Abstract
Objective
Describe the ear and hearing outcomes in Aboriginal infants in an Australian urban area.
Design
Aboriginal infants enrolled in the Djaalinj Waakinj prospective cohort study had ear health screenings at ages 2-4, 6-8 and 12-18 months and audiological assessment at ∼12 months of age. Sociodemographic, environmental characteristics, otoscopy, otoacoustic emissions, tympanometry and visual reinforcement audiometry data were collected.
Study Sample
125 infants were enrolled in the study; 67 completed audiological assessment, 62, 54, and 58 of whom attended ear screenings at 2-4, 6-8 and 12-18 months.
Results
Of the children that attended the audiological assessment, 36.5%, 50% and 64.3% of infants had otitis media (OM) at 2-4, 6-8 and 12-18 months. Using a 10 dB correction factor, 44.8% of infants had hearing loss (HL) (≥ 25 dB HL) at ∼ 12 months of age. More males (X2=5.4 (1df, p = 0.02)) and infants with OM at audiological assessment (X2=5.8 (1df, p = 0.02)) had HL. More infants that used a pacifier at 12-18 months of age had HL (X2=4.7 (1df, p = 0.03)).
Conclusion
Aboriginal infants in an urban area have high rates of HL and OM, which requires early surveillance and timely treatment to reduce the medical and developmental impacts of OM and HL.
Introduction
OM is a common middle ear condition which impedes sound transmission and reduces hearing sensitivity and can result in mild to moderate conductive hearing loss in children (Cai et al. Citation2018), which can have debilitating effects on speech, language, education, cognition, and social development, impacting socio-economic circumstances, health and well-being in both childhood and adulthood (Williams and Jacobs Citation2009; Brennan-Jones et al. Citation2020). Longitudinal studies show early life OM (untreated) can impact hearing and binaural processing later in life as well as educational and attention behaviours (Altamimi et al. Citation2023; Graydon et al. Citation2017). A data linkage study by Su et al. (Citation2020) showed that Aboriginal children in the Northern Territory with hearing impairment had increased risk for developmental vulnerability. Schönweiler et al. (Citation1998) showed that increased speech and language pathologies and lower auditory perception skills were found in pre-school aged children with fluctuating conductive hearing loss, which was often not detected early enough (Schönweiler et al., 1998). However, findings from a longitudinal pregnancy cohort study in the general Western Australian population showed contradicting evidence that demonstrate no long-term benefit of ventilation tube insertion with regards to hearing and middle ear health (Alenezi et al. Citation2022).
Conductive hearing loss is more common in Aboriginal and/or Torres Strait Islander children than the general Australian child population (Australian Institute of Health and Welfare & Australian Institute of Family Studies Citation2014). To date, most research in this area has focused on the prevalence of conductive hearing loss in remote areas (Kaspar and Leach Citation2020). However, most (79%) Aboriginal and/or Torres Strait Islander people live in urban areas (Jennings et al. Citation2021). Consequently, investigating the burden of conductive hearing loss in an urban Aboriginal and/or Torres Strait Islander population is important, as demographic, geographic, climatic, historical, and social factors, including access to services, are different to those living in rural and remote areas (Eades et al. Citation2010). Guidelines have now been developed for Aboriginal and/or Torres Strait Islander infants in Western Australia (WA) for early OM screening at routine child health visits from two months of age (Western Australia Child and Adolescent Health Services Citation2020). However, this is still in the process of being implemented and does not include a routine hearing assessment before school age.
OM has been found to occur in up to 95% of Aboriginal infants as early as 2 months of age living in remote areas (Boswell and Nienhuys Citation1996), and continues to have a high prevalence after the first year of life when compared to non-Aboriginal children (Jervis-Bardy et al., Citation2014). To our knowledge, there is only one published community-based prospective study of the prevalence of hearing loss in Aboriginal and/or Torres Strait Islander infants under one year of age (Lehmann et al. Citation2008a). The Kalgoorlie Otitis Media Research Project (KOMRP) was conducted in and around a regional mining town in WA and found that more than 60% of Aboriginal infants had hearing loss at 6-11 months of age compared to 20% of non-Aboriginal infants of the same age. The prevalence of hearing loss and impact of OM in young infants has not been explored globally and not yet reported for young Aboriginal infants living in an Australian urban area. The Djaalinj Waakinj cohort study enrolled urban Aboriginal infants soon after birth and followed them to 12-18 months of age to determine the prevalence of and risk factors for OM and hearing loss. The proportion of children with OM was 35% at 2-4 months of age, and 49% at 6-8 and 12-18 months of age (Richmond et al. Citation2023). Among children in the cohort, prior OM was a strong predictor of OM at 12-18 months of age (Richmond et al. Citation2023). In a brief research letter, we have reported that 68.9% of 67 infants in this cohort had hearing loss at ∼12 months of age (Veselinović et al. Citation2022). However, in view of the issues around visual reinforcement audiometry (VRA) interpretation in infants, correction factors should be considered for a more accurate estimation of hearing loss in the cohort (British Society of Audiology Citation2014). This is because a minimum response level (MRL) rather than a hearing threshold estimate in infants under 12 months is often obtained with VRA (Norrix Citation2015; Widen Citation1990). The British Society of Audiology clinical guidelines suggest that for 7-12-month-olds tested in a free field environment, such as in the study of Veselinović et al. (Citation2022), responses are approximately 10 dB better relative to adult thresholds (from 500 – 4000 Hz). As such, the estimated proportion of infants with hearing loss previously reported using VRA may be an over-estimation of infants with hearing loss.
This paper presents i) ear and hearing outcomes from the Djaalinj Waakinj cohort study in greater depth than previously published in a brief research letter (Veselinović et al., Citation2022), ii) associations between sociodemographic/environmental characteristics and prior documented OM and subsequent hearing loss at ∼12 months of age and iii) a comparison between our study classification of hearing loss based on VRA responses (with a correction factor) and other international classifications.
