Abstract
Objective
To estimate the incidence of uterine rupture in the Netherlands and evaluate risk indicators prelabour and during labor of women with adverse maternal and/or perinatal outcome.
Methods
This is a population-based nationwide study using the Netherlands Obstetrics Surveillance System (NethOSS). We performed a two-year registration of pregnant women with uterine rupture. The first year of registration included both women with complete uterine rupture and women with incomplete (peritoneum intact) uterine rupture. The second year of registration included women with uterine rupture with adverse maternal and/or perinatal outcome. We collected maternal and obstetric characteristics, clinical signs, and symptoms during labor and CTG abnormalities. The main outcome measures were incidence of complete uterine rupture and uterine rupture with adverse outcome and adverse outcome defined as major obstetric hemorrhage, hysterectomy, embolization, perinatal asphyxia and/or (neonatal) intensive care unit admission.
Results
We registered 41 women with a complete uterine rupture (incidence: 2.5 per 10,000 births) and 35 women with uterine rupture with adverse outcome (incidence: 0.9 per 10,000 births). No adverse outcomes were found among women with incomplete uterine rupture. Risk indicators for adverse outcome included previous cesarean section, higher maternal age, gestational age <37 weeks, augmentation of labor, migration background from Sub-Saharan Africa or Asia. Compared to women with uterine rupture without adverse outcomes, women with adverse outcome more often expressed warning symptoms during labor such as abdominal pain (OR 3.34, 95%CI 1.26-8.90) and CTG abnormalities (OR 9.94, 95%CI 2.17-45.65). These symptoms were present most often 20 to 60 min prior to birth.
Conclusion
Uterine rupture is a rare condition for which several risk indicators were identified. Maternal symptoms and CTG abnormalities are associated with adverse outcomes and time dependent. Further analysis could provide guidance to expedite delivery.
Introduction
Uterine rupture is a rare obstetric complication characterized by disruption of two or three layers of the uterine wall. It is more common after previous uterine surgery, such as previous cesarean section, and is associated with severe adverse maternal and perinatal outcomes. Examples are postpartum hemorrhage, peripartum hysterectomy and perinatal asphyxia and mortality [Citation1–7]. The incidence of uterine rupture varies between high-income countries, ranging from 1.6 to 7.8 per 10,000 births [Citation6]. In countries with a higher TOLAC rate the incidence of uterine rupture is higher, but the cesarean section rate in the general pregnant population generally lower. Cesarean sections, and particularly repeated cesarean sections, can result in short-term and long-term risks for woman and baby [Citation8,Citation9]. Therefore, from a public health perspective, maternity care should aim to reduce the incidence of severe adverse outcomes of uterine rupture without increasing the cesarean section rate [Citation10].
To achieve this, risk indicators before and during labor could be used to predict the probability of uterine rupture. The following prelabour risk indicators were identified: higher maternal age, small interval between subsequent births, induction of labor, epidural anesthesia, higher body mass index (BMI) and higher birth weight [Citation1,Citation4,Citation11–14]. These may aid the counseling of women on mode of birth after previous cesarean section, which currently focuses only on the probability of successful vaginal birth in the Netherlands.
Risk indicators during labor include maternal symptoms such as abdominal pain and abnormalities of the fetal heart rate on the cardiotocogram (CTG) [Citation3,Citation15–17]. These are often nonspecific, and some symptoms may be considered part of the normal physiology of birth. As a result, risk indicators during labor can be difficult to recognize and hinder rapid decision-making. Outcomes of both woman and child are directly related to decision-to-incision time, and the risk of adverse outcomes increases with every minute delay in this time interval [Citation18].
This study analyzed adverse maternal and/or perinatal outcomes in women with complete (myometrium and peritoneum) and incomplete (myometrium with intact peritoneum) uterine rupture. Our primary aim was to evaluate incidence, risk indicators prelabour and during labor of women with uterine rupture and adverse maternal and/or perinatal outcomes. Secondary aims were to a) analyze maternal and perinatal outcomes of complete uterine rupture; b) assess the incidence of women with complete uterine rupture and of women with uterine rupture and adverse maternal and/or perinatal outcomes.
