Abstract
Objective
To use a standardised reporting tool to identify potential eligible candidates for cochlear implant (CI) referral and quantify the proportion of adults who had a CI referral discussion after presenting with an audiogram within United Kingdom (UK) audiometric criteria.
Design
Retrospective multicentre 6-month audit of Audiology clinic databases.
Study sample
A total of 810 adults from five geographically diverse UK Audiology sites.
Results
Data were collected in late 2019 after UK CI audiometric candidacy criteria changed; one site collected only 3 months of data. The proportion of potential eligible adults (based only on audiometry) considered for CI referral was 64% (521 out of 810) and varied by site (from 50% to 83%). About 24% of patients (123 out of 521) declined CI referral; this also varied across sites (12–45%). The median age of patients where CI referral was not considered was 80 years – significantly higher than the group where CI referral was considered (73 years).
Conclusions
CI referral is dependent on where adults live, and how old they are. Older adults are significantly less likely to be considered for CI referral by Audiologists. Audiology clinics need more support to empower staff to talk to patients about CI referral.
Introduction
Severe to profound deafness can lead to anxiety, depression, and social isolation (Carlsson et al. Citation2015; Kim et al. Citation2017). Hearing loss is the largest of the twelve potentially modifiable risk factors for dementia (Livingston et al. Citation2020). Cochlear implants (CIs) are a cost-effective intervention that generally reduce the economic impact of severe to profound deafness (O'Neill, Lamb, and Archbold Citation2016) and improve quality of life (Crowson et al. Citation2017; World Health Organisation Citation2021).
The uptake of CI by adults globally is disheartening, with only 5–13% of estimated audiometrically eligible adults receiving a CI (De Raeve Citation2016; Nassiri, Sorkin, and Carlson Citation2022; Raine Citation2013) despite evidence showing large, life-changing benefits post-implantation (Gaylor et al. Citation2013; Ng et al. Citation2016). However, estimated figures of unmet need are typically based on prevalence of severe to profound hearing loss, and may not reflect CI candidacy (i.e. outcomes with hearing aids, desire to have a CI) (Looi, Bluett, and Boisvert Citation2017). The new United Kingdom (UK) NICE guidance was published on 7 March 2019 (National Institute for Health and Care Excellence Citation2019), enabling many more people to benefit from a CI. Candidacy for adult cochlear implantation in the UK is guided by audiometry and speech perception results. CIs will not be suitable for everyone with severe to profound deafness, and not all deaf people will want a CI.
The transition from hearing aids to CI represents a continuum of care for adults with severe to profound deafness (Buchman et al. Citation2020; Turton et al. Citation2020). This journey begins in local Audiology services and moves into specialist CI services. Determining CI candidacy, counselling and referral are usually performed by hearing aid Audiologists (not CI specialists), who may lack training on CI candidacy and have low confidence discussing CI (Bierbaum et al. Citation2020; Chundu and Buhagiar Citation2013; Raine Citation2013). The journey from hearing aids to CI is considered complex and convoluted by both patients and clinicians (Rapport et al. Citation2020) which may contribute to low uptake (Looi, Bluett, and Boisvert Citation2017). Cullington et al. (Citation2022) introduced the “leaky pipe” analogy to explain why so many adults with severe to profound deafness do not have a CI. They suggested that adults ‘drop out’ of the process at all stages – from the Audiology clinic (referral not mentioned, patient declined referral) to the CI centre (patient not suitable, patient declined assessment, patient declined surgery). This is supported by Australian data showing that of 18 adults within candidacy criteria, 7 were not referred and only 4 ended up having a CI (Looi, Bluett, and Boisvert Citation2017).
Informed choice and shared decision-making are firmly embedded in National Health Service (NHS) policies (NHS England and NHS Improvement Citation2019) and NICE guidance (National Institute for Health and Care Excellence Citation2021). All adults within candidacy criteria should have CI candidacy explored and explained, with accessible verbal and written information on potential benefits and limitations. Patterns in CI referral in the UK are unknown, but the literature suggests CI referral is limited by a number of patient and professional factors (Bierbaum et al. Citation2020; Ebrahimi-Madiseh et al. Citation2020; Nassiri et al. Citation2021; Raine Citation2013).