Methods
The Djaalinj Waakinj (meaning ‘listening, talking’ in Noongar language) urban Aboriginal birth cohort study was established in 2017 in Perth, WA, to determine the prevalence of OM, OM-related hearing loss and associated risk factors in urban Aboriginal and/or Torres Strait Islander infants. A detailed methodology has been published (Swift et al. Citation2020). Here we discuss the methods used in the study relevant to the assessment of ear and hearing health.
Cultural governance and community engagement
An Aboriginal Community Advisory Group (ACAG) of 12 Aboriginal community members was established to provide a community voice and cultural governance for the project. The ACAG was consulted quarterly regarding all decisions, results, and future directions of the study (Swift et al. Citation2020).
Recruitment and enrolment protocol
Infants were recruited antenatally, soon after birth or referred before 12 weeks of age by health services in the south metropolitan region of Perth. An Aboriginal Research Assistant (ARA) and a Registered Nurse (RN) conducted home visits and explained the study to participants’ parents. The inclusion criteria for the study were: being an Aboriginal and/or Torres Strait Islander child living in the south metropolitan area of Perth, intending to remain in the area for 18 months, and informed consent given. Infants who met the inclusion criteria were enrolled before 12 weeks of age. Demographic, obstetric, social, and environmental data were collected at enrolment and, for time-dependant variables, again at subsequent visits.
Ethical considerations
Ethical approvals to conduct the Djaalinj Waakinj study were obtained from the Western Australian Aboriginal Human Ethics Committee (WAAHEC #759) and Child and Adolescent Health Services Human Research Ethics Committee (CAHS HREC #12).
Routine ear health screening
Routine ear health screenings were to be conducted by the ARA and RN in participants’ homes at 2-4, 6-8 and 12-18 months of age when a clinical history was also taken, and a general health check performed. NBHS results were documented based on available clinical reports at the 2–4-month visit. At each of the three routine ear screenings, otoscopy (using Welch Allyn) was used to inspect the ear canal and tympanic membrane. Tympanometry, using either the Titan Middle Ear Analyser (Interacoustics) or GSI 39 or MI 44 tympanometers (Maico), was performed to assess middle ear function if no ear discharge was seen on otoscopy; 1000 Hz probe tone was used to assess infants at 2-4 months of age and 226 Hz probe tone thereafter. An overall tympanometry classification was based on the results obtained for the worst ear.
At the 2-4-month routine screening, transient-evoked otoacoustic emissions (TEOAEs) with an Otodynamics Otoport or distortion product otoacoustic emissions (DPOAEs) with a Titan Middle Ear Analyser were measured as an objective assessment of inner ear function, with the rationale previously reported (Swift et al. Citation2020). A pass result was obtained if OAEs with a signal-to-noise ratio greater than 3 dB (TEOAEs) or 6 dB (DPOAEs) at four or more of the test frequencies (1-6 kHz) (Norton et al. Citation2000).
Infants with ear discharge visualised at any visit were referred to a general practitioner (GP) and/or Ear, Nose and Throat (ENT) specialists, and the RN/ARA provided advice on tissue spearing/aural toileting and health promotion advice for managing discharging ears. For recurrent or persistent OM as determined by abnormal tympanometry at two consecutive routine ear health screenings, infants were also referred to the above-mentioned health professionals. If there were further concerns at any time, the clinical audiologist referred infants to an appropriate health professional.
Audiological assessment
An audiological assessment was to be conducted at 9-12 months of age by an audiologist in one of three community-based clinical settings. This assessment included otoscopy, tympanometry and VRA. The audiological assessment was at times completed outside the prescribed age range and often at the same time as the scheduled 12-18-month routine ear health screening.
Tympanometry results using a 1000 Hz probe tone (for 2-4-month routine ear health screening only) were classified by audiologists in accordance with the presence of a positive peak indicative of normal middle ear function and a negative or no peak, indicative of middle ear dysfunction (Kei et al. Citation2003). Tympanometry results using a 226 Hz probe tone were classified by an audiologist according to ear canal volume, static compliance and tympanometric peak pressure values. The normative values used are shown in Appendix 1 (Bluestone and Klein Citation2007). The overall tympanometry classification was based on the child’s worst ear. A classification of a type A tympanogram (normal) included bilateral type A or unilateral type A when the contralateral ear tympanogram result was unknown. A type B tympanogram included unilateral or bilateral type B or high-volume B results (the latter indicative of tympanic membrane perforation or grommet in situ). Bilateral type C tympanograms or unilateral type C with type A or unknown results in the contralateral ear were included as an overall type C tympanogram. Infants with overall type B or C tympanogram were classified as having an abnormal tympanogram, indicative of any OM, which was used throughout this paper when categorising infants with or without OM.
An overall diagnosis (for the worst ear) was provided by the audiologist based on otoscopy and tympanometry results. If these could not be confirmed, an unknown diagnosis was noted. The diagnostic classifications were normal, acute otitis media (AOM), eustachian tube dysfunction (ETD), otitis media with effusion (OME) or perforation/grommet. A normal diagnosis was determined by normal otoscopic findings and bilateral Type A tympanograms. AOM was classified by bulging, pink/red TM and type B tympanogram, ETD by normal, retracted, and/or dull TM otoscopic findings and type C tympanogram, and OME by normal, retracted, and/or dull TM and type B tympanogram. Perforation/grommet were classified by otoscopic observation (visible perforation or grommet in situ) and, where possible, with high volume type B tympanograms (at least unilaterally); however, if middle ear discharge was present then tympanometry was not performed but classified as a high-volume B.