Methods
We performed a multi-centre nationwide study of uterine rupture. We identified cases through the Netherlands Obstetric Surveillance System (NethOSS), a registration system for adverse maternal morbidity and mortality in the Netherlands. We sent a monthly email using the NethOSS to an assigned reporting maternity care giver (physician or research midwife) in every hospital with an obstetrician-led maternity unit in the Netherlands (2016-2018, N = 86). We asked to report the number of cases of uterine rupture that had occurred in the past month or to respond with zero if they had nothing to report. If we received notification of a case of uterine rupture, we requested anonymized copies of the patient’s medical files to be sent in. We specifically asked for information on general history, obstetric history, current pregnancy, labor management, and maternal and perinatal outcomes.
We included cases during a two-year registration period from 1 April 2016 to 31 March 2018. In the first year (Period Citation1: 1 April 2016 − 31 March 2017), we included all women with a uterine rupture, both complete (myometrium and peritoneum) and incomplete (myometrium with intact peritoneum) uterine rupture. In the second year (Period Citation2: 1 April 2017 − 31 March 2018), we included women with uterine rupture, both complete and incomplete, but only in case of adverse maternal and/or perinatal outcomes. To focus on the uterine ruptures of highest clinical relevance, we compared women with uterine rupture with adverse maternal and/or perinatal outcomes (“uterine rupture with adverse outcomes”) with women with uterine rupture without adverse maternal and/or perinatal outcomes (“uterine rupture without adverse outcomes”).
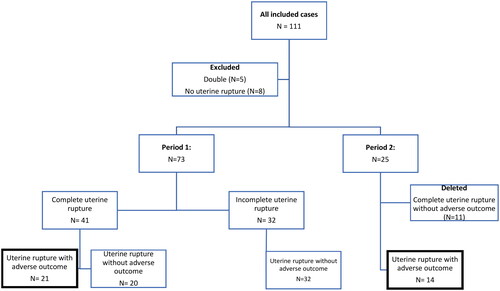
The criteria for adverse maternal outcome were major obstetric hemorrhage (MOH) (>1000 mL blood loss), hysterectomy, embolization and/or intensive care unit (ICU) admission. The criteria for adverse perinatal outcome were perinatal asphyxia and/or admission to a neonatal intensive care unit (NICU) or neonatology ward due to convulsions, hypotonia, ischemia with ultrasonic abnormalities, hypoxic ischemic encephalopathy and/or multiple organ failure. We defined perinatal asphyxia as arterial umbilical cord pH <7.0, base deficit >16mmol/l, Apgar scores (AS) ≤5 at 5 min, resuscitation, or artificial ventilation for >10 min after birth, Thompson score >7 or Sarnat score >1. A flowchart of the registration process is shown in .
Figure 1. Flowchart of included women with uterine rupture.
Period 1. All women with uterine rupture, both complete (myometrium and peritoneum) and incomplete (myometrium with intact peritoneum) uterine rupture
Period 2. Women with uterine rupture, both complete and incomplete, but only in case of adverse maternal and/or perinatal outcomes
Complete uterine rupture was defined as uterine rupture of both the myometrium and the peritoneum. Complete uterine rupture includes women with and without adverse outcomes. We calculated rates of maternal and perinatal outcomes and incidence of complete uterine rupture using data from Period Citation1.
