Abstract
Introduction
It is still no consensus on the use of ropivacaine or bupivacaine in epidural anesthesia for cesarean section (CS), because their anesthetic potency and relative complications remains controversial. This system review and meta-analysis aimed to compare the efficacy of epidural ropivacaine and bupivacaine for elective CSs and investigate relative complications for parturients and neonates.
Methods
We searched PubMed, MEDLINE, Embase, Cochrane Library, Science-Direct, and Google Scholar to June 30, 2023 for randomized controlled trials (RCTs), which compared epidural ropivacaine with bupivacaine for elective CSs. The success rate of epidural anesthesia (EA) was primary outcome. The secondary outcomes included onset times of sensory block, maternal side effects, neonatal Apgar scores and umbilical artery pH.
Results
We analyzed 8 RCTs with 532 parturients. 0.75% ropivacaine is associated with a shorter onset time of sensory block than 0.5% bupivacaine (SMD = −0.43, 95% CI: −0.70 to −0.17; p = .001). 0.5% ropivacaine resulted in a reduced nausea than 0.5% bupivacaine (RR = 0.49, 95% CI: 0.28 to 0.83; p = .008). In addition, there were no significant difference between ropivacaine and bupivacaine groups in terms of success rate of epidural anesthesia, maternal side effects (hypotension, bradycardia, shivering), and neonatal Apgar scores and umbilical artery pH.
Conclusions
The findings suggest that there were no significant difference between epidural ropivacaine and bupivacaine for elective CSs in terms of the success rate (85.9% vs. 83.5), maternal side effects (hypotension, bradycardia, shivering), and neonatal Apgar scores and umbilical artery pH. But compared with 0.5% bupivacaine, epidural 0.75% ropivacaine was mildly effective for reducing onset time of sensory block and 0.5% ropivacaine reduced the incidence of maternal nausea.
Introduction
Cesarean section (CS) is a common surgical procedure worldwide, with 21.1% of parturients have undergone a CS [Citation1]. The rate is increasing steadily, and it is expected that this rate will rise to 28.5% in 2030 [Citation2]. Unlike vaginal delivery, the CS must be performed under anesthesia.
In the majority of cases, the choice of general anesthesia (GA) or neuraxial anesthesia (NA) can be influenced by maternal co-morbidities, urgency, and available equipment and expertise. NA is prevalent and well-accepted because of easy practice, rapid onset, high success rate, and improving maternal and fetal outcomes over general anesthesia (GA) [Citation2]. Unlike SA, it is flexible for epidural anesthesia (EA) to administer the anesthetic effect by intermittent additional local anesthetics (LAs) to epidural space [Citation3]. Meanwhile, the epidural catheter provides a simple and effective channels to use LAs and opioids for postoperative analgesia [Citation3]. Moreover, if a parturient is needed to convert labor analgesia to surgical anesthesia, a technique of “top-up” the epidural will be practiced, which happens to about 18% of emergency CS in the UK [Citation4].
Overall, the choice of epidural LAs is governed by immediate availability and individual practical experience, but not be determined by high-level evidence-based medical researches. Better understanding of the onset times of epidural LAs may improve decision making for CS. A meta-analysis showed that the onset times of epidural 0.75% ropivacaine and 0.5% bupivacaine were longer than 2% lidocaine with bicarbonate, 3% 2-chloroprocaine and 2% lidocaine [Citation5]. Although rapid-onset NA can avoid GA with its associated risks for CS, the goals of obstetric anesthesia include the parturients’s comfort and fetal well-being, focusing on minimizing complications and mortality for both [Citation2].
It is crucial to comprehensively and objectively evaluate the efficacy and complications of epidural LAs for CS, but there are currently few clinical controlled studies on 2% lidocaine with bicarbonate, 3% 2-chloroprocaine and 2% lidocaine. Bupivacaine is a well-established long-acting LA for CS. Ropivacaine has similar pharmacodynamic and pharmacokinetic characteristics to bupivacaine at higher doses, and has similar clinical effects in sensory blockade and slightly less in motor blockade than bupivacaine [Citation6].
Although several studies have compared epidural bupivacaine and ropivacaine for CSs, the conclusions are not consistent. The choice of LAs is an eternal and unchanging challenge in clinical anesthesia for CSs, so we must explore the efficacy and relative complications of epidural ropivacaine and bupivacaine for elective CSs.
Materials and methods
Literature search
This meta-analysis adhered to the guidelines of the Preferred Reporting Items for Systematic Reviews and Meta-Analysis (PRISMA) [Citation7]. Institutional review board approval was not required. Articles of epidural ropivacaine versus bupivacaine for CS were identified by searching electronic databases (PubMed, Embase, MEDLINE, Cochrane Library, Science-Direct, and Google Scholar) up to 30 June 2023. Key words and Medical Subject headings (MESH) descriptor terms were: “epidural,” “ropivacaine,” “bupivacaine,” “cesarean section” were used in a variety of sequences. Unpublished studies were not investigated. There was no limitation on language and sample size.