VRA was performed in a sound-treated room, and frequency-specific hearing response levels were obtained in the free field using observed localisation cues. GSI 61, Interacoustics Equinox and MedRx Avant audiometers were used with warble or filtered narrowband noise stimuli at 500, 1000, 2000 and 4000 Hz presented through loudspeakers at a 1 m distance and an angle of 90° from the child’s ears. Average hearing responses, calculated from thresholds obtained for the better hearing ear, were used to classify degree of hearing loss. For this study, an average hearing response of 25 dB HL was classified as normal hearing, 26-40 dB HL as mild hearing loss, 41-60 dB HL as moderate hearing loss, 61-90 dB HL as severe hearing loss and >90 dB HL as profound hearing loss (Clark Citation1981).
Data analysis
Filemaker Pro version 15 was used for data storage. IBM SPSS Statistics software v28.0.0.0 (190) and Microsoft Excel were used for data analysis. Descriptive statistics were used to report demographic, obstetric and environmental risk factors, as well as otoscopy and OAE findings. For categorical variables, Pearson’s X2 test and Fisher’s Exact test (where the expected cell count was <5) were used to determine differences between groups of interest. Due to the small sample size, more detailed analysis of risk factors for hearing loss could not be conducted.
A correction factor of 10 dB was applied to overall and frequency-specific minimum hearing response levels (British Society of Audiology Citation2014), which was then used to recalculate mean hearing responses. We compared the above-mentioned classification of hearing loss used in this study with other classifications: applying the Global Burden of Disease (GBD) Hearing Loss Expert Group guidelines for hearing loss classification, the average hearing response ≤20 dB HL were re-classified as normal hearing, 21-34 dB HL as mild HL, 35-50 dB HL as moderate HL, 51-64 dB HL as moderately severe HL, 65-80 dB as severe HL and >80 dB HL as profound hearing loss (Stevens et al. Citation2013). Hearing responses were also re-classified as disabling hearing impairment, which was determined by average responses greater than 30 dB HL (World Health Organisation 2018). Independent samples t-test was used to compare mean hearing responses (four-frequency average) between groups of interest. One-way ANOVA was used to compare mean hearing loss of infants in those with 0, 1 or 2 prior OM events. In all analyses, a P value <0.05 was considered statistically significant.
Results
Characteristics of all study participants
In the Djaalinj Waakinj cohort, 52.8% (66/125) of participants were male. Twenty-one (16.8%) of the 125 enrolled infants were not seen at the 2-4-month visit. Of the 99 infants with documented NBHS results, 96.9% (96/99) passed (bilaterally) and 3.1% (3/99) did not pass (at least unilaterally).
Although OAEs were part of the first ear health screening test battery, for 53.5% (53/99) of the infants, OAEs could not be performed at the home visit and for 25.3% (25/99) of infants the results were not valid (likely due to background noise in the home environment). Successful results were therefore only obtained (at least unilaterally) in 26.3% (26/99) of infants. Of these 26 infants, 92.3% (24/26) passed and 7.7% (2/26) failed.
Appendix 2 shows the sociodemographic, obstetric, and environmental characteristics of the 67 infants who attended the audiological assessment at ∼12 months of age compared to those infants who did not attend. More male infants had an audiological assessment than females (X2= 4.1 (1 df, p = 0.04)), and more infants whose mothers were non-smokers were tested than infants of mothers who smoked (X2= 4.8 (1 df, p = 0.03)).
Characteristics of audiological assessment participants
shows the sociodemographic, obstetric, and environmental characteristics (collected at enrolment or on day of assessment) of the 67 study participants who had an audiological assessment. The mean age of infants who had an audiological assessment was 12.1 ± 2.3 months (SD) (range = 9.0 to 20.6 months). More males (21/43 (76.6%) had hearing loss than female (7/26 (23.3%) infants (X2= 5.4 (1 df, p = 0.02)) and more infants with OM (35/65 (53.8%)) on the day of audiological assessment had hearing loss (X2 = 5.8 (1 df, p = 0.02). Information on time-dependent variables (exposure to cigarette smoke, pacifier use and feeding) collected at the 12–18-month visit showed that more infants currently using a pacifier (13/26 (50%)) had hearing loss on the day of audiological assessment than those who were not currently using a pacifier (7/31 (22.5%); X2 = 4.7 (1 df, p = 0.03)). Smoking and feeding characteristics at 12-18 months did not differ between normal and hearing loss groups ((smoking: X2 = 0.58 (1 df, p = 0.45)); (feeding: X2 = 0.192 (1 df, p = 0.66)).
Table 1. Sociodemographic, obstetric, environmental characteristics at enrolment or at 2-4-month visit and presence or absence of OM at time of audiological assessment at ∼12 months of age among the 67 infants with and without hearing loss.
Otoscopic findings
Of the 67 infants who had an audiological assessment at ∼12 months of age, 43.3% (29/67) had bilateral normal TM observations, 8.9% (6/67) had bilateral bulging TMs and 6.0% (4/67) had bilateral retraction. Approximately 18% (12/67) of infants had unilaterally normal TM observations with either bulging of the TM in the other ear (2.9% (2/67)), retraction in the other ear (1.5% (1/67)) or the other ear’s TM observation was unknown (13.4% (9/67)). TM observation was unknown bilaterally for 19.4% (13/67) of infants, and for those with a known result in at least one ear (n = 54), 75.9% (41/54) had normal otoscopic results, 14.8% (8/54) had bulging TM, and 9.3% (5/54) had retraction observed.
Tympanometry findings
shows the tympanometry results obtained at each routine ear screening and during the audiological assessment among the 67 infants who had the audiology assessment. The mean age of routine ear health screenings was 2.7 ± 0.5, 7.1 ± 1.1, and 13.1 ± 1.8 months. Among those with valid tympanometry at 2-4, 6-8 and 12-18 months, 36.5% (19/52), 50.0% (25/50) and 64.3% (36/56) had abnormal tympanograms, respectively. At the audiological assessment, 53.8% (35/65) of infants had abnormal tympanograms. Of the infants with OM, 77.1% (27/35) had bilateral OM, and 22.9% (8/35) had unilateral OM.