Aims
This pilot audit used a standardised reporting tool to identify potential eligible candidates for CI referral in the UK’s National Health Service; it aimed to quantify the proportion of adults who had a CI referral discussion after presenting with an audiogram within NICE audiometric criteria.
Audiometry is only part of the candidacy criteria in the UK; this pilot audit only examined audiometry.
Research questions
What proportion of adults with an audiogram meeting NICE criteria had CI referral discussion?
Did the proportions differ across five UK Audiology clinics?
Did patient age, sex, pure tone average (PTA) in better ear or degree of audiometric slope affect CI referral being discussed?
What proportion of those offered CI referral declined referral?
Did the proportions differ across five UK Audiology clinics?
Did patient age, sex, PTA in better ear or degree of audiometric slope affect the proportion of patients who declined CI referral?
Materials and methods
Development of the Crystal report
An Auditbase Crystal report was developed by Auditdata and Cochlear®, with input from authors. Auditbase is the most common UK Audiology patient management system (PMS).
Participating sites
Five Audiology sites developed and tested the report, based on their previous experiences with building and running Crystal reports and/or interest in auditing CI referral in their clinic. Three sites had an integrated CI programme (same database) (sites C, D and E), and two referred to external CI centres (sites A and B). The sites represented diverse geographical locations and covered inner city and rural areas. NHS hospitals do not have clearly defined geographical areas as patients can attend any hospital, so it is difficult to define their catchment population. However, the hospital locations ranged from being situated in the 10% most deprived location of England to the 20% least deprived (gov.uk Citation2019).
Data collection and categorisation
The Crystal report was retrospectively run from 1 July 2019 to 31 December 2019 (6 months) at four sites (A, B, C, E), and from 1 September to 30 November 2019 (3 months) at one site (D). The report returned a spreadsheet of all adults with audiograms done during this period within NICE criteria for CI. The authors then searched for information regarding whether CI referral had been discussed with the patient at any point in their current pathway (typically the last 3–5 years), using hand searching of paper notes and/or digital records within Auditbase. Auditbase has a search function in the ‘journal’ area, so that may have been used, but the exact method used to interrogate Auditbase was not noted. All cases were then retrospectively categorised (). If there was no documentation that a CI discussion had occurred, it was assumed it had not happened.
Table 1. Audit categories.
Additional non-analysed categories were: patient seen in ENT but not seen in Audiology service/not under the Audiology service (e.g. private hearing aids) (CI6), subsequently deceased (CI7), using CI already (CI8), and missing data (CI9). These data were removed from the analysis, as the aim was to assess whether Audiologists were discussing referral with potential eligible patients.
Categories CI1–CI4 represent cases where CI discussion was documented. To meet the gold standard that all suitable adults are considered for referral, all cases should be in categories CI1–CI4.
Research and development approval
Audits were registered with local NHS Trust R&D.
Analysis
The number of patients in each category (CI1–CI5) and patient age and sex were obtained at each site. Pure tone air conduction thresholds were averaged at 500, 1000, 2000, 3000 and 4000 Hz. If there was no response, 5 dB was added to the last measured value e.g. no response at 120 dB was recoded to 125 dB. If data were missing for 500, 1000, 2000 or 4000 Hz, the average was not calculated. Pure tone average in better ear was chosen as the lowest of the two averages, or the only average if one was missing. In one case where the 2000 Hz threshold was missing in an ear with much better hearing, a better ear average was not included. To estimate audiometric slope, the magnitude of the difference between the thresholds at 500 and 4000 Hz was calculated for each ear, and the largest value taken. (The magnitude was used to cater for reverse slope audiograms where the 4000 Hz threshold is better (lower) than the 500 Hz threshold).
Data distribution of the categorical variables was assessed using p-p plots (age, audiometric threshold in better ear, slope); all showed violation from normal distribution and were analysed using non-parametric tests (Mann–Whitney). Differences in proportion were assessed using the Chi-square test. The p value was set to 0.05. Analysis was done using IBM SPSS version 29 (SPSS Inc., Chicago, IL).