Inclusion and exclusion criteria
Eligibility criteria was designed according to PICOS criteria: the parturients underwent elective CSs (P); epidural anesthesia is the only mode of anesthesia (I); local anesthetics included ropivacaine and bupivacaine (C); the success rate of anesthesia, onset time of sensory block, maternal side effects (hypotension, bradycardia, shivering, nausea), and neonatal Apgar scores and umbilical artery pH (O); and randomized controlled trials (RCTs) (S). Studies were excluded if they were met the following criteria: (1) the parturients of converting labor analgesia to surgical anesthesia; (2) incomplete data which could not be used for statistical analysis; (3) unpublished studies, parallel and crossover randomized design studies; (4) for duplicate data used for several studies, the studies with incomplete data were excluded.
Data extraction
Two researchers (T. Kang and Jw. Tao) individually screened the literature in accordance with the inclusion and exclusion criteria and extracted data. Disagreements were resolved through consultation or discussion with a third person (Xt Wang). The data extracted from the RCTs included basic characteristics (authors, published year, sample size, age, height, weight, American Society of Anesthesiologists physical status, interventions, epidural blockade level for surgery), primary outcome (success rate of epidural anesthesia. It was defined as pregnant women did not receive supplemental analgesics or not convert to general anesthesia during operation), secondary outcomes (onset times of sensory block, maternal side effects, and neonatal Apgar scores and umbilical artery pH).
Quality assessment
Two independent investigators assessed the risk of bias for the included studies. The methodological quality was assessed by using the Cochrane Risk of Bias review criteria for randomized studies in Review Manager, which includes of sequence generation, allocation sequence concealment, blinding of participants and personal, blinding of outcome assessment, incomplete outcome data, selective outcome reporting and other potential sources bias [Citation8]. We evaluated evidence with the GRADE framework [Citation9].
Statistical analysis
Statistical analysis was carried out in REVMAN V.5.3 software. The onset times of surgical block was expressed as a continuous variable and as standardized mean difference (SMD) with 95% confidence intervals (CI) to estimate the summary effect size (SES), whereas neonatal umbilical artery pH was presented using mean difference (MD). For dichotomous variables (categorical data) was expressed as risk ratios (RRs) with 95% CI to estimate the SES. Overall effect sizes were illustrated by forest plots. Subgroup analysis was based on the concentrations of ropivacaine versus bupivacaine in the included studies.
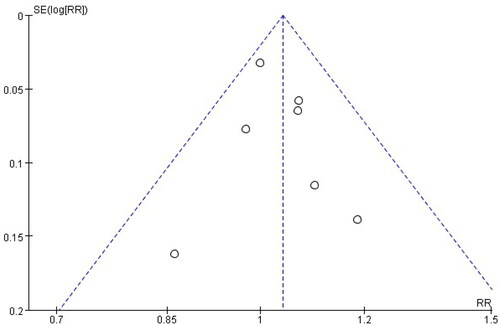
Heterogeneity value was assessed by I2 statistics. I2 < 50% and I2 ≥ 50% were considered insignificant and significant heterogeneity, performed via fixed-effects model and random-effects model, respectively [Citation10]. If heterogeneity was significant, we performed a leave-one-out sensitivity analysis by sequentially removing individual study and reanalyzing its effect on the overall heterogeneity [Citation11]. p Values < .05 were considered as statistical significance in all analyses. A funnel plot using the success rate of EA as endpoints did not indicate the presence of publication bias ().
