized using the Apnea-Hypopnea Index (AHI), defined as the number of all apneas and hypopnea per hour of estimated sleep, accompanied by ≥3% oxygen desaturation. The threshold of AHI ≥ 5 defined the presence of SDB. SDB was included as three dichotomous variables: (1) present at either evaluation during the pregnancy (SDB ever), (2) SDB at visit 1 early-pregnancy SDB (6–15 weeks), or (3) SDB at visit 3 mid-pregnancy SDB (22–31 weeks); the first was analyzed as primary and the latter two as secondary outcomes.
Risk factors
We considered the following maternal characteristics as risk factors: maternal age, BMI, education, obesity, parity, prior miscarriages, previous bleeding in the first trimester, previous abdominal surgery, neck circumference, craniofacial abnormalities, hypothyroidism, acromegaly, smoking, alcohol use, federal poverty level, and health insurance status.
Self-reported race was used as a social construct and considered as a proxy for social experience, systematic racism, and other unmeasured social determinants of health that potentially manifest through chronic stress. Race included people of self-reported non-Hispanic Black, and non-Hispanic White, Hispanic, Asian, Native American, Native Hawaiian, Multiracial, and additional racial and ethnic groups.
Statistical analysis
Maternal characteristics were summarized by allostatic load and SDB, with differences in proportions using chi-square. To test the association between allostatic load and SDB, odds ratios and adjusted (aORs) with CIs were reported from multivariable logistic regression models adjusted for potential confounding variables chosen either a priori based on reported associations [Citation21–23] and or risk factors with an association of p-value <0.10.
Primary analysis
We tested the association of allostatic load and SDB ever; at either visit (i.e. anytime during the pregnancy), with early-pregnancy SDB and mid-pregnancy SDB analyzed as secondary outcomes. As an exploratory analysis, we modeled each allostatic load component with each time point of SDB to determine which allostatic load components were most associated with SDB; yielding 36 multivariable comparisons, in which adjustments for multiple comparisons were made and analyses were interpreted as exploratory.
Moderation analysis
We tested effect modification by race including an interaction between race (non-Hispanic Black vs. “Non-Hispanic White, Hispanic, Asian, Native American, and Native Hawaiian, multiracial and other racial backgrounds”) and allostatic load. A significant interaction of a p-value <0.05 would demonstrate a difference in the association between allostatic load and SDB for individuals of non-Hispanic Black race versus people of “Non-Hispanic White, Hispanic, Asian, Native American, and Native Hawaiian, multiracial and other racial backgrounds” (i.e. moderation). We then conducted a sensitivity analysis of the moderation analysis, limiting the analytical population to individuals from non-Hispanic Black and non-Hispanic White groups.
Sensitivity analysis: removing BMI from the allostatic load definition
To assess whether the relationship between allostatic load and SDB is primarily driven by BMI, we excluded BMI from the allostatic load score index. We then tested whether obesity moderates the relationship between allostatic load and SDB using multivariable logistic regression. A significant interaction of a p-value <0.05 demonstrates a difference in the association between high allostatic load and SDB for individuals with obesity (i.e. effect modification). This was repeated for secondary SDB outcomes. We then adjusted for BMI and potential confounders included in the primary analysis.
Primary comparisons are interpreted at α = 0.05. For secondary comparisons, we adjust for the two secondary outcomes and 12 exposures, yielding the Bonferroni adjusted α = 0.002. All tests performed were 2-sided. Data analyses were conducted using SAS 9.4 software (SAS Institute Inc., Cary, NC).
Results
Primary analysis results
A total of 10,038 were enrolled in the nuMoM2b study, of which 3705 individuals were enrolled in the SDB ancillary sub-study. Baseline characteristics were similar between nuMoM2b individuals who did and did not participate in the SDB ancillary study [Citation7,Citation11,Citation15,Citation16]. In the primary analysis, we included 1771 individuals who met all inclusion criteria (). High allostatic load was present in 35.0% of the cohort. Maternal age, preexisting diabetes, and chronic hypertension were associated with high allostatic load ().
Table 1. Demographic and clinical characteristics between participants with high allostatic and low allostatic loads in pregnancy.
SDB ever occurred in 8.3% (n = 147/1771) individuals; 70/1683(4.2%) had SDB in early-pregnancy, 115/1390 (8.3%) had SDB in mid-pregnancy, and 38/1771 (2.1%) had SDB in both early and mid-pregnancy. Baseline demographic characteristics in association between SDB ever, early-pregnancy, and mid-pregnancy were reported in (Supplemental Table 1), (Supplemental Table 2), (Supplemental Table 3), respectively.
