ABSTRACT
Ischaemic preconditioning (IPC), brief periods of ischaemia immediately followed by reperfusion applied to a vascular bed, has emerged as a method to improve exercise performance. There is, however, a lack of research exploring repeated episodes of IPC on anaerobic performance. The aim of this study was to determine if a 2-week repeated IPC intervention could enhance anaerobic performance in male academy football players. Eight male academy football players completed two, 2-week intervention trials: six IPC episodes (4 × 5 min at 220 mmHg per episode), and six SHAM episodes (4 × 5 min at 20 mmHg per episode). Prior to and following each intervention trial, the participants completed assessments of anaerobic performance (Running Anaerobic Sprint Test [RAST]), and superficial femoral artery endothelial function (flow-mediated dilation [FMD]). IPC significantly enhanced peak and mean power output by 12% (p = 0.026) and 11% (p = 0.019) and significantly improved superficial femoral artery FMD (p = 0.049). The increase in endothelial function suggests that this may be a mechanism contributing to this enhancement of anaerobic performance. The present study supports the use of repeated IPC prior to matches and training sessions to enhance anaerobic performance.
Introduction
Ischaemic preconditioning (IPC) is an intervention that consists of short periods of ischaemia which is immediately followed by reperfusion (Murry et al., Citation1996). Whilst ischaemia causes an insufficient amount of blood supply to the organs and tissues, the following period of reperfusion, enables the restoration of perfusion and reoxygenation in the specific organ that is being preconditioned (Hausenloy & Yellon, Citation2009). Although IPC has been utilized as a clinical method to protect certain organs and delay cell injury, it may also benefit exercise performance via mechanisms such as increased blood flow and enhanced endothelial function (Bailey et al., Citation2012; de Groot et al., Citation2010).
IPC can enhance many aspects of exercise performance, however, compared to aerobic exercise performance, less research has investigated the effects of IPC on anaerobic exercise performance (Caru et al., Citation2019). The physical anaerobic performance demands of football require outfield players to perform numerous accelerations, decelerations, high-speed running and change of directions throughout the duration of a match (Gualtieri et al., Citation2020). Additionally, these physical demands are linked to repeated sprint ability (RSA), which involves the ability to produce high-intensity, short duration efforts, followed by short (≤60 s) recovery periods (Bishop et al., Citation2011). Indeed, enhanced RSA performance is regarded as a critical determinant of success in football (Bishop et al., Citation2011; Rampinini et al., Citation2009). Outfield players are expected to produce and reproduce maximal or close to maximal effort sprints lasting for 1–7 s followed by very short recovery times (Rampinini et al., Citation2009). For example, in elite English football, players on average perform 1.7 to 5.2 successive sprints per game, depending on playing position (Ade et al., Citation2016). Recently, acute IPC improved repeated sprint performance in Division I collegiate basketball players (Cheng et al., Citation2021). However, other research using an acute IPC intervention and team sport athletes, including football players, has observed no improvements in repeated sprint performance (Gibson et al., Citation2013). The disparity in findings between studies could be attributed to differences in the dose of IPC. Gibson et al. (Citation2013) administered three sets of 5-min occlusions, whilst Cheng et al. (Citation2021) administered a higher dose of four sets of 5-min occlusions, which is viewed as the traditional IPC dose (Cocking et al., Citation2018).
The application of IPC, in terms of single or repeated dose, may also influence its effectiveness. Although significant performance improvements following a single dose of IPC have been observed, this is not a universal finding (Caru et al., Citation2019). A possible explanation for these equivocal findings following a single dose of IPC could be due to a failure to meet the metabolic threshold required to induce the beneficial effects of IPC (Cocking et al., Citation2018). Therefore, to ensure that this metabolic threshold is met, a loading period of repeated IPC applications may be required. Whilst there is minimal research assessing repeated IPC and exercise performance, it has been shown to be a useful ergogenic aid in the few studies that have adopted this treatment method (Foster et al., Citation2014; Jeffries et al., Citation2019; Lindsay et al., Citation2017). Furthermore, repeated IPC has led to greater performance enhancements compared to studies only implementing a single dose of IPC prior to exercise. For example, a single dose of IPC improved aerobic performance by 3% and peak power output by 1.6% (de Groot et al., Citation2010) whilst 7 days of repeated IPC improved aerobic performance by 9.5% and anaerobic performance by 11% (Lindsay et al., Citation2017). Consequently, repeated applications of IPC may be required to enhance RSA in football players, yet this is unknown.