Results
Literature search screening and inclusion and bias evaluation
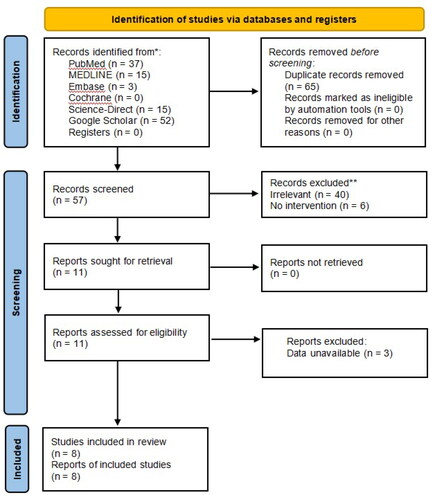
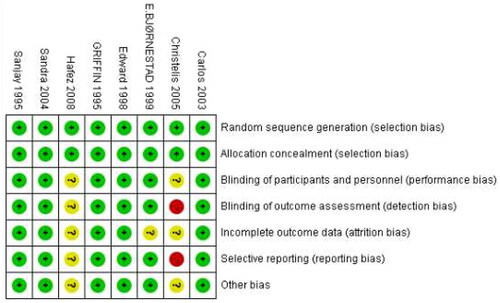
We obtained 57 articles on a preliminary search of the literature. Ultimately, a total of eight articles (n = 532 patients) [Citation12–19] were included in quantitative analysis, between ropivacaine (n = 289 patients) and bupivacaine (n = 243 patients) groups. The inclusion and exclusion processes are shown in . Among the eight RCTs, four trials [Citation12–14,Citation19] used 0.5% ropivacaine versus 0.5% bupivacaine, two trials [Citation15,Citation17] used 0.75% ropivacaine versus 0.5% bupivacaine, one trial [Citation18] used 0.75% ropivacaine versus a mixture of 0.5% bupivacaine with fentanyl, one trial [Citation16] used a mixture of 0.75% ropivacaine with fentanyl versus a mixture of 0.5% bupivacaine with fentanyl and 1:200,000 epinephrine, a total volume between 20 and 30 ml. The basic characteristics of the included studies are displayed in . The methodological quality and judgment about each risk of bias domain as a percentage across all included studies is presented in . A summary of evidence certainty in .
Table 1. Baseline demographics of included studies.
Table 2. Summary of evidence for epidural ropivacaine versus bupivacaine for cesarean sections.
Success rate of epidural anesthesia
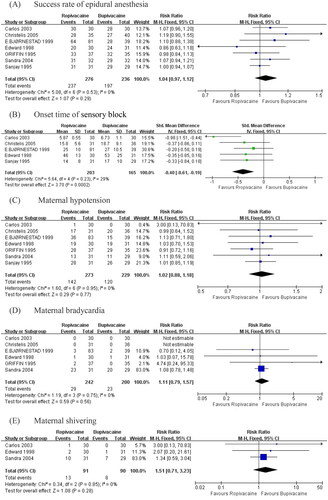
The pooled data of seven studies [Citation12–18] did not suggest that ropivacaine had a significant effect on the success rate of epidural block with bupivacaine (RR = 1.04, 95% CI: 0.97 to 1.12; p = .29; I2 = 0%; ).
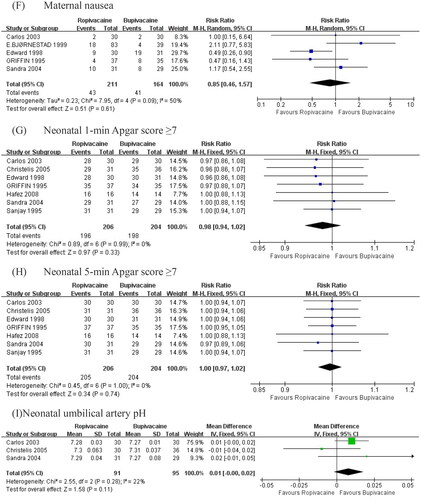
Figure 4. Summary effect size of primary and secondary outcomes among RC Ts. (A) Success rate of epidural block. (B) Onset time of surgical block. (C) Maternal hypotension. (D) Maternal bradycardia. (E) Maternal shivering. (F) Maternal nausea. (G) Neonatal 1-min Apgar score ≥. (H) Neonatal 5-min Apgar score ≥. (I) Neonatal umbilical artery pH.
Onset time of surgical block
The pooled data of five studies [Citation12–16,Citation18] examining the result of ropivacaine on onset time for epidural block with bupivacaine revealed a statistically significant effect (SMD = −0.40, 95% CI: −0.61 to −0.19; p = .0002; I2 = 29%; ).
Maternal side effects
Compared with bupivacaine, the ropivacaine group showed no detectable difference in terms of hypotension (RR = 1.02, 95% CI: 0.88 to 1.18; p = 0.77; I2 = 0%; ), bradycardia (RR = 1.11, 95% CI: 0.79 to 1.57; p = .56; I2 = 0%; ), shivering (RR = 1.51, 95% CI: 0.71 to 3.23; p = .28; I2 = 0%; ).
The pooled data of five studies [Citation12,Citation14–17] did not suggest that ropivacaine had a significant effect on nausea with bupivacaine (RR = 0.85, 95% CI 0.46 to 1.57; p = .61; I2 = 50%, ). To investigate this significant heterogeneity (I2 = 50%), we calculated the overall effects using a random-effects model. A sensitivity analysis was performed with exclusion of the outlier, and the heterogeneity reduced to 15% without alteration to the P value or the direction of the overall effect. The source of heterogeneity is that patients experienced vomiting was counted in the numbers of patients experienced nausea in this article [Citation14].