Results
A total of 875 adults across the five centres who had done an audiogram in the time period met the NICE audiometric criteria for CI referral. A total of 65 patients were excluded from analysis because they had not been seen in Audiology, they had since died, they already had a CI or their data were missing (categories CI6–CI9). This left 810 patients aged from 18 to 105 years (354 female, 451 male, 5 unknown) where a discussion about CI referral should have been considered.
Research question
What proportion of adults with an audiogram meeting NICE criteria had CI referral discussion?
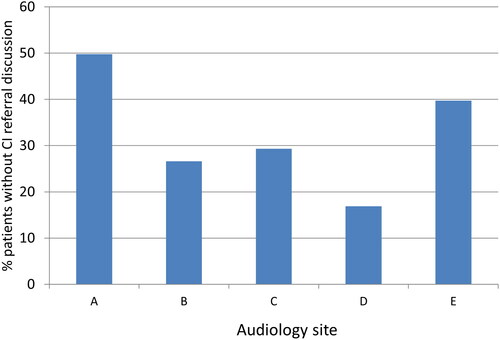
There was documented consideration of CI referral (categories CI1 to CI4) in 521 out of 810 cases (64%); CI referral discussion did not occur (CI5) in 289 out of 810 patients (36%).
Did the proportions differ across five UK Audiology clinics?
The proportion of people where CI referral discussion was considered varied significantly from 50% to 83% across the five centres (Chi-square (4, n = 810) = 38.5 p = 0.000). This means that in 17–50% of cases of severe to profound hearing loss, there was no documentation that CI discussion had occurred ().
Did patient age, sex, pure tone average in better ear or degree of audiometric slope affect CI referral being discussed?
Figure 1. Percentage of cases of severe to profound hearing loss where CI referral was not discussed (numbers at each site are A: n = 97 out of 195; B: n = 29 out of 109, C: n = 54 out of 184, D: n = 14 out of 83, E: n = 95 out of 239).
Age
The median age of patients where CI referral was considered was 73 years (range = 18–105 years; sd = 20 years); the median age for patients where CI referral was not considered was 80 years (range = 18–105; sd = 16 years). Those patients where CI referral was considered were significantly younger than those where CI referral was not considered (Mann–Whitney U = 57188.5, Z = −5.674, p = 0.000).
Sex
There was no significant difference in the sex of people in the group where CI discussion occurred and the group where it did not occur (Chi-square (1, n = 805) = 2.488 p = 0.115).
Pure tone average (PTA)
The median pure tone average in the better ear for patients where CI referral was considered was 85 dBHL (range = 46–123 dBHL; sd = 16 dB); median pure tone average in the better ear for patients where CI referral was not considered was 77 dBHL (range = 43–118 dBHL; sd = 14 dB). Those patients where CI referral was considered had significantly worse (higher) pure tone average in the better ear than those where CI referral was not considered (U = 53952.0, Z = −6.619, p = 0.000).
Slope
The median slope was 35 dB (range = 0–110 dB; sd = 24 dB) in the group where CI referral was considered and 40 dB (range = 0–100 dB; sd = 24 dB) in the group where CI referral was not considered. There was not a statistically significant difference in the slope parameter in patients where CI referral was and was not considered (Mann–Whitney U = 69429.0, Z = −1.797, p = 0.072).
What proportion of those offered CI referral declined referral?
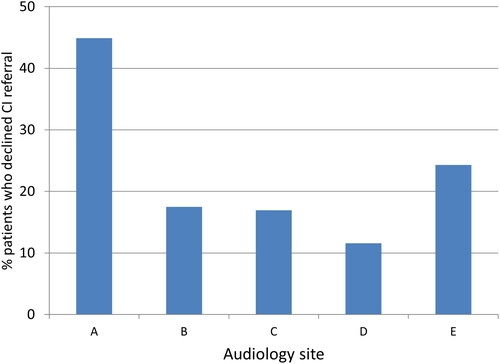
172 out of 810 patients (21%) were referred for CI assessment; 123 out of 521 patients (24%) declined CI referral.
Did the proportions differ across five UK Audiology clinics?
The Audiology centres differed significantly in the proportion of people who declined referral; this ranged from 12% to 45% (Chi-square (4, n = 521) = 35.1 p = 0.000) ().
Did patient age, sex, pure tone average in better ear or degree of audiometric slope affect the proportion of patients who declined CI referral?