Table 2. Number and proportion of infants with hearing loss according to different classifications, using corrected and uncorrected VRA responses in the Djaalinj Waakinj cohort study (n = 67).
Table 3. Tympanometry results for each assessment time-point among 67 infants who had an audiological assessment at ∼12 months of age.
Overall diagnosis
Based on overall diagnoses (as per audiologist based on otoscopy and tympanometry), 40.3% (27/67) of infants had normal ears at their audiological assessment. The most common middle ear diagnosis was OME (35.8% (24/67)) while 6.0% (4/67) had AOM and 9.0% (6/67) had ETD; 1.5% (1/67) had a wet perforation and 1.5% (1/67) had patent grommets. For 6.0% (4/67) of infants, a diagnosis was not clear.
Detailed VRA findings
The overall average corrected hearing response was significantly worse in infants with OM (defined by type B or C tympanogram) (30.35 dB HL (95% CI: 27.24, 34.59)) than in those without OM (20.69 dB HL (95% CI: 18.04, 23.51)) on the day of audiological assessment (p < 0.001). Of the infants that had OM on the day, 40.0% (14/35) had normal hearing and 60.0% (21/35) had hearing loss. Of the infants with an overall OME diagnosis, 70.8% had hearing loss; infants with ETD diagnosis, 33.3% had hearing loss and all children with an AOM diagnosis had hearing loss. Of the infants without OM on the day of audiological assessment, 70.0% (21/30) had normal hearing and 30.0% (9/30) had a hearing loss.
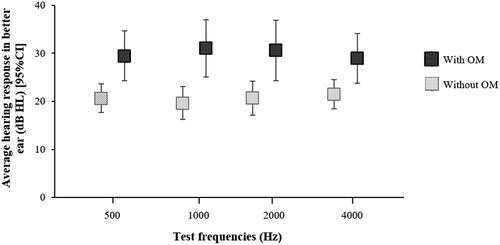
Infants with OM on the day of audiological assessment had average corrected frequency-specific responses of 29.50 dB HL (95% CI: 24.32, 34.68), 31.06 dB HL (95% CI: 25.14, 36.98), 30.62 dB HL (95% CI: 24.35, 36.89) and 28.97 dB HL (95% CI: 23.81, 34.12) at 500, 1000, 2000 and 4000 Hz, respectively, compared to the infants without OM on the day, who had corrected frequency-specific responses of 20.67 dB HL (95% CI: 17.66, 23.68), 19.67 dB HL (95% CI: 16.28, 23.06), 20.69 dB HL (95% CI: 17.14, 24.24), and 21.50 dB HL (95% CI: 18.43, 24.57) at 500, 1000, 2000 and 4000 Hz, respectively (). The difference in hearing responses between those with and without OM was significant at all tested frequencies; 500 Hz (p = 0.003), 1000 Hz (p < 0.001), 2000 Hz (p = 0.004) and 4000 Hz (p = 0.005).
Figure 1. Average corrected hearing responses (dB HL) of infants in the Djaalinj Waakinj study with OM (n = 35) and without OM (n = 30) at ∼12 month of age.
Using the study hearing loss classification with corrected VRA responses, 55.3% (37/67) of infants had normal hearing on the day, 35.8% (24/67) had a mild hearing loss, and 9.0% (6/67) had a moderate hearing loss. shows the proportion of infants with hearing loss (corrected or uncorrected VRA responses) according to our study classification compared with results using the GBD classification or the WHO disabling hearing impairment classification. Using the GBD classification 49.2% (33/67) had normal hearing while 20.9% (14/67) had a mild hearing loss, and 29.8% (20/67) had moderate hearing loss. Using the WHO disabling hearing impairment classification, 38.8% (26/67) had disabling hearing loss (using corrected responses).
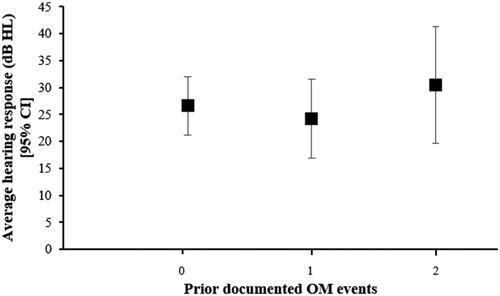
The impact of prior OM events on average corrected hearing responses at 12 months of age was analysed comparing infants with prior documented OM at 2-4 and/or 6-8 months of age with infants who had no reported prior OM (). This analysis included only those infants who attended both prior ear health screening appointments (n = 38). Of the infants who had no prior OM, 50.0% (8/16) had hearing loss (≥26 dB) at ∼12 months of age with a mean corrected hearing response of 26.61 dB HL (95% CI: 21.22, 32.01) for this group. Of the infants with OM at either 2-4 or 6-8 months, 33.3% (5/15) had subsequent hearing loss at ∼12 months of age with a mean corrected hearing reponse of 24.19 dB HL (95% CI: 16.91, 31.48). Of the infants that had OM at both 2-4 and 6-8 months, 57.1% (4/7) had subsequent hearing loss at ∼12 months of age with a mean corrected hearing response of 30.48 dB HL (95% CI: 19.64, 41.30); however, this was not significantly different to the mean hearing responses in other groups (one-way ANOVA (F (2, 36) = 0.709, p = 0.499)).
Discussion
This paper presents the audiological findings from the Djaalinj Waakinj cohort study, which is the first study in Australia to provide estimates of the proportion of urban Aboriginal infants with OM and hearing loss and to apply the recommended correction for VRA responses in infants. Using corrected VRA responses we found 44.8% of tested infants had hearing loss (≥26 dB HL) at ∼12 months of age. Infants with OM at the time of assessment had significantly worse hearing responses compared to those without OM (). The previously published work describing hearing loss in this study population showed a higher proportion of infants with hearing loss (68.9% of infants ≥26 dB HL) in at least the better ear (Veselinović et al. Citation2022). These earlier estimates were comparable to those in another study conducted in Kalgoorlie WA, which reported >60% of hearing loss using the same method and classification for infants of a similar age (Lehmann et al. Citation2008a). However, both reports did not consider a correction factor for VRA responses as we have applied here, which is recommended by the British Society of Audiology (British Society of Audiology Citation2014) and the Barnsley Neonatal Hearing Screening Protocol for VRA testing (Day et al. Citation2008).