We compared women with uterine rupture with adverse maternal and/or perinatal outcomes with two groups. We created the first group, a general reference group of pregnant women, using all pregnant women in 2016 collected by the Dutch Perinatal Registry (Perined). This first group was used to calculate incidence and evaluate risk indicators. The Perined registry contains population-based information of up to 99% of all pregnancies in the Netherlands [Citation19]. Of the 166,694 births in 2016, 14,789 women had a history of a previous cesarean section, of whom 7,201 women underwent TOLAC. Of the 165,628 births in 2017, 14,393 women had a history of previous cesarean section, of whom 6,813 underwent TOLAC. We calculated the incidence of uterine rupture with adverse outcomes separately for Periods Citation1 and Citation2.
We created the second group, women with uterine rupture without adverse outcomes, using the NethOSS cohort of all included women with uterine rupture. This second group was used to evaluate risk indicators and signs and symptoms of women with uterine rupture with adverse outcomes. We performed a nested case control study. The nested case control study compared women with uterine rupture with adverse outcomes (N = 35) to women with uterine rupture without adverse outcomes (N = 63). We evaluated signs and symptoms by comparing the reported maternal signs and symptoms, and the fetal heart rate in the two hours prior to birth of women with uterine rupture with adverse outcomes and women with uterine rupture without adverse outcomes.
We included several outcome measures for analysis: total blood loss, hospital and/or ICU admission, medical and/or surgical therapies and perinatal characteristics (birth weight, Apgar scores). We subdivided women into one of five groups based on their BMI: underweight (<18.5 kg/m2), normal (18.5-24.9 kg/m2), overweight (25.0-29.9 kg/m2), obese (30.0-34.9 kg/m2) and morbidly obese (>35.0 kg/m2) [Citation20]. Country of origin was based on the definition of Statistics Netherlands. We defined the geographical ethnic origin of a woman by the geographical origin of her parents. We used “mixed” if the parents were not from the same geographical origin. Interval between births was the time between the date of previous birth and current birth rounded to months. Analgesia during labor was divided into epidural, oral or intravenous opioids or no analgesia. If a patient received both epidural and opioids, we included her in both categories. Maternal symptoms of uterine rupture were abdominal pain, pain in the area of the cesarean section scar, uterine hypertonia, acute loss of contractions, abnormal vaginal blood loss, and hematuria. We distinguished abdominal pain from uterine contractions by the presence of abdominal pain in between contractions. We based the analysis of these symptoms on the notes of the physician, midwife, or nurse present during labor.
Fetal characteristics were based on fetal monitoring through CTG. Two researchers (EO and NH) independently analyzed the CTG according to the FIGO guidelines to identify fetal heart rate (FHR) abnormalities [Citation21]. The researchers compared their analyses and discussed any disagreements to reach a consensus. FHR abnormalities included complicated decelerations, uncomplicated decelerations >60 beats, uncomplicated decelerations <60 beats, fetal tachycardia (>160 bpm), fetal bradycardia (<110 bpm), loss of variability and loss of accelerations. If CTG tracings were missing or unavailable, we identified abnormalities based on the clinician’s notes. We analyzed the course of clinical symptoms and CTG tracings across time in intervals of 20 min, starting from two hours up until birth. If a pregnant woman arrived at the hospital within two hours before birth, CTG tracings were included from the time of arrival at the hospital.
Statistical analyses were carried out using IBM SPSS 25 (SPSS Inc., Chicago, IL, USA). We performed descriptive analyses. Proportions are presented as percentages and skewed distributions as medians with interquartile ranges (IQR). Unadjusted odds ratios (ORs) with 95% confidence intervals (95% CI) were calculated in univariate analysis. Differences were compared for statistical significance using a Chi Square test.