Neonatal Apgar scores and umbilical artery pH
Compared with bupivacaine, the ropivacaine group showed no detectable difference in terms of 1-min Apgar score ≥7 (RR = 0.98, 95% CI: 0.94 to 1.02; p = .33; I2 = 0%, ), 5-min Apgar score ≥7 (RR = 1.00, 95% CI: 0.97 to 1.02; p = .74; I2 = 0%; ). The pooled data of three studies [Citation15–17] did not suggest that ropivacaine had a significant effect on umbilical artery pH of epidural block with bupivacaine (MD = 0.01, 95% CI: 0.00 to 0.02; p = .11; I2 = 22%; ).
Subgroup analysis: concentrations of LAs
Among the eight trials [Citation12–19], patients in four trials [Citation15–18] received either 0.75% ropivacaine or 0.5% bupivacaine, and the other four [Citation12–14,Citation19] received either 0.5% ropivacaine or 0.5% bupivacaine. Summary results of the subgroup analysis are shown in .
Table 3. Summary results of subgroup analysis.
Discussion
Anesthetic options of CS can broadly be divided into NA and GA. In contrast to GA, the NA has many irreplaceable advantages which includes a birth partner can be present in the operating room, a skin-to-skin contact can be established after delivery, and postoperative analgesia and so on [Citation20]. Spinal anesthesia (SA) has been used widely for CS because it is easier and quicker than EA, and it uses fewer drugs than GA [Citation21]. EA is convenient for converting labor analgesia to surgical anesthesia and postoperative analgesia. However, the GA will be performed when the NA is ineffective or contraindicated, the incidence about 1.7% [Citation22]. The commonest indications for GA include maternal anticoagulation, thrombocytopenia and/or coagulopathy, hemorrhage, unstable cardiac disease, cardiorespiratory compromise, and fetal bradycardia with imminent fetal demise [Citation23]. It is worth noting that parturients have higher risks of difficult airway, failed tracheal intubation, hypoxemia, and pulmonary aspiration during GA induction and maintenance [Citation24].
Our meta-analysis included 8 RCTs (532 parturients), the result confirmed that the success rates of EA were similar between ropivacaine and bupivacaine groups (85.9% vs. 83.5). A retrospective study revealed that patient-related risk factors of EA failure included a higher BMI, younger age, previous epidural experience, prolonged labor, breakthrough pain during labor analgesia, urgency during cesarean section, an increasing number of top-ups, maternal height, and insufficient waiting time before surgery [Citation25,Citation26]. It was worth noting that history of epidural catheterization could increase the risk of EA failure by 2.6-fold, because of significant inflammatory changes and adhesions in the epidural space [Citation27]. The traumatic changes would result in disturbance in recatheterization, the cephalic diffusion of drugs, LAs penetration to nerve roots, and further lead to inadequate anesthesia or block failure [Citation27]. Other risk factors of EA failure included anesthesia performed by non-obstetric anesthesiologists, determination of epidural space, and the operability of catheterization [Citation28]. Failure of EA required extra drugs to achieve an satisfied anesthesia and even conversion to GA immediately, which would be dangerous for parturients because of potentially difficult airways and pulmonary aspiration [Citation29].
Bupivacaine is an amino-amide LA and has become the standard LA in NA for CS. It has higher binding to α1-glycoprotein and longer duration of action compared with ropivacaine [Citation30]. Cardiac and central nervous system toxicity limited the use of large doses of bupivacaine, the maximum safe dosage is 2 mg/kg [Citation31]. Ropivacaine is the propyl homologue of bupivacaine but is prepared as the pure S(–)-enantiomer [Citation32]. Ropivacaine has lower lipid solubility and sodium channel affinity, which is associate with shorter duration of motor block [Citation33]. Our meta-analysis confirmed that the onset time of EA varied from 5.87 ± 0.55 to 53 ± 25 min depending on the definition used, and 0.75% ropivacaine was associated with a shorter onset time of sensory block than 0.5% bupivacaine. A meta-analysis revealed that the mean (95% CrI) onset time of epidural 2% lidocaine with bicarbonate was the fastest, which faster than 0.5% bupivacaine about 6.4 (3.3–9.6) min [Citation5]. The onset time of 0.5% bupivacaine was 19.8 (17.3–22.4) min, and it was similar to 0.75% ropivacaine 1.6 (−1.4 to 4.8) min faster [Citation5]. Although rapid-onset NA can avoid GA for CS, goals of anesthesia for CS are to ensure the parturients’s comfort and fetal well-being, focusing on minimizing complications and mortality for both [Citation2].