Age
The median age for patients who accepted CI referral was 73 years (range = 18–105 years; sd = 20 years); the median age for patients who declined CI referral was also 73 years (range = 18–100; sd = 21 years). There was no difference in age between patients who accepted referral and those who declined it (Mann–Whitney U = 24291.0, Z = −0.127, p = 0.899).
Sex
Almost identical numbers of men and women declined CI referral (61 female, 59 male, 3 unknown) (Chi-square (1, n = 517) = 1.448 p = 0.229).
PTA
The group of patients who declined CI referral had worse pure tone thresholds in the better ear than those who accepted referral (Mann–Whitney U = 17059.0, Z = −4.942, p = 0.000). Median pure tone average in the better ear for patients who declined CI referral was 93 dBHL (range = 56–121 dBHL; sd = 14 dB); in the group who accepted referral the median was 83 dBHL (range = 46–123 dBHL; sd = 6 dB).
Slope
Slope was also not a significant factor in the groups where patients accepted (median = 35 dB; range = 0–110 dB; sd = 24 dB) or declined CI referral (median = 38 dB; range = 0–100 dB; sd = 24 dB) (Mann–Whitney U = 23751.5, Z = −0.363, p = 0.716).
Discussion
Discussion about CI should happen for all adults meeting NICE audiometric criteria. In some cases, this may be a discussion about why a CI is not appropriate, for example speech perception testing is out of criteria, or the patient does not want an implant. At the time of data collection, there was very little speech perception testing documented, so the report was unable to include these data. This audit showed that a documented CI discussion occurred in only 64% of cases (521 out of 810 patients) – with significant variation between the five centres (50–83%).
Potential reasons for results
The centre with the highest rate of CI referral discussion had an attached CI centre; the centre with the lowest did not. It is not surprising that Audiologists working alongside a CI centre were more likely to consider CI.
CI referral was declined by the patient in 24% of cases (123 out of 521 patients); decline reason was not collected. The report was not designed to extract this information, but as the audit progressed we began to try to collect the reason. However, there was often insufficient or missing information. The referral decline rate varied considerably between centres (12–45%) suggesting local factors (both patient and professional) may be involved. The hospital with the highest decline rate was in the least deprived location of the five sites. Future work should consider whether sociodemographic factors of the hospital areas impact referral and decline patterns.
and show roughly similar patterns, suggesting those centres that had higher rates of documenting CI referral discussion also have a lower referral decline rate.
Audiologists were less likely to discuss referral with older patients. Australian data with smaller patient numbers showed no significant difference in age between those referred and not referred – however, their data were not analysed for ‘referral discussed’ like the current work. Age was not a factor influencing whether adults themselves accepted or declined CI referral, also shown by Looi, Bluett, and Boisvert (Citation2017). Evidence shows outcomes with CI are as good in adults aged 85+ as they are in younger adults (Wong, Moran, and O'Leary Citation2016). Sex was not a factor influencing CI referral.
Our results showed that Audiologists were less likely to discuss referral with patients with better hearing thresholds – unlike Australian data which showed little effect of PTA (Looi, Bluett, and Boisvert Citation2017). It is possible that Audiologists were unfamiliar with the new NICE guidance, or perhaps other data (e.g. speech perception) suggested the patients were “too good” for CI referral, although speech tests were not documented. Conversely, those patients with better hearing thresholds were less likely to decline CI referral. Perhaps they were more reliant on their remaining hearing, and thus more motivated to optimise it. Previous work suggested that patients with ski slope losses (good low frequency thresholds) may not be considered for CI referral (Raine et al. Citation2016). However, our data showed no effect of audiometric slope.
The UK NICE guidance defines severe to profound hearing loss as pure tone audiometric thresholds equal to or greater than 80 dBHL at two or more frequencies (500, 1000, 2000, 3000 and 4000 Hz) bilaterally. The British Society of Audiology recommended procedure for pure tone audiometry lists test frequencies as 250, 500, 1000, 2000, 4000 and 8000 Hz – only advising testing intermediate frequencies such as 3000 Hz “where needed and practicable” (British Society of Audiology Citation2018). Omitting 3000 Hz testing may, therefore, result in a patient not being in criteria for CI. The current data showed that out of 875 audiograms, 3000 Hz was not measured in both ears in 211 cases (24%). However, these were cases that were already within audiometric criteria for CI, so may not represent all audiograms being done.