Results
Incidence
We registered 111 women in the two-year study period, of whom 13 were excluded because they were registered double (N = 5) or did not have uterine rupture (N = 8). In total, 98 women were included for analysis. presents an overview of the included cases. In total, we included 55 women with complete uterine rupture: 20 women had a complete uterine rupture without adverse outcomes and 35 had a uterine rupture with adverse outcomes. We included 32 women with incomplete uterine rupture, none of whom had adverse outcomes. We included 21 women with uterine rupture with adverse outcomes in period 1 and 14 with uterine rupture with adverse outcome in Period Citation2. The incidence was calculated based on the annual number of women who had given birth and the annual number of women who underwent TOLAC as reported by Perined. The overall incidence of complete uterine rupture in Period Citation1 was 2.5 per 10,000 births and 5.7 per 1,000 women who underwent TOLAC. The incidence of uterine rupture with adverse outcome in Period Citation1 was 1.1 per 10,000 births and 2.9 per 1,000 women who underwent TOLAC. The incidence of uterine rupture with adverse outcome in Period Citation2 was 0.7 per 10,000 births and 2.1 per 1,000 women who underwent TOLAC.
Pre-labor risk indicators for uterine rupture with adverse outcome
presents the characteristics of all women who had uterine rupture with adverse outcome compared to the general pregnant population of 2016. Significant pre-labor risk indicators of uterine rupture with adverse outcome were previous cesarean section, age above 35 years, migration background from Sub-Saharan Africa or Asia, gestational age <37 weeks, and augmentation of labor. The risk of uterine rupture with adverse outcomes was lower for women with two or more previous births, age below 35 years, Western country of origin and women at term.
Table 1. Characteristics of pregnant women with uterine rupture with adverse outcomes.
Risk indicators during labor for uterine rupture with adverse outcome
presents the risk indicators during labor of women in Period Citation1 who had uterine rupture with adverse outcome compared to women who had uterine rupture without adverse outcome. Clinicians reported maternal symptoms significantly more often in women with uterine rupture with adverse outcome compared to women with uterine rupture without adverse outcomes (OR 3.34, 95%CI 1.26-8.90). Out of all maternal symptoms, abdominal pain was most strongly associated with adverse outcome (OR 5.00, 95%CI 1.85-13.48). Clinicians registered maternal symptoms in 25 women (25/35, 74%) with uterine rupture with adverse outcomes, of whom 20 women (20/25, 80%) presented with a combination of symptoms. Only one woman (1/35, 3%) presented with acute loss of contractions. CTG abnormalities were more common for women with uterine rupture with adverse outcome (OR 9.94, 95%CI 2.17-45.65), and the type of CTG abnormality most often found was bradycardia (OR 5.42, 95%CI 2.09-14.08). Two women with adverse outcome (2/35, 6%) were not monitored during labor. One woman was transferred from another hospital and immediately went for surgery, and one woman gave birth after antepartum death at 21 weeks’ gestational age.
Table 2. Risk indicators during labor for adverse outcomes.
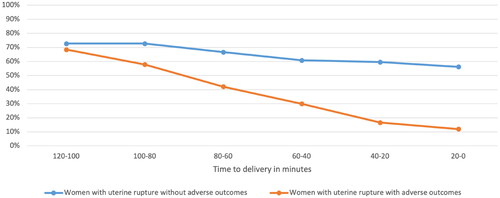
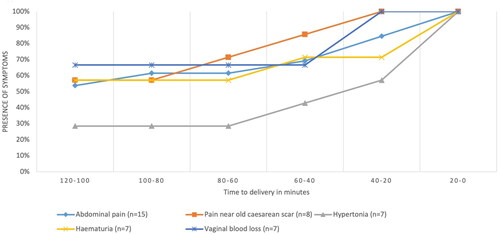
shows the course of all symptoms combined over time for women with uterine rupture with and without adverse outcomes. The median time between start of complaints and time of birth was 63 min (range 34-294 min). In twelve women (12/98, 12%) symptoms were present more than two hours prior to birth. Symptoms were expressed more often within 60 min prior to birth. shows the course of each symptom within two hours prior to birth in women with uterine rupture with adverse outcomes. Abdominal pain was the most common symptom, while pain in the area of the old cesarean scar was a relatively early symptom.
Figure 2. Maternal symptoms of women with uterine rupture with and without adverse outcomes in the two hours prior to birth.