Hypotension and bradycardia pose life-threatening risks of NA during CS, which occur in 90% of parturients. Hypotension is a decrease in systolic arterial pressure below accepted low values, pressures less than 90 mmHg or 20% below the baseline are recognized as hypotensive [Citation34]. Bradycardia was defined as heart rate less than 50/min [Citation35]. Reduced systemic vascular resistance are caused by sympathetic blockade and supine hypotensive syndrome can further worsen maternal hemodynamics [Citation36], leading to serious maternal adverse effects, such as dizziness, nausea and vomiting, loss of consciousness, heart failure, apnea, and aspiration of gastric contents [Citation37]. In the current meta-analysis, the incidences of maternal hypotension and bradycardia were similar between ropivacaine and bupivacaine groups. Therefore, there is no conclusive evidence supporting one LA over the other for minimizing the risk of hypotension and bradycardia.
Perioperative shivering is a common undesirable symptom of NA. Its occurrence rate up to 40–80% for cesarean section in SA [Citation38], but the incidence of EA below 32.3%. Shivering is a thermoregulatory reflex triggered by hypothermia and core temperature variability, the pathophysiology is associated with blocking sympathetic nerves in NA [Citation39]. When the core temperature decreases 0.5 °C or to the shivering threshold, the hypothalamus affects the activity of the thermogenic organs, which represented by skeletal muscles involuntary and repetitive actions [Citation40]. A severe and persistent shivering could increase plasma catecholamines and oxygen consumption, the depth of pain, as well as interfering with monitoring of noninvasive blood pressure, electrocardiogram, and pulse oximetry [Citation41]. Our meta-analysis confirmed that the incidences of maternal shivering were similar between ropivacaine and bupivacaine groups, which suggested that they had equally clinical influence for blocking sympathetic nerves. Previous studies [Citation42,Citation43] have demonstrated that this uncomfortable experience could be alleviated by various chemical and physical strategies such as intravenous fentanyl, dexmedetomidine, ketamine, meperidine, and heated infusion and warmed body.
Nausea was defined as a subjective sensation suggesting that a patient desires to vomit. Besides causing pregnant women discomfort, it interferes with surgery and increases procedure time, risk of bleeding, inadvertent surgical trauma, and the risk of pulmonary aspiration of gastric contents [Citation44]. The incidence of nausea is variable, and it is more frequent in a mass of blood loss in a few minutes, uterus exteriorization, using uterotonic agents [Citation45]. In this meta-analysis, the incidence of nausea was reduced in 0.5% ropivacaine compared with 0.5% bupivacaine (19.4% vs. 40.9%, p = .008). Therefore, epidural 0.5% ropivacaine may be recommended for parturients of full stomach.
The Apgar score has been used as a rapid index for assessing a neonate immediately after birth and in response to resuscitation [,Citation46]. Neonatal umbilical artery pH may reflect accurately to the degree of acidosis [Citation47]. It is more reasonable to evaluate the degree of asphyxia by combining Apgar score with neonatal umbilical artery pH. In this study, the ratio of 1-min and 5-min Apgar score ≥ 7 are 96.1% and 99.8% respectively, the umbilical artery pH varies from 7.27 ± 0.01 to 7.31 ± 0.037. The above results suggest that epidural ropivacaine and bupivacaine does not aggravate the degree of ischemia and hypoxia. Sustained maternal hypotension-induced uteroplacental blood flow decreases is associated with fetal intrauterine distress and fetal acidosis [Citation48]. Preventing hypotension is essential for both maternal safety and comfort, and neonatal outcome. A international consensus [Citation49] recommended that using alpha-agonist drugs, drugs with mixed alpha- and beta-agonist activity, left uterine displacement, and intravenous infusion to increase the blood pressure during cesarean section. Recent researches [Citation34,Citation50] has confirmed that vasopressors reduce the incidence of hypotension, nausea and vomiting whereas intravenous infusion did not. In addition, metaraminol is the most effective vasopressor, phenylephrine can cause maternal bradycardia and ephedrine can cause tachycardia [Citation50]. We need to further investigate the best doses of every vasopressor, and the best intervention strategies in high risk parturients.
There are several potential limitations of this meta-analysis and systematic review to highlight. First, the varying definitions of onset times among included studies may lead to misjudging the relationship between epidural LA types and the onset time. Though we have adopted a unified definition in one study, it should be cautious in explaining the onset times between two studies. Second, we have made a subgroup analysis for concentration between ropivacaine and bupivacaine groups, but it was not ignore that the test doses, volumes and speeds of injection, height, vertebral length addition of fentanyl and epinephrine could affect the anesthetic potency of each LA. Third, considering the impact of labor analgesia on the success rate and the onset time of sensory block for epidural anesthesia, we did not include those parturients who were needed to convert labor analgesia to surgical anesthesia. Our results could increase the precision of data analysis but reduce the generalizability of the conclusions. Moreover, because of all the included trials were conducted on EA for CS, our findings may not apply to other patients. Four, as the included studies did not provide sufficient data, we did not evaluate the accurate time of the sensory and motor recovery after EA.