What has changed since 2019 data collection?
These results were collected in 2019, and it is important to note several changes to the audiology and CI landscape in the UK that have occurred subsequently:
The British Academy of Audiology (BAA) and the British Cochlear Implant Group (BCIG) launched the CI Champions scheme in November 2019 to train and support Audiologists on CI referral (British Academy of Audiology Citation2022).
The impact of the Covid-19 pandemic on CI referral is unknown. The number of people receiving CI in the UK is increasing but still not up to pre-pandemic levels (British Cochlear Implant Group Citation2022).
A global consensus aims to highlight evidence and unite professionals in the aim of improving access to CI (Buchman et al. Citation2020).
An international advocacy group CIICA (Cochlear Implant International Community of Action) was formed in 2021 to raise awareness and reduce the global gap in CI provision.
The UK ENT Trainee Research Network recently completed a study assessing CI referral in several UK sites, although results are not yet published (INTEGRATE, The UK ENT Trainee Research Network Citation2020).
Limitations
CI referral discussion may have occurred in cases where it was not documented.
UK CI candidacy for adults is based on audiometric thresholds and speech perception testing. Some patients where CI referral discussion was omitted may have been out of criteria on speech perception, although many Audiology sites do not have the facility for speech perception testing.
The five centres involved were those with experience of Crystal reports and/or interest in auditing CI referral, and may not be representative of all UK Audiology centres.
Three of the five Audiology services were linked to CI teams and shared the same PMS. Both internal and external referrals for CI assessment would have occurred, but this version of the Crystal report did not separate out different referral sources.
Learning from this work
Future work should explore reasons that patients decline CI referral.
Improve education and empowerment of Audiologists.
Raise awareness of the benefits of CI for older adults.
3000 Hz should be tested in cases of severe to profound hearing loss.
The Crystal report has now been modified when run in a joint Audiology/CI centre to ensure that adults referred from external sources are coded separately.
Enable Audiology departments to run speech perception testing.
Future versions of the report should allow categories to be assigned by the Audiologist shortly after or while seeing the patient, making the audit quicker to run.
The BCIG has just started collecting annual UK referral data (British Cochlear Implant Group Citation2022) which will allow assessment of the efficacy of referral initiatives.
The standardised reporting tool shows promise as a measure of CI referral in NHS Audiology services. A regular national audit would support better understanding of access to CI and barriers to referral.
Author contributions
Development of the Crystal Report: Joseph Blackaby, Ann-Marie Dickinson, Lisa Kennedy, Unai Martinez de Estibariz, Katie McNeill, Sara O’Neill.
Leading data collection: Joseph Blackaby, Lisa Kennedy, Unai Martinez de Estibariz, Katie McNeill, Sara O’Neill.
Analysis and write-up of results: Helen Cullington, Ann-Marie Dickinson, Unai Martinez de Estibariz.
Declaration and ethics statements
Data were anonymised. This project was an audit to find out if healthcare was provided in line with clinical guidance (NICE), and therefore ethical approval was not required. The participating centres registered the audit with their local hospital Trusts.
Acknowledgements
Thank you to members of the Audiology departments who collected data.
Disclosure statement
Cochlear® funded the development of the Crystal Report, supported Audiologists to run the report and paid the second author Ann-Marie Dickinson to plan the study and write up the paper. Cochlear did not have access to the data once collected and did not contribute to the analysis or write-up of the results. Unai Martinez de Estibariz now works for Advanced Bionics GmbH. The other authors have no relevant financial or non-financial competing interests to report.
Data availability statement
The participants of this study did not give written consent for their data to be shared publicly, so due to the sensitive nature of the research supporting data are not available.