SDB ever occurred more frequently in people with high compared to low allostatic load (17.1%, n = 105, vs 3.6% n = 54, p-value <0.001). Among people with high allostatic load, early-pregnancy SDB occurred in 9.5% (n = 56), vs. 1.3% (n = 14); p-value <0.001. Among people with high allostatic load, SDB at mid-pregnancy SDB occurred at 17.6% (n = 84) vs 3.4% (n = 31): p-value <0.001. High allostatic load was significantly associated with SDB ever, early-pregnancy SDB, and mid-pregnancy SDB. The associations between allostatic load and SDB ever, early-pregnancy SDB, and mid-pregnancy SDB were only slightly attenuated but remained strong after adjustment for maternal age, smoking status, gravidity, smoking status, alcohol use, poverty level, preexisting diabetes, chronic hypertension, and government health insurance status ().
Figure 2. Adjusted logistic regression estimating the association between sleep-disordered breathing and allostatic load. Abbreviations: Ever, (SDB ever); CI confidence interval. *Adjusted Odds ratios and CIs were reported using unconditional logistic regression after adjusting for maternal age, education level, gravida, smoking status, health insurance status, preexisting diabetes, and chronic hypertension.
In exploratory analyses, SDB ever, early-pregnancy SDB, and mid-pregnancy SDB were significantly associated with BMI, DBP, SBP, hsCRP, HDL, and LDL. Glucose, insulin, creatinine, and triglycerides (Supplementary Table 4).
Moderation analysis results
The association of allostatic load and each SDB outcome was not significantly different comparing non-Hispanic Black to all other races (“Non-Hispanic White, Hispanic, Asian, Native American, Native Hawaiian, multiracial and other racial backgrounds”) (Supplemental Table 5). When restricting the analysis to people of non-Hispanic Black or non-Hispanic White race, the association of allostatic load and SDB ever, early-pregnancy SDB remained not significant (Supplemental Table 6).
Sensitivity analysis results: removing BMI from the allostatic load definition
The association between allostatic load and SDB was not significantly different for people with and without obesity when we excluded BMI from the allostatic load (i.e. moderation) (). Obesity, along with confounders from the primary analysis were then included in a model assessing allostatic load and SDB as confounding covariates. Results were smaller in magnitude compared to the primary analysis; however, high allostatic load remained statistically associated with SDB ever, early-pregnancy SDB and mid-pregnancy SDB ().
Table 2. Moderation of obesity in the association between sleep-disordered breathing outcomes and high allostatic load (≥ 4), unadjusted and adjusted logistic regression model estimates.
Table 3. Adjusted logistic regression estimating the association between sleep-disordered breathing and allostatic load.
In a sensitivity moderation analysis where we excluded BMI from the allostatic load score, results remained similar for SDB ever and early-pregnancy SDB. When BMI was excluded from the allostatic load, the interaction between race and allostatic load was no longer significant (data not shown).
Discussion
This study found that a high allostatic load during early-pregnancy was associated with increased odds for SDB ever, in early-pregnancy, and mid-pregnancy. After adjusting for confounders and excluding BMI from the allostatic load score, the relationship between high allostatic load and SDB had smaller associations but remained significant. Our findings are consistent with studies examining the relationship between allostatic load and SDB, which have reported associations of high AL with sleep apnea, sleep apnea symptoms, insomnia component, short sleep duration, and diagnosed sleep disorder [Citation24]. The components of allostatic load most strongly associated with SDB ever, early-pregnancy SDB and mid-pregnancy SDB were BMI, DBP, SBP, hs-CRP, HDL, and LDL. Glucose, insulin creatinine, and triglycerides were associated with SDB ever and mid-pregnancy.
These findings support growing evidence that cumulative stress is associated with SDB. One review found that a higher incidence of pregnancy-related SDB involving a recurrent episodic partial or complete cessation of breathing at night impacted maternal-fetal outcomes [Citation25]. Others proposed that poor sleep quality acts as a physiologic stressor that may impair neurophysiological function leading to allostatic load changes by increasing inflammation, oxidative stress, cortisol, and insulin [Citation26].
Another study demonstrated an association between allostatic load and SES and sleep quality [Citation17]. A study using NHANES data demonstrated that lower SES was associated with higher allostatic load [Citation27]. Chronic stress is believed to be a risk factor shared by both sleep and allostatic load and as such, some researchers have considered sleep as a component of allostatic load. Prolonged SDB-associated chronic stress may contribute to allostatic load, and in turn high allostatic load might contribute to sleep-related disturbances. There may be a bi-directional association between allostatic load and SDB [Citation24–26].
Allostatic load is a potential pathway to explain some health disparities because stressors due to structural racism, such as poverty, educational disadvantage, and perceived stress are more prevalent in Black individuals [Citation28]. A “moderation” in effect may be due to social factors that accumulate based on racism that have a complex interplay in the relationship of race, allostatic load, and SDB, but we did not observe race to significantly moderate the relationship between allostatic load and SDB ever, mid-pregnancy and early-pregnancy SDB.