This study, therefore, aimed to explore if a 2-week repeated IPC intervention could enhance anaerobic performance via improved RSA in academy football players. Such information could elucidate whether a repeated IPC intervention prior to matches and training sessions could be adopted to enhance anaerobic performance. It was hypothesized that the repeated IPC intervention would enhance RSA, via increases in peak and mean power output.
Methods
Participants
Eight male, full-time, non-elite, under-23 football academy student-athletes were recruited, and written informed consent was obtained. Participants were of a mean age of 20.8 ± 1.0 years, with a mean height of 177.8 ± 5.6 cm, a mean body mass of 78.3 ± 14.3 kg, and a mean body mass index of 24.6 ± 3.4 kg·m−2. All participants were experienced players who were involved in football training and matches on at least 5 days per week for 6 months prior to taking part in the study. Participants had a mean academy playing experience of 3.0 ± 0.9 years, and a typical training load of 630 ± 0 A.U. Additionally, three participants were defenders, two participants were midfielders, two participants were centre-forwards, and one participant was a winger. Sample size was informed by existing research exploring the effect of IPC on exercise performance, which recruited between six and eight healthy, trained participants (Foster et al., Citation2014; Garcia et al., Citation2017). Inclusion and exclusion criteria were clearly stated in the participant information sheet that was given to all participants who expressed an interest in completing the study. Each recruited participant was an outfield player (no goalkeepers) and aged between 18 and 24 years. Participants were screened prior to testing using the Physical Activity Readiness Questionnaire (PAR-Q). Furthermore, participants regularly taking medication, diagnosed with a chronic disease, with a resting blood pressure that exceeded 140/100 mmHg (systolic/diastolic) (Cheng et al., Citation2021) or who were recently injured (>2 weeks), were not eligible to take part. The study procedures were approved by the York St John Ethics Committee (RECELP00006) and adhered to the Declaration of Helsinki (1964).
Experimental design
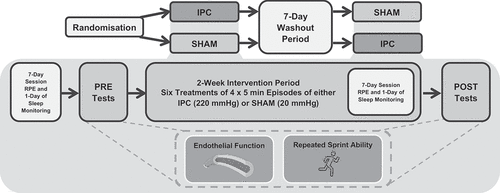
A randomized, SHAM-controlled, and single-blinded crossover trial design was conducted (). Each intervention trial lasted 2-weeks. The participants were randomly allocated to the order they completed trials by the principal researcher using computer-generated random numbers. Prior to beginning each 2-week intervention trial, participants were required to attend the laboratory to obtain anthropometric measurements and baseline measurements of anaerobic performance and endothelial function. Following baseline measurements, participants were separated into one of the two trial intervention groups: IPC or SHAM-controlled. Throughout the intervention, participants visited the laboratory on six separate occasions within a 2-week period to receive IPC or SHAM-controlled treatment. A 2-week intervention period was chosen as previous research has shown that in healthy, young males, endothelial function increases following six episodes of IPC over a 2-week period (Jones et al., Citation2015). Additionally, the less frequent number of IPC treatments compared to daily IPC treatments is informed by the timing of the late phase of protection against endothelial ischaemia-reperfusion injury that IPC can provide (Loukogeorgakis et al., Citation2007). At the end of the 2-weeks, anaerobic performance and endothelial function measurements were repeated. There was a minimum 7-day washout period before the crossover of the IPC and SHAM-controlled trial intervention groups (Cocking et al., Citation2017).
Laboratory testing visits
Prior to each laboratory testing visit, participants were asked to complete an overnight fast, to refrain from caffeine and the consumption of alcohol, and to avoid strenuous physical activity for 24-hr (Incognito et al., Citation2016). Additionally, each participant was asked to go to sleep and wake up at a similar time before each trial to standardize sleep. To standardize dietary intake, participants recorded the food they ate the day before each visit in a food diary and were asked to keep this the same for subsequent laboratory visits, which was verified by the lead researcher. At the start of visit 1, height, weight and resting blood pressure were recorded. Following this, participants rested supine for 20-min before endothelial function was assessed via non-invasive vascular ultrasound. Participants then completed the Running-Based Anaerobic Sprint Test (RAST) to achieve a baseline measurement of anaerobic performance. After completion of the 2-week intervention, all baseline measurements were repeated.