We have compared VRA classification (with and without correction factor) used in our study with different international classifications of hearing loss (). It is a serious concern that 39% to 51% of the infants (depending on the classification used) had hearing loss at ∼12 months of age after adjusting with the recommended correction factor. There are difficulties with VRA interpretation, the overestimation of hearing loss with behavioural responses, and overall, how VRA responses are used to determine MRL rather than thresholds (Norrix Citation2015). However, as no alternative behavioural tests are currently available to assess infants in a community setting, the use of the VRA with the correction factor could overcome some of these challenges and may provide a more accurate estimate of the burden of hearing loss in infants. Given most infants with hearing loss in this study had OM at the time of assessment, these findings demonstrate the impact of OM on Aboriginal infants with the known impacts of fluctuating hearing loss in this infant population of notable concern (Schönweiler et al., 1998).
To our knowledge, no studies have reported prevalence of hearing loss in First Nations infants. However, a recent study in older school aged Canadian First Nations children reported a hearing loss prevalence of ∼23.5%, largely related to OM (Fitzpatrick et al. Citation2021). Another study conducted in USA investigated the prevalence of OM-related hearing loss in the general paediatric population and found that 15% had hearing loss (>20 dB HL) at one year of age, 40% of whom had confirmed OME, while more than half of the infants with hearing loss did not have OM on the day (Gravel and Wallace Citation2000). As found by Gravel and Wallace (Citation2000) many infants at ∼12 months without OM, showed to have hearing loss, further affirming the possible need to use a correction factor when using VRA to establish more accurate hearing responses in infants, and improve prevalence estimates. A recent study of urban Aboriginal children showed ∼20% of the children aged 3.5-6 years had hearing loss, and half of the children had OM from 6 months − 3.5 years (DeLacy et al. Citation2023), with two-thirds of children in the study with OM and hearing impairment. DeLacy et al. (Citation2023), as with other OM studies, did not evaluate the hearing responses in young infants (<12 months), making it difficult to compare the prevalence reported between studies.
The proportion of infants with documented demographic and environmental risk factors was generally similar among infants with and without hearing loss in this cohort (), aside from sex, OM at time of audiology assessment and pacifier use at 12-18 months of age. These findings are consistent with previous literature that report higher OM prevalence in males (Kvestad et al. Citation2004), which is the case for many infectious diseases (Muenchhoff and Goulder Citation2014). OM is the most significant contributing factor to conductive hearing loss in children (Gunasekera et al. Citation2009), and the higher hearing loss prevalence among infants with OM in this cohort was in line with the literature. Infants that used a pacifier had a high proportion of hearing loss than non-users, which in line with pacifier use being a risk factor for OM as it is thought to cause reflux of nasopharyngeal secretions into the middle ear as well as lead to dysfunction to the Eustachian tube (Rovers et al. Citation2008).
The otoscopic findings and overall diagnoses in this study show that most infants had OME, with over two-thirds with hearing loss, and likely the main cause of hearing loss in this cohort. Gunasekera et al. (Citation2009) has also reported OME as a significant contributor to hearing loss in early childhood for Aboriginal and/or Torres Strait Islander children (Gunasekera et al. Citation2009). In Aboriginal and/or Torres Strait Islander infants in particular, this high prevalence of OME should prompt increased efforts for early detection as OME is often asymptomatic, but the associated hearing loss can result in delayed development (Su et al. Citation2020). It is likely that the low numbers of AOM reflect the study design and more regular surveillance would be required to detect the incidence of AOM, due to the acute episodic nature of the disease – unlike OME, which is often persistent.
In this study, we did not find an association between prior documented OM episodes and subsequent hearing loss in the small subset of 38 infants seen at all timepoints (); however, 4 of the 7 infants who had two or more prior OM events had hearing loss at ∼12 months of age. Future studies with a larger sample size, more frequent assessments in the short-term and including a long-term follow-up would provide more insight on the impact of prior OM on hearing responses later in life.
The main limitation of this study is the small sample size with limited follow-up to the age of 12 months, despite the intensive efforts made by two local Aboriginal researchers (VW, NM) and a nurse well-known to the community (JD) to contact families frequently during the day and evening through home visits, phone calls, health services and contact with family members. Approximately half (53.6%) of infants enrolled in the study had audiological assessments, which is a lower attendance than the comparable KOMRP study (62%), and lower than a WA urban Aboriginal longitudinal cohort (65%) (Lehmann et al. Citation2008b; Eades Citation2003); however, both cohort studies were conducted ∼20 years ago. Recruitment, follow-up, and audiological assessments ceased during the COVID-19 pandemic, which contributed to lower attendance in the Djaalinj Waakinj cohort study. Cohort studies involving infants are challenging to complete due to the competing priorities present for young families caring for their children. This is particularly true for Aboriginal and/or Torres Strait Islander families, who may face numerous conflicting daily commitments; details of reasons for loss to follow-up have been described elsewhere (Swift et al. Citation2020). While larger studies are required, our findings reaffirm the importance of early surveillance, with prompt referral to audiology services in accordance with the most recent OM guidelines (Leach et al. Citation2021), and the critical role First Nations workers play in ear and hearing health surveillance (Poirier et al. Citation2022).