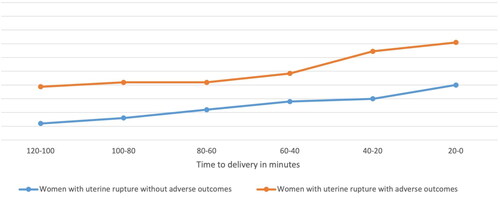
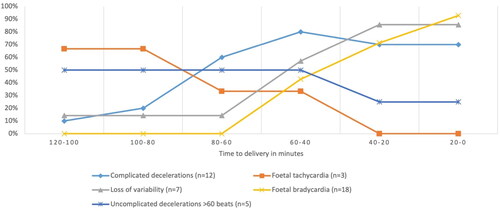
Figure 3. Maternal symptoms present in women with uterine rupture with adverse outcomes in the two hours prior to birth.
and present the CTG assessment over time in the two hours prior to birth. shows that the percentage of women with a normal CTG was lower in women with uterine rupture with adverse outcomes compared to women with uterine rupture without adverse outcomes. The difference between the two groups increases over time. The results in , which suggests that in the hour preceding birth, the presence of fetal bradycardia and loss of variability increases whereas fetal tachycardia and uncomplicated decelerations decreases.
Adverse maternal and perinatal outcomes of complete uterine rupture
We analyzed the presence of adverse maternal and perinatal outcomes after complete uterine rupture. summarizes adverse maternal and perinatal outcomes for the 41 women who had a complete uterine rupture in Period Citation1. None of the women included in this study died. We found adverse maternal and/or perinatal outcomes in 22 women (54%). Adverse maternal outcome occurred in 14 women (33%), adverse perinatal outcomes in 16 women (38%) and of these women we found maternal and perinatal morbidity in 8 women (19%). Maternal morbidity was caused by major obstetric hemorrhage in all these women (14/41, 33%), one of whom had to be admitted to the ICU (1/41, 2%). Perinatal mortality occurred in three fetuses (3/41, 5%). One fetus died antepartum at 21 weeks of gestation, and two neonates died peripartum after term pregnancies. Two neonates with AS ≤5 at 5 min were not admitted to the NICU, but instead remained at the labor ward; one because of an extreme preterm birth <24 weeks and one due to a diagnosis of a chromosomal anomaly with expectant management.
Table 3. Maternal and perinatal adverse outcomes after uterine rupture.
Discussion
Main findings
This study identified the incidence and risk indicators pre-labor and during labor of women with uterine rupture with adverse outcomes in the Netherlands. The incidence of complete uterine rupture was 2.5 per 10,000 births. The incidence of uterine rupture with adverse outcomes was 0.9 per 10,000 births. Incomplete uterine rupture did not lead to adverse events and was therefore not considered clinically relevant. The risk of uterine rupture with adverse outcome increased for women with a previous cesarean section, maternal age above 35 years, migrant background from Sub-Saharan Africa or Asia, gestational age <37 weeks, and augmentation of labor. The risk of uterine rupture with adverse outcome decreased for women with two or more previous vaginal births, maternal age below 35 years, non-migrant background, and term pregnancies. We found an increased risk of adverse outcome if any maternal symptoms were reported and especially if abdominal pain was reported. We also found an increased risk of adverse outcome with an abnormal CTG and especially with fetal bradycardia. We reported no maternal and three perinatal deaths, two of which were a direct result of the uterine rupture. Of all women with complete uterine rupture considerable proportions had adverse maternal and/or perinatal outcome.
Interpretation
The global incidence of uterine rupture varies depending on the type of study and country. In previous research, the overall incidence in high-income countries was 3.3 (range 3.1-3.5) per 10 000 births [Citation6]. The incidence of complete uterine rupture in the Netherlands in the time period 2004 – 2006 was 5.9 (range 5.1-6.7) per 10 000 births [Citation4]. In our study, the incidence of complete uterine rupture was lower than the previously reported incidence, which may be explained by a lower TOLAC rate, which went down from 66% in 2009 to 51% in 2019 [Citation4,Citation7]. In addition to the decline in TOLAC rates, Perined reports a small increase in the percentage of cesarean sections after onset of labor for women who underwent TOLAC: 27% in 2009 to 29% in 2019. This could represent increased alertness for signs of uterine rupture during labor.