Conclusion
Our systematic review and meta-analysis suggests that there were no significant difference between epidural ropivacaine and bupivacaine for elective CSs in terms of the success rate (85.9% vs. 83.5), maternal side effects (hypotension, bradycardia, shivering), and neonatal Apgar scores and umbilical artery pH. But compared with 0.5% bupivacaine, epidural 0.75% ropivacaine was mildly effective for reducing onset time of sensory block and 0.5% ropivacaine reduced the incidence of maternal nausea. Further studies are needed to determine the optimal LAs that minimize side-effects, the most effective dosage and timing of administration, and the role of adjuvants (e.g. fentanyl and epinephrine).
Ethics statement
The data comes from published articles and does not require ethical approval.
Authors note
We declare that the views expressed in the articles submitted are our own and not the official position of the institution or funder.
Disclosure statement
No potential conflict of interest was reported by the author(s).
Data availability statement
The data generated for this study are available from the corresponding authors upon reasonable request.
Additional information
Funding
References
- Betran AP, Ye J, Moller AB, et al. Trends and projections of caesarean section rates: global and regional estimates. BMJ Glob Health. 2021;6(6):1. doi: 10.1136/bmjgh-2021-005671.
- Watson SE, Richardson AL, Lucas DN. Neuraxial and general anaesthesia for caesarean section. Best Pract Res Clin Anaesthesiol. 2022;36(1):53–11. doi: 10.1016/j.bpa.2022.04.007.
- Halliday L, Nelson SM, Kearns RJ. Epidural analgesia in labor: a narrative review. Int J Gynaecol Obstet. 2022;159(2):356–364. doi: 10.1002/ijgo.14175.
- Bamber JH, Lucas DN, Russell R, et al. Obstetric anaesthetic practice in the UK: a descriptive analysis of the national obstetric anaesthetic database 2009-14. Br J Anaesth. 2020;125(4):580–587. doi: 10.1016/j.bja.2020.06.053.
- Reschke MM, Monks DT, Varaday SS, et al. Choice of local anaesthetic for epidural caesarean section: a Bayesian network meta-analysis. Anaesthesia. 2019;75(5):674–682. doi: 10.1111/anae.14966.
- Calder A, Bell GT, Andersson M, et al. Pharmacokinetic profiles of epidural bupivacaine and ropivacaine following single-shot and continuous epidural use in young infants. Paediatr Anaesth. 2012;22(5):430–437. doi: 10.1111/j.1460-9592.2011.
- Liberati A, Altman DG, Tetzlaff J, et al. The PRISMA statement for reporting systematic reviews and meta-analyses of studies that evaluate health care interventions: explanation and elaboration. PLoS Med. 2009;6(7):e1000100. doi: 10.1371/journal.pmed.1000100.
- Higgins JPT, Altman DG, Gøtzsche PC, et al. The Cochrane Collaboration’s tool for assessing risk of bias in randomised trials. BMJ. 2011;343(oct18 2):d5928. doi: 10.1136/bmj.d5928.
- Guyatt G, Oxman AD, Akl EA, et al. GRADE guidelines: 1. Introduction-GRADE evidence profiles and summary of findings tables. J Clin Epidemiol. 2011;64(4):383–394. doi: 10.1016/j.jclinepi.2010.04.026.
- Danielle LC, Mark CK, Zachary LM, et al. Pectoral nerve blocks and postoperative pain outcomes after mastectomy: a meta-analysis of randomized controlled trials. Reg Anesth Pain Med. 2019;44(10):923–928. doi: 10.1136/rapm-2019-100658.
- Zhang YJ, Zhang L, Chen SY, et al. Association between VDR polymorphisms and multiple sclerosis: systematic review and updated meta-analysis of case-control studies. Neurol Sci. 2018;39(2):225–234. doi: 10.1007/s10072-017-3175-3.
- Griffin RP, Reynolds F. Extradural anaesthesia for caesarean section: a double-blind comparison of 0.5% ropivacaine with 0.5% bupivacaine. Br J Anaesth. 1995;74(5):512–516. doi: 10.1093/bja/74.5.512.
- Datta S, Camann W, Bader A, et al. Clinical effects and maternal and fetal plasma concentrations of epidural ropivacaine versus bupivacaine for cesarean section. Anesthesiology. 1995;82(6):1346–1352. doi: 10.1097/00000542-199506000-00004.
- Crosby E, Sandler A, Finucane B, et al. Comparison of epidural anaesthesia with ropivacaine 0.5% and bupivacaine 0.5% for caesarean section. Can J Anaesth. 1998;45(11):1066–1071. doi: 10.1111/anae.14966.
- Bjørnestad E, Smedvig JP, Bjerkreim T, et al. Epidural ropivacaine 7.5 mg/ml for elective caesarean section: a double-blind comparison of efficacy and tolerability with bupivacaine 5 mg/ml. Acta Anaesthesiol Scand. 1999;43(6):603–608. doi: 10.1034/j.1399-6576.1999.430602.x.