The current national guidelines in Australia outline audiological assessment and management strategies from birth to 5 years for Aboriginal and/or Torres Strait Islander children (Leach et al. Citation2021; https://otitismediaguidelines.com). The OM guidelines recommend ear health surveillance at childhood immunisation visits, child health checks, and opportunistically at other clinical interactions, which may include otoscopy, tympanometry and then audiometry for children >3.5 years. Resource limitations in community-based settings impede access to VRA testing for children <3.5 years necessitating prompt referral for audiological assessment is for Aboriginal and/or Torres Strait Islander children with recurrent AOM or persistent OME. Due to resource limitations in community-based settings, VRA testing for children <3.5 years may not be feasible and as such, prompt referral for external audiological assessment is required for Aboriginal and/or Torres Strait Islander children with recurrent AOM or persistent OME.
The Djaalinj Waakinj study highlights the high rates of hearing loss and OM in Aboriginal infants. It demonstrates the need for early detection of OM and timely and appropriate ear health services to prevent OM-associated hearing loss and improve developmental and educational outcomes for Aboriginal and/or Torres Strait Islander children in urban areas. This paper explores the use of a VRA correction factor for young Aboriginal and/or Torres Strait Islander infants, which may be more appropriate for the interpretation of hearing responses in young infants; however, further validation of the correction factor is required due to its limited mainstream clinical applications internationally. Further research is needed to determine the long-term developmental consequences of early life hearing loss in urban Aboriginal and/or Torres Strait Islander children. The findings from this study will help inform national policy and practice to improve access to ear and hearing screening in very early life for Aboriginal and/or Torres Strait Islander infants. Policymakers should implement the recommendation by the OM guidelines for routine, early screening for OM in urban Aboriginal and/or Torres Strait Islander infants and prompt referral for audiological assessment by 12 months of age as outlined in the national guidelines (Leach et al. Citation2021).
Supplemental Material
Download MS Word (41.5 KB)Acknowledgements
The authors acknowledge that this research was conducted on Whadjuk Noongar Boodja, and we pay our respects to Aboriginal and/or Torres Strait Islander Elders past, present and emerging. The authors thank all the families who agreed to take part in the study. The authors thank the members of the Aboriginal Community Advisory Group (the late Eric Wynne, Leon Hayward, Doris Getta, Hannah Nelson, Ricki Lee Dabb, Raelene Hayward, Doreen Nelson, Helen Kickett, Glenys Yarran, Justin Kickett, Chantale Yarran, O’Sheala Yarran, Glen Hayden, Helen Walley-Stack) for their contribution to this project since its inception and for their continuing guidance and wisdom. ENT specialists Drs Francis Lannigan, George Sim and Anton Hinton-Barr provided clinical support and expertise and we thank Kirsty Tomlinson and Tooey Tran, Perth Children’s Hospital (PCH) for promoting the study with their peers and supporting the families to attend appointments at PCH. We thank Clory Carrello who enabled us to establish our office at Cockburn Integrated Health (CIH) and is committed to establishing a strong ear health program. The authors thank the staff at Hearing Australia, Telethon Speech & Hearing, Child Adolescent Health Services-Child Development Services for their ongoing support and provision of clinical facilities to conduct hearing assessments. We thank the staff at Boodjari Yorgas Midwifery Group Practice at Armadale Hospital, South Coastal Babbingur Mia, South Metropolitan Health Services’ Community Health services, and Fiona Stanley Hospital for promoting the study and referring families to the study, and to staff at Moorditj Koort Aboriginal Health Wellbeing Service and Derbarl Yerrigan Health Services for their ongoing support. The authors thank Danny Ford from Kambarang Services for facilitating our initial community forums in Armadale and Kwinana.
Disclosure statement
No potential conflict of interest was reported by the author(s).
References
- Alenezi, E. M., M. Robinson, R. S. Choi, T. Veselinović, P. C. Richmond, R. H. Eikelboom, and C. G. Brennan-Jones. 2022. “Long-Term Follow-Up After Recurrent Otitis Media And Ventilation Tube Insertion: Hearing Outcomes And Middle-Ear Health At Six Years Of Age.” International Journal of Pediatric Otorhinolaryngology 163:111379. https://doi.org/10.1016/j.ijporl.2022.111379
- Altamimi, A. A., M. Robinson, E. J. McKinnon, E. M. Alenezi, T. Veselinović, R. S. Choi, and C. G. Brennan-Jones. 2023. “The Association Between Otitis Media In Early Childhood With Later Behaviour And Attention Problems: A Longitudinal Pregnancy Cohort.” International Journal of Pediatric Otorhinolaryngology 168:111545. https://doi.org/10.1016/j.ijporl.2023.111545
- Australian Institute of Health and Welfare & Australian Institute of Family Studies. 2014. “Ear Disease in Aboriginal and Torres Strait Islander children.” Closing the Gap Clearinghouse. Canberra: Australian Institute of Health and Welfare & Melbourne: Australian Institute of Family Studies.
- Bluestone, C. D., and J. O. Klein. 2007. Otitis Media in Infants and Children (4th ed). Canada: BC Decker.
- Brennan-Jones, C. G., A. J. O. Whitehouse, S. D. Calder, C. D. Costa, R. H. Eikelboom, W. Swanepoel, and S. E. Jamieson. 2020. “Does otitis media affect later language ability? A prospective birth cohort study.” Journal of Speech, Language, and Hearing Research : JSLHR 63 (7):2441–2452. https://doi.org/10.1044/2020_JSLHR-19-00005
- British Society of Audiology. 2014. “Recommended Procedure: Visual Reinforcement Audiometry.” British Society of Audiology p. 17 http://www.thebsa.org.uk/wp-content/uploads/2014/04/BSA_VRA_24June2014_Final.pdf.