Our study specifically focused on women with uterine rupture in whom maternal and perinatal morbidity and mortality were found. The rate of hysterectomy, major obstetric hemorrhage and maternal and perinatal mortality in our study are comparable or even lower than previous findings [Citation1,Citation4,Citation7,Citation13,Citation17,Citation18]. Adverse outcomes occurred in 54% of women with complete uterine rupture. This should be incorporated in the counseling of women with previous cesarean section, which currently often focuses on incidence of complete uterine rupture.
An unscarred uterus, older maternal age, parity ≥3 and short inter-delivery interval are linked to a higher risk of adverse outcomes after uterine rupture [Citation5,Citation13,Citation14,Citation22]. Short delivery interval did not lead to significantly more adverse outcomes in our study. Since all women with uterine rupture and adverse outcomes had a previous cesarean section we were unable to analyze uterine rupture in the unscarred uterus. Augmentation of labor using oxytocin significantly increased the risk of uterine rupture with adverse outcomes. We noticed large inter-institutional differences in dosage of oxytocin used for augmentation of labor but were unable to evaluate how dosage of oxytocin affected the development of uterine rupture due to the large variety in dosage of oxytocin reported. The effect of dosage of oxytocin has also remained unclear in previous studies [Citation23].
Adverse outcomes of uterine rupture often develop after women have been admitted to hospital and fetal heart rate monitoring has started. An important focus has been on recognizing signs and symptoms preceding uterine rupture by the midwife or obstetrician involved. Professionals can even be held responsible for missing these signs and symptoms. Since 2010, 5 cases have been presented to the Dutch Medical Disciplinary Court (DMDC) in the Netherlands. In these cases, the DMDC was asked to determine if the quality of care provided by the midwife or gynecologist preceding the uterine rupture was adequate [Citation10]. These cases concerning uterine rupture in the DMDC are exemplary of the focus on signs and symptoms that predict uterine rupture. They may, however, be difficult to recognize. The presence of abdominal pain increased the risk of adverse outcomes, but is hard to distinguish from abdominal pain due to contractions [Citation5,Citation22]. CTG abnormalities associated with uterine rupture can also be difficult to distinguish from abnormalities due to a physiologic response to prolonged labor or engagement of the fetal head. CTG abnormalities are common in patients undergoing TOLAC with and without uterine rupture [Citation24]. The percentage of normal CTGs was significantly lower for women undergoing TOLAC with uterine rupture compared to women without uterine rupture. The risk of adverse outcomes also increased after an abnormal CTG, especially when fetal bradycardia was present [Citation5,Citation22,Citation25]. Comparing CTG tracings of women undergoing TOLAC with and without uterine rupture could provide new insights into the specific types of CTG abnormalities associated with uterine rupture.
Birth within 30 min after the start of signs and symptoms of uterine rupture is considered necessary to prevent uterine rupture [Citation13,Citation18]. Although in the present study we did not calculate optimal time-to-birth interval, we noticed a wide range in the time between complaints and birth (34 to 294 min). It would be interesting to analyze which factors influenced this range. Complaints increased sharply within the hour prior to birth and signs of fetal distress according to FHR on CTG appeared and increased between 60 and 40 min before birth. Therefore, birth within 30 min after onset of symptoms could prevent and minimize the duration of fetal distress and we suggest that imminent intervention is necessary when uterine rupture is suspected.