Our study has several limitations. We only evaluated allostatic load during the first trimester. In addition, we only used a 12-factor allostatic load index and were unable to assess other biomarkers (i.e. C-peptide insulin) [Citation29,Citation30]. We did not assess the severity of SDB due to small sample size. Our cohort also lacked generalizability since it was limited to nulliparous participants who could access tertiary medical care centers and had the means to participate in a complex longitudinal study. We did not pre-pregnancy BMI owing to self-report, which may have introduced inaccuracy. However, we had objectively measured BMI in early pregnancy which was accurate and close to pre-pregnancy BMI. We were unable to adjust for the presence of craniofacial abnormalities or acromegaly, insomnia, and or depression/anxiety. We were unable to measure sleep quality or cortical arousals (and hypopneas associated with these arousals), which in turn have implications for chronic stress. We might be missing pregnant individuals with upper airway resistance syndrome, who may also have higher levels of sympathetic activation and inflammatory biomarkers. Finally, it is important to note that the relationship between allostatic load and SDB is complex and likely bidirectional.
Our study also had major strengths. This was a large, well-characterized prospective cohort with standardized data collection; these characteristics limited bias and enhanced accuracy. Our prospective study design limited bias since other studies used cross-sectional or retrospective designs. Our objective measures of SDB using the Embletta-Gold device were a strength. Each allostatic load biomarker component was weighted equally, a scientifically sound approach. Evidence suggests no significant difference between empirical and clinical cutoff assessments [Citation5,Citation31]. Our population was geographically, racially, and ethnically diverse.
The association between allostatic load and SDB was independent of critical confounders such as BMI. The association of allostatic load and mid-pregnancy SDB was significantly different by race. The complex and likely bidirectional relationship between chronic stress and SDB deserves further study in hopes of reducing SDB. Allostatic load is a potential risk reduction target for individuals with SDB.
Supplemental Material
Download MS Word (34.8 KB)Disclosure statement
No potential conflict of interest was reported by the author(s).
Data availability statement
Data sharing is not applicable to this article as no new data were created or analyzed in this study.
Additional information
Funding
References
- Ayyar L, Shaib F, Guntupalli K. Sleep-disordered breathing in pregnancy. Sleep Med Clin. 2018;13(3):1–8. doi: 10.1016/j.jsmc.2018.04.005.
- Memon J, Manganaro SN. Obstructive sleep-disordered breathing. In: StatPearls. Treasure Island (FL): StatPearls Publishing; 2022.
- Facco FL, Chan M, Patel SR. Common sleep disorders in pregnancy. Obstet Gynecol. 2022;140(2):321–339. doi: 10.1097/AOG.0000000000004866.
- Kapur VK, Koepsell TD, deMaine J, et al. Association of hypothyroidism and obstructive sleep apnea. Am J Respir Crit Care Med. 1998;158(5 Pt 1):1379–1383. doi: 10.1164/ajrccm.158.5.9712069.
- McEwen BS. Stress, adaptation, and disease. Allostasis and allostatic load. Ann N Y Acad Sci. 1998;840(1):33–44. doi: 10.1111/j.1749-6632.1998.tb09546.x.
- Carbone JT, Clift J, Alexander N. Measuring allostatic load: approaches and limitations to algorithm creation. J Psychosom Res. 2022;163:111050. doi: 10.1016/j.jpsychores.2022.111050.
- Haas DM, Ehrenthal DB, Koch MA, et al. Pregnancy as a window to future cardiovascular health: design and implementation of the nuMoM2b heart health study. Am J Epidemiol. 2016;183(6):519–530. doi: 10.1093/aje/kwv309.
- Viljoen M, Claassen N. Allostatic load and heart rate variability as health risk indicators. Afr Health Sci. 2017;17(2):428–435. doi: 10.4314/ahs.v17i2.17.
- Bobba-Alves N, Juster RP, Picard M. The energetic cost of allostasis and allostatic load [published online ahead of print, 2022 Oct 8]. Psychoneuroendocrinology. 2022;146:105951. doi: 10.1016/j.psyneuen.2022.105951.
- Murkey JA, Watkins BX, Vieira D, et al. Disparities in allostatic load, telomere length and chronic stress burden among African American adults: a systematic review. Psychoneuroendocrinology. 2022;140:105730. doi: 10.1016/j.psyneuen.2022.105730.
- Facco FL, Parker CB, Reddy UM, et al. NuMoM2b sleep-disordered breathing study: objectives and methods. Am J Obstet Gynecol. 2015;212(4):542.e1–542.e127. doi: 10.1016/j.ajog.2015.01.021.
- Mullington JM, Haack M, Toth M, et al. Cardiovascular, inflammatory, and metabolic consequences of sleep deprivation. Prog Cardiovasc Dis. 2009;51(4):294–302. doi: 10.1016/j.pcad.2008.10.003.