- Côrtes CAF, Oliveira AS, Castro LFL, et al. Comparative study between 0.5% bupivacaine, 0.5% enantiomeric mixture of bupivacaine (S75-R25) and 0.75% ropivacaine, all associated to fentanyl, for epidural cesarean section anesthesia. Rev Bras Anestesiol. 2003;53(2):177–187. doi: 10.1590/s0034-70942003000200005.
- Kampe S, Tausch B, Paul M, et al. Epidural block with ropivacaine and bupivacaine for elective caesarean section: maternal cardiovascular parameters, comfort and neonatal well-being. Curr Med Res Opin. 2004;20(1):7–12. doi: 10.1185/030079903125002649.
- Christelis N, Harrad J, Howell PR. A comparison of epidural ropivacaine 0.75% and bupivacaine 0.5% with fentanyl for elective caesarean section. Int J Obstet Anesth. 2005;14(3):212–218. doi: 10.1016/j.ijoa.2005.01.002.
- Hafez M, Helmy N. Epidural ropivacaine versus bupivacaine for cesarean section: effect on maternal and fetal hemodynamica. Reg Anesth Pain Med. 2008;33(Sup 1):e14. doi: 10.1097/00000542-199506000-00004.
- Pérez-Jiménez JM, Luque-Oliveros M, Gonzalez-Perez D, et al. Does immediate skin-to-skin contact at caesarean sections promote uterine contraction and recovery of the maternal blood haemoglobin levels? A randomized clinical trial. Nurs Open. 2023;10(2):649–657. doi: 10.1002/nop2.1331.
- Klimek M, Rossaint R, van de Velde M, et al. Combined spinal-epidural vs. spinal anaesthesia for caesarean section: meta-analysis and trial-sequential analysis. Anaesthesia. 2018;73(7):875–888. doi: 10.1111/anae.14210.
- Stav M, Matatov Y, Hoffmann D, et al. Incidence of conversion to general anaesthesia and need for intravenous supplementation in parturients undergoing caesarean section under spinal anaesthesia: a retrospective observational study. Acta Anaesthesiol Scand. 2023;67(1):29–35. doi: 10.1111/aas.14146.
- Devroe S, Van de Velde M, Rex S. General anesthesia for caesarean section. Curr Opin Anaesthesiol. 2015;28(3):240–246. doi: 10.1097/ACO.0000000000000185.
- Pombo A, Cardoso TM, Araújo AM, et al. Airway approach for caesarean section under general anaesthesia: a national survey. Int J Obstet Anesth. 2023;56:103920. doi: 10.1016/j.ijoa.2023.103920.
- Kula AO, Riess ML, Ellinas EH. Increasing body mass index predicts increasing difficulty, failure rate, and time to discovery of failure of epidural anesthesia in laboring patients. J Clin Anesth. 2017;37:154–158. doi: 10.1016/j.jclinane.2016.11.010
- Eley VA, Chin A, Tham I, et al. Epidural extension failure in obese women is comparable to that of non-obese women. Acta Anaesthesiol Scand. 2018;62(6):839–847. doi: 10.1111/aas.13085.
- Chao WH, Cheng WS, Hu LM, et al. Risk factors for epidural anesthesia blockade failure in cesarean section: a retrospective study. BMC Anesthesiol. 2023;23(1):338. doi: 10.1186/s12871-023-02284-w.
- Mankowitz SK, Gonzalez Fiol A, Smiley R. Failure to extend epidural labor analgesia for cesarean delivery anesthesia: a focused review. Anesth Analg. 2016;123(5):1174–1180. doi: 10.1213/ANE.0000000000001437.
- Ratnayake G, Patil V. General anaesthesia during caesarean sections: implications for the mother, foetus, anaesthetist and obstetrician. Curr Opin Obstet Gynecol. 2019;31(6):393–402. doi: 10.1097/GCO.0000000000000575.
- Feng G, Wang Y, Feng J, et al. The relationship between core temperature and perioperative shivering during caesarean section under intrathecal anesthesia with bupivacaine and ropivacaine: a randomized controlled study. J Anesth. 2021;35(6):889–895. doi: 10.1007/s00540-021-02995-9.
- Schug SA, Saunders D, Kurowski I, et al. Neuraxial drug administration a review of treatment options for anesthesia and analgesia. CNS Drugs. 2006;20(11):917–933. doi: 10.2165/00023210-200620110-00005.
- Althaus J, Wax J. Analgesia and anesthesia in labor. Obstet Gynecol Clin North Am. 2005;32(2):231–244. doi: 10.1016/j.ogc.2005.01.002.