- Boswell, J. B., and T. G. Nienhuys. 1996. “Patterns of Persistent Otitis Media In The First Year Of Life In Aboriginal And Non-Aboriginal Infants.” The Annals of Otology, Rhinology, and Laryngology 105 (11):893–900. https://doi.org/10.1177/000348949610501110
- Cai, T., B. McPherson, C. Li, and F. Yang. 2018. “Pure Tone Hearing Profiles In Children With Otitis Media With Effusion.” Disability and Rehabilitation 40 (10):1166–1175. https://doi.org/10.1080/09638288.2017.1290698
- Clark, J. G. 1981. “Uses and Abuses of Hearing Loss Classification.” American Speech-Language-Hearing Association 23:493–500.
- Day, J., R. Green, K. Munro, G. Parry, P. Shaw, S. Wood, E. Brown, and G. Sutton. 2008. “Newborn HEARING and Assessment. Visual Reinforcement Audiometry Testing for Infants: A Recommended Test Protocol.” https://eratraining.co.uk/onewebmedia/NHSP%20VRA_protocol.pdf
- DeLacy, J., L. Burgess, M. Cutmore, S. Sherriff, S. Woolfenden, K. Falster, E. Banks, A. Purcell, K. Kong, H. Coates, et al. 2023. “Ear Health and Hearing In Urban Aboriginal Children.” Australian and New Zealand Journal of Public Health 47 (4):100075. https://doi.org/10.1016/j.anzjph.2023.100075
- Eades, S. J., B. Taylor, S. Bailey, A. B. Williamson, J. C. Craig, and S. Redman, SEARCH Investigators 2010. “The Health of Urban Aboriginal people: Insufficient Data to Close The Gap.” The Medical Journal of Australia 193 (9):521–524. https://doi.org/10.5694/j.1326-5377.2010.tb04036.x
- Eades, S. J. 2003. “Bibbulung Gnarneep (Solid Kid). A Longitudinal Study Of A Population-Based Cohort of Urban Aboriginal Children In Western Australia.“” Determinants of health outcomes during early childhood of Aboriginal children residing in an urban area.”., PhD thesis., Perth: The University of Western Australia
- Fitzpatrick, E. M., L. McCurdy, J. Whittingham, R. Rourke, F. Nassrallah, V. Grandpierre, F. Momoli, and V. Bijelic. 2021. “Hearing Loss Prevalence And Hearing Health Among School-Aged Children In The Canadian Arctic.” International Journal of Audiology 60 (7):521–531. https://doi.org/10.1080/14992027.2020.1731616
- Gravel, J. S., and I. F. Wallace. 2000. “Effects of Otitis Media with Effusion on Hearing in the First 3 Years of Life.” Journal of Speech, Language, and Hearing Research : JSLHR 43 (3):631–644. https://doi.org/10.1044/jslhr.4303.631
- Graydon, K., G. Rance, R. Dowell, and B. Van Dun. 2017. “Consequences of Early Conductive Hearing Loss On Long-Term Binaural Processing.” Ear and Hearing 38 (5):621–627. https://doi.org/10.1097/AUD.0000000000000431
- Gunasekera, H., P. S. Morris, P. McIntyre, and J. C. Craig. 2009. “Management of Children With Otitis Media: A Summary Of Evidence From Recent Systematic Reviews.” Journal of Paediatrics and Child Health 45 (10):554–563. https://doi.org/10.1111/j.1440-1754.2009.01564.x
- Hearing Australia. 2022. “HAPEE ears for early years. https://www.hearing.com.au/Hearing-loss/HAPEE.
- Jennings, W., G. Spurling, B. Shannon, N. Hayman, and D. Askew. 2021. “Rapid Review Of Five Years Of Aboriginal And Torres Strait Islander Health Research In Australia - Persisting Under-Representation Of Urban Populations.” Australian and New Zealand Journal of Public Health 45 (1):53–58. https://doi.org/10.1111/1753-6405.13072
- Jervis-Bardy, J., L. Sanchez, and A. S. Carney. 2014. “Otitis Media In Indigenous Australian Children: Review Of Epidemiology And Risk Factors.” The Journal of Laryngology and Otology 128 Suppl 1 (S1):S16–S27. / https://doi.org/10.1017/S0022215113003083
- Kaspar, A., and A. J. Leach. 2020. “Hearing Loss Among Australian Aboriginal Infants And Toddlers: A Systematic Review.” Public Health in Practice (Oxford, England) 1:100048. https://doi.org/10.1016/j.puhip.2020.100048
- Kei, J., J. Allison-Levick, J. Dockray, R. Harrys, C. Kirkegard, J. Wong, M. Maurer, J. Hegarty, J. Young, D. Tudehope, et al. 2003. “High-Frequency (1000 Hz) Tympanometry In Normal Neonates.” Journal of the American Academy of Audiology 14 (1):20–28. https://doi.org/10.3766/jaaa.14.1.4
- Kong, K., and H. L. Coates. 2009. “Natural history, Definitions, Risk Factors And Burden Of Otitis Media.” The Medical Journal of Australia 191 (S9):S39–S43. https://doi.org/10.5694/j.1326-5377.2009.tb02925.x
- Kvestad, E., K. J. Kvaerner, E. Røysamb, K. Tambs, J. R. Harris, and P. Magnus. 2004. “Otitis Media: Genetic Factors And Sex Differences.” Twin Research : The Official Journal of the International Society for Twin Studies 7 (3):239–244. https://doi.org/10.1375/136905204774200514
- Leach, A. J., P. S. Morris, H. L. Coates, S. Nelson, S. J. O'Leary, P. C. Richmond, H. Gunasekera, S. Harkus, K. Kong, C. G. Brennan-Jones, et al. 2021. “Otitis Media Guidelines For Australian Aboriginal And Torres Strait Islander Children: Summary Of Recommendations.” The Medical Journal of Australia 214 (5):228–233. https://doi.org/10.5694/mja2.50953