Additional information
Funding
References
- Bierbaum, M., C. M. McMahon, S. Hughes, I. Boisvert, A. Y. S. Lau, J. Braithwaite, and F. Rapport. 2020. “Barriers and Facilitators to Cochlear Implant Uptake in Australia and the United Kingdom.” Ear and Hearing 41 (2):374–385. https://doi.org/10.1097/AUD.0000000000000762
- British Academy of Audiology. 2022. Cochlear Implant Champions. Retrieved 20 May 2022 from https://www.baaudiology.org/professional-information/cochlear-implant-champions/
- British Cochlear Implant Group. 2022. Annual UK Update. Retrieved 27 September 2022 from https://www.bcig.org.uk/annual-uk-update/
- British Society of Audiology. 2018. Recommended Procedure. Pure-tone air-conduction and Bone-conduction Threshold Audiometry with and Without Masking. https://www.thebsa.org.uk/wp-content/uploads/2018/11/OD104-32-Recommended-Procedure-Pure-Tone-Audiometry-August-2018-FINAL.pdf
- Buchman, C. A., R. H. Gifford, D. S. Haynes, T. Lenarz, G. O'Donoghue, O. Adunka, A. Biever, R. J. Briggs, M. L. Carlson, P. Dai, Jr., et al. 2020. “Unilateral Cochlear Implants for Severe, Profound, or Moderate Sloping to Profound Bilateral Sensorineural Hearing Loss: A Systematic Review and Consensus Statements.” JAMA Otolaryngology- Head & Neck Surgery 146 (10):942–953. https://doi.org/10.1001/jamaoto.2020.0998
- Carlsson, P. I., J. Hjaldahl, A. Magnuson, E. Ternevall, M. Edén, Å. Skagerstrand, and R. Jönsson. 2015. “Severe to Profound Hearing Impairment: Quality of Life, Psychosocial Consequences and Audiological Rehabilitation.” Disability and Rehabilitation 37 (20):1849–1856. https://doi.org/10.3109/09638288.2014.982833
- Chundu, S., and R. Buhagiar. 2013. “Audiologists’ Knowledge of Cochlear Implants and their Related Referrals to the Cochlear Implant Centre: Pilot Study Findings from UK.” Cochlear Implants International 14 (4):213–224. https://doi.org/10.1179/1754762812y.0000000025
- Crowson, M. G., Y. R. Semenov, D. L. Tucci, and J. K. Niparko. 2017. “Quality of Life and Cost-Effectiveness of Cochlear Implants: A Narrative Review.” Audiology & Neuro-Otology 22 (4–5):236–258. https://doi.org/10.1159/000481767
- Cullington, H. E., A. M. Dickinson, U. Martinez de Estibariz, and M. O’Driscoll. 2022. 27 April 2022). Improving adult CI referral in the UK [oral presentation]. British Cochlear Implant Group Annual Conference, Cardiff. https://drive.google.com/file/d/1Hck0WnFvZU7I3D1n_pn_E459C3DBT3YX/view?usp=drive_link
- De Raeve, L. 2016. “Cochlear Implants in Belgium: Prevalence in Paediatric and Adult Cochlear Implantation.” European Annals of Otorhinolaryngology, Head and Neck Diseases 133 (1):S57–S60. https://doi.org/10.1016/j.anorl.2016.04.018
- Ebrahimi-Madiseh, A., R. H. Eikelboom, R. J. Bennett, G. S. Upson, P. L. Friedland, W. Swanepoel, C. Psarros, W. K. Lai, and M. D. Atlas. 2020. “What Influences Decision-Making for Cochlear Implantation in Adults? Exploring Barriers and Drivers from a Multistakeholder Perspective.” Ear and Hearing 41 (6):1752–1763. https://doi.org/10.1097/AUD.0000000000000895
- Gaylor, J. M., G. Raman, M. Chung, J. Lee, M. Rao, J. Lau, and D. S. Poe. 2013. “Cochlear Implantation in Adults: A Systematic Review and Meta-analysis.” JAMA Otolaryngology- Head & Neck Surgery 139 (3):265–272. https://doi.org/10.1001/jamaoto.2013.1744
- gov.uk. 2019. English Indices of Deprivation 2019. https://www.gov.uk/government/statistics/english-indices-of-deprivation-2019
- INTEGRATE, The UK ENT Trainee Research Network. 2020. CIRCA: Cochlear Implant Referral Criteria Audit. Retrieved 1 November 2022 from https://entintegrate.co.uk/circa