- Kakunje R, Sethuramachandran A, Parida S, et al. Effects of adding low-dose clonidine to intrathecal hyperbaric ropivacaine: a randomized double-blind clinical trial. Anesth Essays Res. 2016;10(1):38–44. doi: 10.4103/0259-1162.
- Fitzgerald JP, Fedoruk KA, Jadin SM, et al. Prevention of hypotension after spinal anaesthesia for caesarean section: a systematic review and network meta-analysis of randomised controlled trials. Anaesthesia. 2020;75(1):109–121. doi: 10.1111/anae.14841.
- Sharkey AM, Siddiqui N, Downey K, et al. Comparison of intermittent intravenous boluses of phenylephrine and norepinephrine to prevent and treat Spinal-Induced hypotension in cesarean deliveries: randomized controlled trial. Anesth Analg. 2019;129(5):1312–1318. doi: 10.1213/ANE.0000000000003704.
- Langesæter E, Dyer RA. Maternal haemodynamic changes during spinal anaesthesia for caesarean section. Curr Opin Anaesthesiol. 2011;24(3):242–248. doi: 10.1097/ACO.0b013e32834588c5.
- Lee A, Ngan Kee W. Effects of vasoactive medications and maternal positioning during cesarean delivery on maternal hemodynamics and neonatal Acid-Base status. Clin Perinatol. 2019;46(4):765–783. doi: 10.1016/j.clp.2019.08.009.
- Abdollahpour A, Azadi R, Bandari R, et al. Effects of adding midazolam and sufentanil to intrathecal bupivacaine on analgesia quality and postoperative complications in elective cesarean section. Anesth Pain Med. 2015;5(4):e23565. doi: 10.5812/aapm.23565.
- Jain A, Gray M, Slisz S, et al. Shivering treatments for targeted temperature management: a review. J Neurosci Nurs. 2018;50(2):63–67. doi: 10.1097/JNN.0000000000000340.
- Morrison SF, Nakamura K. Central neural pathways for thermoregulation. Front Biosci. 2011;16(1):74–104. doi: 10.2741/3677.
- Wódarski B, Chutkowski R, Banasiewicz J, et al. Risk factors for shivering during caesarean section under spinal anaesthesia. A prospective observational study. Acta Anaesthesiol Scand. 2020;64(1):112–116. doi: 10.1111/aas.13462.
- Dinges HC, Al-Dahna T, Rücker G, et al. Pharmacologic interventions for the therapy of postanesthetic shivering in adults: a systematic review and network meta-analysis. Minerva Anestesiol. 2023;89(10):923–935. doi: 10.23736/S0375-9393.23.17410-4.
- Liu J, Wang Y, Ma W. Shivering prevention and treatment during cesarean delivery under neuraxial anesthesia: a systematic review. Minerva Anestesiol. 2018;84(12):1393–1405. doi: 10.23736/S0375-9393.18.12478-3.
- Griffiths JD, Gyte GM, Paranjothy S, et al. Interventions for preventing nausea and vomiting in women undergoing regional anaesthesia for caesarean section. Cochrane Database Syst Rev. 2017;2012(9):CD007579. doi: 10.1002/14651858.CD007579.pub2.
- Niu K, Liu H, Chen R-W, et al. Use of propofol for prevention of post-delivery nausea during cesarean section: a double-blind, randomized, placebo-controlled trial. J Anesth. 2018;32(5):748–755. doi: 10.1007/s00540-018-2549-x.
- Chen H-Y, Chauhan SP. Apgar score at 10 minutes and adverse outcomes among low-risk pregnancies. J Matern Fetal Neonatal Med. 2022;35(25):7109–7118. doi: 10.1080/14767058.2021.1943659.
- Ogunyemi D, Jovanovski A, Friedman P, et al. Temporal and quantitative associations of electronic fetal heart rate monitoring patterns and neonatal outcomes. J Matern Fetal Neonatal Med. 2019;32(18):3115–3124. doi: 10.1080/14767058.2018.1456523.
- Aksoy M, Dostbil A, Aksoy AN, et al. Granisetron or ondansentron to prevent hypotension after spinal anesthesia for elective cesarean delivery: a randomized placebo-controlled trial. J Clin Anesth. 2021;75:110469. doi: 10.1016/j.jclinane.2021.110469.
- Kinsella SM, Carvalho B, Dyer RA, et al. International consensus statement on the management of hypotension with vasopressors during caesarean section under spinal anaesthesia. Anaesthesia. 2018;73(1):71–92. doi: 10.1111/anae.14080.
- Singh PM, Singh NP, Reschke M, et al. Vasopressor drugs for the prevention and treatment of hypotension during neuraxial anaesthesia for caesarean delivery: a Bayesian network meta-analysis of fetal and maternal outcomes. Br J Anaesth. 2020;124(3):e95–e107. doi: 10.1016/j.bja.2019.09.045.