- Zee PC, Turek FW. Sleep and health: everywhere and in both directions. Arch Intern Med. 2006;166(16):1686–1688. doi: 10.1001/archinte.166.16.1686.
- Reutrakul S, Chen H, Chirakalwasan N, et al. Metabolomic profile associated with obstructive sleep apnoea severity in obese pregnant women with gestational diabetes mellitus: a pilot study. J Sleep Res. 2021;30(5):e13327. doi: 10.1111/jsr.13327.
- Lucchini M, Rayport Y, Valeri L, et al. Racial/ethnic disparities in sleep-disordered breathing during pregnancy in the nuMoM2b study. Obesity (Silver Spring). 2023;31(4):923–933. doi: 10.1002/oby.23697.
- Haas DM, Parker CB, Wing DA, et al. A description of the methods of the Nulliparous Pregnancy Outcomes Study: monitoring mothers-to-be (nuMoM2b). Am J Obstet Gynecol. 2015;212(4):539.e1–539.e24. doi: 10.1016/j.ajog.2015.01.019.
- Gruenewald TL, Karlamangla AS, Hu P, et al. History of socioeconomic disadvantage and allostatic load in later life. Soc Sci Med. 2012;74(1):75–83. doi: 10.1016/j.socscimed.2011.09.037.
- Geronimus AT, Hicken M, Keene D, et al. “Weathering” and age patterns of allostatic load scores among blacks and whites in the United States. Am J Public Health. 2006;96(5):826–833. doi: 10.2105/AJPH.2004.060749.
- Frei R, Haile SR, Mutsch M, et al. Relationship of serum vitamin D concentrations and allostatic load as a measure of cumulative biological risk among the US population: a cross-sectional study. PLoS One. 2015;10(10):e0139217. doi: 10.1371/journal.pone.0139217.
- Lueth AJ, Allshouse AA, Blue NM, et al. Allostatic load and adverse pregnancy outcomes. Obstet Gynecol. 2022;140(6):974–982. doi: 10.1097/AOG.0000000000004971.
- Paul K, Boutain D, Agnew K, et al. The relationship between racial identity, income, stress and C-reactive protein among parous women: implications for preterm birth disparity research. J Natl Med Assoc. 2008;100(5):540–546. doi: 10.1016/s0027-9684(15)31300-6.
- Räisänen S, Lehto SM, Nielsen HS, et al. Risk factors for and perinatal outcomes of major depression during pregnancy: a population-based analysis during 2002-2010 in Finland. BMJ Open. 2014;4(11):e004883. Published 2014 Nov 14. doi: 10.1136/bmjopen-2014-004883.
- Dunkel Schetter C, Tanner L. Anxiety, depression and stress in pregnancy: implications for mothers, children, research, and practice. Curr Opin Psychiatry. 2012;25(2):141–148. doi: 10.1097/YCO.0b013e3283503680.
- Chen X, Redline S, Shields AE, et al. Associations of allostatic load with sleep apnea, insomnia, short sleep duration, and other sleep disturbances: findings from the National Health and Nutrition Examination Survey 2005 to 2008. Ann Epidemiol. 2014;24(8):612–619. doi: 10.1016/j.annepidem.2014.05.014.
- Pien GW, Schwab RJ. Sleep disorders during pregnancy. Sleep. 2004;27(7):1405–1417. doi: 10.1093/sleep/27.7.1405.
- Tremblay A, Chaput JP. Obesity: the allostatic load of weight loss dieting. Physiol Behav. 2012;106(1):16–21. doi: 10.1016/j.physbeh.2011.05.020.
- Bird CE, Seeman T, Escarce JJ, et al. Neighbourhood socioeconomic status and biological ‘wear and tear’ in a nationally representative sample of US adults. J Epidemiol Community Health. 2010;64(10):860–865. doi: 10.1136/jech.2008.084814.
- Wallace ME, Harville EW. Allostatic load and birth outcomes among white and black women in New Orleans. Matern Child Health J. 2012;17(6):1025–1029. doi: 10.1007/s10995-012-1083-y.
- Morrison S, Shenassa ED, Mendola P, et al. Allostatic load may not be associated with chronic stress in pregnant women, NHANES 1999–2006. Ann Epidemiol. 2013;23(5):294–297. doi: 10.1016/j.annepidem.2013.03.006.
- Li Y, Dalton VK, Lee SJ, et al. Exploring the validity of allostatic load in pregnant women. Midwifery. 2020;82:102621. doi: 10.1016/j.midw.2019.102621.
- Hux VJ, Roberts JM, Okun ML. Allostatic load in early pregnancy is associated with poor sleep quality. Sleep Med. 2017;33:85–90. doi: 10.1016/j.sleep.2016.09.001.