Conclusion
We found a lower incidence of uterine rupture in the Netherlands compared to 2006. Adverse events were present in 54% of women with uterine rupture. Risk indicators for uterine rupture with adverse outcomes are previous cesarean section, migrant background from Sub-Saharan Africa or Asia, preterm birth and augmentation of labor. Risk indicators during labor included maternal signs and symptoms, such as abdominal pain and CTG abnormalities. They are associated with adverse outcomes. Birth should be persued without delay when these signs or symptoms are present. Future research into analyses of CTG tracings and onset of maternal symptoms over time, might provide further insight into the improved management of uterine rupture.
Authors’ contributions
EO, KB and TS participated in the outline, design and coordination of the study. EO contacted hospitals, registered, and analyzed cases. NH and EO extracted data from hospital files, analyzed and interpreted data and wrote the article draft. AR extracted data for the reference group. EO, NH, KB, TvdA, TS, AR, TV and JZ contributed to interpretation of data edited and approved the final version of the article.
Details of ethical approval
All procedures performed in studies involving human participants were in accordance with ethical standards of the institutional and/or national research committee and with the 1964 Helsinki declaration and its later amendments or comparable ethical standards. The NethOSS registration system is part of the National Perinatal Registry foundation in the Netherlands (Perined). This study did not require specific ethical approval and Informed consent of participants was not obtained since Perined is allowed administrative permission in the Netherlands to access patient information from patient charts if the information used is not personally identifiable, concerns large numbers of participants and it is not feasible to trace and contact individual participants.
Acknowledgments
We would like to thank all health care givers in Dutch hospitals for cooperating with the registration process and filling in the case report forms.
Disclosure statement
The authors report no conflict of interest.
Data availability statement
The data that support the findings of this study are available from the corresponding author upon reasonable request.
Additional information
Funding
References
- Ronel D, Wiznitzer A, Sergienko R, et al. Trends, risk factors and pregnancy outcome in women with uterine rupture. Arch Gynecol Obstet. 2012;285(2):1–10. doi:10.1007/s00404-011-1977-8.
- Ofir K, Sheiner E, Levy A, et al. Uterine rupture: risk factors and pregnancy outcome. Am J Obstet Gynecol. 2003;189(4):1042–1046. doi:10.1067/s0002-9378(03)01052-4.
- Guiliano M, Closset E, Therby D, et al. Signs, symptoms and complications of complete and partial uterine ruptures during pregnancy and delivery. Eur J Obstet Gynecol Reprod Biol. 2014;179:130–134. doi:10.1016/j.ejogrb.2014.05.004.
- Zwart JJ, Richters JM, Ory F, et al. Uterine rupture in The Netherlands: a nationwide population-based cohort study. BJOG. 2009;116(8):1069–1080. doi:10.1111/j.1471-0528.2009.02136.x.
- Barger MK, Nannini A, Weiss J, et al. Severe maternal and perinatal outcomes from uterine rupture among women at term with a trial of labor. J Perinatol. 2012;32(11):837–843. doi:10.1038/jp.2012.2.
- Vandenberghe G, Bloemenkamp K, Berlage S, et al. The international network of obstetric survey systems study of uterine rupture: a descriptive multi-country populationbased study. BJOG. 2019;126(3):370–381. doi:10.1111/1471-0528.15271.
- Kwee A, Bots ML, Visser GH, et al. Uterine rupture and its complications in The Netherlands: a prospective study. Eur J Obstet Gynecol Reprod Biol. 2006;128(1-2):257–261. doi:10.1016/j.ejogrb.2006.02.005.
- Sandall J, Tribe RM, Avery L, et al. Short-term and long-term effects of caesarean section on the health of women and children. Lancet. 2018;392(10155):1349–1357. doi:10.1016/S0140-6736(18)31930-5.
- Betran AP, Torloni MR, Zhang J, et al. What is the optimal rate of caesarean section at population level? A systematic review of ecologic studies. Reprod Health. 2015;12(1):57. doi:10.1186/s12978-015-0043-6.