- Kim, S. Y., H. J. Kim, E. K. Park, J. Joe, S. Sim, and H. G. Choi. 2017. “Severe Hearing Impairment and Risk of Depression: A National Cohort Study.” PLoS One 12 (6):e0179973. https://doi.org/10.1371/journal.pone.0179973
- Livingston, G., J. Huntley, A. Sommerlad, D. Ames, C. Ballard, S. Banerjee, C. Brayne, A. Burns, J. Cohen-Mansfield, C. Cooper, et al. 2020. “Dementia Prevention, Intervention, and Care: 2020 Report of the Lancet Commission.” Lancet (London, England) 396 (10248):413–446. https://doi.org/10.1016/S0140-6736(20)30367-6
- Looi, V., C. Bluett, and I. Boisvert. 2017. “Referral Rates of Postlingually Deafened Adult Hearing Aid Users for a Cochlear Implant Candidacy Assessment.” International Journal of Audiology 56 (12):919–925. https://doi.org/10.1080/14992027.2017.1344361
- Nassiri, A. M., J. P. Marinelli, D. L. Sorkin, and M. L. Carlson. 2021. “Barriers to Adult Cochlear Implant Care in the United States: An Analysis of Health Care Delivery.” Seminars in Hearing 42 (4):311–320. https://doi.org/10.1055/s-0041-1739281
- Nassiri, A. M., D. L. Sorkin, and M. L. Carlson. 2022. “Current Estimates of Cochlear Implant Utilization in the United States.” Otology & Neurotology 43 (5):e558–e562. https://doi.org/10.1097/MAO.0000000000003513
- National Institute for Health and Care Excellence. 2019. Cochlear Implants for Children and Adults with Severe to Profound Deafness. https://www.nice.org.uk/guidance/TA566
- National Institute for Health and Care Excellence. 2021. Shared Decision Making. https://www.nice.org.uk/guidance/ng197
- Ng, Z. Y., B. Lamb, S. Harrigan, S. Archbold, S. Athalye, and S. Allen. 2016. “Perspectives of Adults with Cochlear Implants on Current CI Services and Daily Life.” Cochlear Implants International 17 (1):89–93. https://doi.org/10.1080/14670100.2016.1157314
- NHS England and NHS Improvement 2019. Shared Decision Making: Summary Guide. https://www.england.nhs.uk/publication/shared-decision-making-summary-guide/
- O’Neill, C., B. Lamb, and S. Archbold. 2016. “Cost Implications for Changing Candidacy or Access to Service Within a Publicly Funded Healthcare System?” Cochlear Implants International 17 Suppl 1 (sup1):31–35. https://doi.org/10.1080/14670100.2016.1161123
- Raine, C. 2013. “Cochlear Implants in the United Kingdom: Awareness and Utilization.” Cochlear Implants International 14 (1):S32–S37. https://doi.org/10.1179/1467010013Z.00000000077
- Raine, C., H. Atkinson, D. R. Strachan, and J. M. Martin. 2016. “Access to Cochlear Implants: Time to Reflect.” Cochlear Implants International 17 (1):42–46. https://doi.org/10.1080/14670100.2016.1155808
- Rapport, F., S. E. Hughes, I. Boisvert, C. M. McMahon, J. Braithwaite, M. Faris, and M. Bierbaum. 2020. “Adults’ Cochlear Implant Journeys through Care: A Qualitative Study.” BMC Health Services Research 20 (1):457. https://doi.org/10.1186/s12913-020-05334-y
- Turton, L., P. Souza, L. Thibodeau, L. Hickson, R. Gifford, J. Bird, M. Stropahl, L. Gailey, B. Fulton, N. Scarinci, et al. 2020. “Guidelines for Best Practice in the Audiological Management of Adults with Severe and Profound Hearing Loss.” Seminars in Hearing 41 (3):141–246. https://doi.org/10.1055/s-0040-1714744
- Wong, D. J., M. Moran, and S. J. O’Leary. 2016. “Outcomes After Cochlear Implantation in the Very Elderly.” Otology & Neurotology 37 (1):46–51. https://doi.org/10.1097/MAO.0000000000000920
- World Health Organisation. 2021. World Report on Hearing. https://www.who.int/publications/i/item/9789240020481