- Lehmann, D., S. Weeks, P. Jacoby, D. Elsbury, J. Finucane, A. Stokes, R. Monck, and H. Coates, Kalgoorlie Otitis Media Research Project Team 2008a. “Absent Otoacoustic Emissions Predict Otitis Media In Young Aboriginal Children: A Birth Cohort Study In Aboriginal And Non-Aboriginal Children In An Arid Zone Of Western Australia.” BMC Pediatrics 8 (1):32. & https://doi.org/10.1186/1471-2431-8-32
- Lehmann, D., A. Arumugaswamy, D. Elsbury, J. Finucane, A. Stokes, R. Monck, C. Jeffries-Stokes, D. McAullay, H. Coates, and F. J. Stanley. 2008b. “The Kalgoorlie Otitis Media Research Project: Rationale, Methods, Population Characteristics And Ethical Considerations.” Paediatric and Perinatal Epidemiology 22 (1):60–71. https://doi.org/10.1111/j.1365-3016.2007.00891.x
- Muenchhoff, M., and P. J. Goulder. 2014. “Sex Differences in Pediatric Infectious Diseases.” The Journal of Infectious Diseases 209 (Suppl 3):S120–S126. https://doi.org/10.1093/infdis/jiu232
- Norrix, L. W. 2015. “Hearing Thresholds, Minimum Response Levels, and Cross-Check Measures in Pediatric Audiology.” American Journal of Audiology 24 (2):137–144. https://doi.org/10.1044/2015_AJA-14-0095
- Norton, S. J., M. P. Gorga, J. E. Widen, R. C. Folsom, Y. Sininger, B. Cone-Wesson, B. R. Vohr, K. Mascher, and K. Fletcher. 2000. “Identification of Neonatal Hearing Impairment: Evaluation Of Transient Evoked Otoacoustic Emission, Distortion Product Otoacoustic Emission, And Auditory Brain Stem Response Test Performance.” Ear and Hearing 21 (5):508–528. https://doi.org/10.1097/00003446-200010000-00013
- Poirier, B., L. Quirino, M. Allen, R. Wilson, and J. Stephens. 2022. “The Role Of Indigenous Health Workers In Ear Health Screening Programs For Indigenous Children: A Scoping Review.” Australian and New Zealand Journal of Public Health 46 (5):604–613. / https://doi.org/10.1111/1753-6405.13291
- Richmond, H. J., V. M. Swift, J. E. Doyle, N. R. Morrison, S. A. Weeks, T. Veselinović, P. Jacoby, C. G. Brennan-Jones, P. C. Richmond, and D. Lehmann. 2023. “Early Onset Of Otitis Media Is A Strong Predictor Of Subsequent Disease In Urban Aboriginal Infants: Djaalinj Waakinj Cohort Study.” Journal of Paediatrics and Child Health 59 (5):729–734. https://doi.org/10.1111/jpc.16378
- Rovers, M. M., M. E. Numans, E. Langenbach, D. E. Grobbee, T. J. Verheij, and A. G. Schilder. 2008. “Is Pacifier Use A Risk Factor For Acute Otitis Media? A dynamic cohort study.” Family Practice 25 (4):233–236. https://doi.org/10.1093/fampra/cmn030
- Schönweiler, R., M. Ptok, and H. J. Radü. 1998. “A Cross-Sectional Study of Speech-And Language-Abilities of Children With Normal Hearing, Mild Fluctuating Conductive Hearing Loss, or Moderate to Profound Sensorineural Hearing Loss.” International Journal of Pediatric Otorhinolaryngology 44 (3):251–258. https://doi.org/10.1016/S0165-5876(98)00075-5
- Stevens, G., S. Flaxman, E. Brunskill, M. Mascarenhas, C. D. Mathers, and M. Finucane, Global Burden of Disease Hearing Loss Expert Group 2013. “Global and Regional Hearing Impairment Prevalence: An Analysis of 42 Studies in 29 Countries.” European Journal of Public Health 23 (1):146–152. https://doi.org/10.1093/eurpub/ckr176
- Su, J.-Y., S. Guthridge, V. Y. He, D. Howard, and A. J. Leach. 2020. “Impact of Hearing Impairment on Early Childhood Development in Australian Aboriginal children: A data Linkage Study.” Journal of Paediatrics and Child Health 56 (10):1597–1606. https://doi.org/10.1111/jpc.15044
- Swift, V. M., J. E. Doyle, H. J. Richmond, N. R. Morrison, S. A. Weeks, P. C. Richmond, C. G. Brennan-Jones, D. Lehmann, and D. Waakinj Team, Djaalinj Waakinj Team. 2020. “Djaalinj Waakinj (listening talking): Rationale, Cultural Governance, Methods, Population Characteristics–an Urban Aboriginal birth Cohort Study of Otitis Media.” Deafness & Education International 22 (4):255–274. & https://doi.org/10.1080/14643154.2020.1826101
- Veselinović, T., S. A. Weeks, V. M. Swift, D. Lehmann, and C. G. Brennan-Jones. 2022. “High Prevalence of Hearing Loss in Urban Aboriginal Infants: the Djaalinj Waakinj Cohort Study.” The Medical Journal of Australia 217 (1):46–47. https://doi.org/10.5694/mja2.51534
- Widen, J. E. 1990. “Behavioral Screening of High-Risk Infants Using Visual Reinforcement Audiometry.” Seminars in Hearing 11 (04):342–355. https://doi.org/10.1055/s-0028-1085514
- Williams, C. J., and A. M. Jacobs. 2009. “The Impact of Otitis Media On Cognitive And Educational Outcomes.” The Medical Journal of Australia 191 (S9):S69–S72. https://doi.org/10.5694/j.1326-5377.2009.tb02931.x
- Western Australia Child and Adolescent Health Services 2020. Hearing and Ear Health Guidelines. https://www.cahs.health.wa.gov.au/-/media/HSPs/CAHS/Documents/Community-Health/CHM/Hearing-and-ear-health.pdf?thn=0
- World Health Organization. 2018. Addressing the Rising Prevalence of Hearing Loss. Geneva. https://www.who.int/publications/i/item/addressing-the-rising-prevalence-of-hearing-loss