- Tuchtrecht O. Available from: https://tuchtrecht.overheid.nl/
- Kaczmarczyk M, Sparén P, Terry P, et al. Risk factors for uterine rupture and neonatal consequences of uterine rupture: a population‐based study of successive pregnancies in Sweden. BJOG. 2007;114(10):1208–1214. doi:10.1111/j.1471-0528.2007.01484.x.
- Landon MB, Hauth JC, Leveno KJ, et al. Maternal and perinatal outcomes associated with a trial of labor after prior cesarean delivery. N Engl J Med. 2004;351(25):2581–2589. doi:10.1056/NEJMoa040405.
- Al-Zirqi I, Daltveit AK, Vangen S. Infant outcome after complete uterine rupture. Am J Obstet Gynecol. 2018;219(1):109.e1–e8. doi:10.1016/j.ajog.2018.04.010.
- Al-Zirqi I, Daltveit AK, Vangen S. Maternal outcome after complete uterine rupture. Acta Obstet Gynecol Scand. 2019;98(8):1024–1031. doi:10.1111/aogs.13579.
- Andonovová V, Hruban L, Gerychová R, et al. Uterine rupture during pregnancy and delivery: risk factors, symptoms and maternal and neonatal outcomes - restrospective cohort. Ceska Gynekol. 2019;84(2):121–128.
- Ouzounian JG, Quist-Nelson J, Miller DA, et al. Maternal and fetal signs and symptoms associated with uterine rupture in women with prior cesarean delivery. J Matern Fetal Neonatal Med. 2015;28(11):1270–1277. doi:10.3109/14767058.2014.954537.
- Calì G, Timor-Tritsch IE, Palacios-Jaraquemada J, et al. Outcome of cesarean scar pregnancy managed expectantly: systematic review and meta-analysis. Ultrasound Obstet Gynecol. 2018;51(2):169–175. doi:10.1002/uog.17568.
- Holmgren C, Scott JR, Porter TF, et al. Uterine rupture with attempted vaginal birth after cesarean delivery: decision-to-delivery time and neonatal outcome. Obstet Gynecol. 2012;119(4):725–731. doi:10.1097/AOG.0b013e318249a1d7.
- Goossen WT, Arns-Schiere AM. Information architecture for perinatal registration in The Netherlands. J Obstet Gynecol Neonatal Nurs. 2017;46(2):310–321. doi:10.1016/j.jogn.2016.11.011.
- Engin A. The definition and prevalence of obesity and metabolic syndrome. Adv Exp Med Biol. 2017;960:1–17. doi:10.1007/978-3-319-48382-5_1.
- Ayres-de-Campos D, Spong CY, Chandraharan E. FIGO consensus guidelines on intrapartum fetal monitoring: cardiotocography. Int J Gynaecol Obstet. 2015;131(1):13–24. doi:10.1016/j.ijgo.2015.06.020.
- Markou GA, Muray JM, Poncelet C. Risk factors and symptoms associated with maternal and neonatal complications in women with uterine rupture. A 16 years multicentric experience. Eur J Obstet Gynecol Reprod Biol. 2017;217:126–130. doi:10.1016/j.ejogrb.2017.09.001.
- Smith JG, Merrill DC. Oxytocin for induction of labor. Clin Obstet Gynecol. 2006;49(3):594–608. doi:10.1097/00003081-200609000-00019.
- Andersen MM, Thisted DLA, Amer-Wåhlin I, et al. Can intrapartum cardiotocography predict uterine rupture among women with prior caesarean delivery?: A population based case-control study. PLoS One. 2016;11(2):e0146347. doi:10.1371/journal.pone.0146347.
- Desseauve D, Bonifazi-Grenouilleau M, Fritel X, et al. Fetal heart rate abnormalities associated with uterine rupture: a case–control study: a new time-lapse approach using a standardized classification. Eur J Obstet Gynecol Reprod Biol. 2016;197:16–21. doi:10.1016/j.ejogrb.2015.10.019.