Treatment visits
For each treatment visit, participants attended the laboratory for approximately 40-min to receive either SHAM-controlled treatment (blood pressure cuffs inflated to 20 mmHg) or IPC treatment (blood pressure cuffs inflated to 220 mmHg). Although it can be difficult to effectively SHAM-control treatment in human subjects, previous literature recommends that research studies should acknowledge the participants’ understanding of IPC, whilst potentially utilizing deception (Incognito et al., Citation2016). Therefore, participants were not made aware which condition was expected to be performance-enhancing. Bilateral occlusion was performed simultaneously on the right and left thighs. Participants laid in the supine position for all treatments, and blood pressure cuffs (20.5–28 cm, M1753A, Phillips, the Netherlands) were positioned on the proximal portion of the thighs. Cuffs were inflated for 5-min at the required pressure, using an automatic rapid cuff inflator (Vascular Assessment Pressure Cuff Controller, Moor Instruments, Devon, UK). Four sets of 5-min of inflation (4 × 5 min) were completed. Following each set of 5-min, the cuffs were deflated for 5-min to allow reperfusion to occur, before administering another set. After the four sets of SHAM/IPC treatments were finished, that concluded one day of treatment. This occlusion/reperfusion protocol has been used in previous studies assessing repeated IPC and sports performance (Cheng et al., Citation2021; Incognito et al., Citation2016). Three treatments per week were administered for 2-weeks (Jones et al., Citation2015). Participants were supervised for each treatment.
During the 2-week treatment period, participants were able to complete their habitual exercise and dietary patterns. Participants were asked to report their training load using a training diary. Participants reported the number of hours they trained each day and rated the intensity of each training session on a 0–10 scale. The training load was then calculated using the session-RPE approach, by multiplying the minutes of training and the intensity of training (Foster et al., Citation2001) Session-RPE has been validated as an approach to measure internal training load (Haddad et al., Citation2017).
Measurements
Demographics
Participants’ age, typical training load, and years of football academy playing experience were recorded. Typical training load was assessed subjectively via the previously described session-RPE method.
Sleep
The day prior to each laboratory testing visit participants’ sleep was monitored, since acute changes in sleep can influence subsequent athletic performance (Walsh et al., Citation2021). Obtaining the sleep pattern of participants therefore enabled the researcher to account for any differences in RAST performance that may be explained by changes in sleep. Sleep was assessed via actigraphy, an approach widely used to objectively assess sleep based on movement patterns (Cellini et al., Citation2013). Compared to the gold standard measure of assessing sleep, polysomnography, actigraph devices have obtained various agreement rates of sleep and wake epochs ranging from 77% to 87% (Kawada, Citation2008). Participants were required to wear an accelerometer (ActiGraph GT3X, Pensacola, Florida) on their non-dominant wrist approximately an hour before bedtime and to remove it upon getting out of bed. Participants also completed the Consensus Sleep Diary (Carney et al., Citation2012) as recommended in conjunction with actigraphy to prevent periods of non-wear time being incorrectly classed as sleep (Sadeh & Acebo, Citation2002). The Consensus Sleep Diary was used to set bedtime and get up time during analyses. Data from the accelerometer were downloaded in 60-s epochs and analysed utilizing ActiLife Software (Version 6.13.4) and using the Sadeh algorithm, which is validated in a young (10–25 years) population (Sadeh et al., Citation1994). Each night of sleep was analysed for the sleep parameters: total minutes in bed, sleep duration, sleep efficiency, and sleep fragmentation index (Cellini et al., Citation2013).
Anthropometrics
Stature and body mass were recorded on visit 1. Participants were required to stand as still as possible underneath a stadiometer (SECA, Hamburg, Germany) to obtain a measure of stature, recorded to the nearest 0.1 cm. In minimal clothing and without shoes, body mass was measured to the nearest 0.1 kg using an electronic scale (Tanita BC-543 Body Composition Monitor, Amsterdam, the Netherlands). Body mass index (BMI) was subsequently calculated (mass/stature2).
Resting heart rate and blood pressure
Resting heart rate and systolic and diastolic blood pressure were assessed using an oscillometric cuff at the left brachial artery (Carescape V100, Dinamap, GE Healthcare, UK). Measurements were obtained after participants had completed the 20-min supine rest.
Superficial femoral artery endothelial function
Peripheral superficial femoral artery endothelial function was assessed using the standardized flow-mediated dilation (FMD) technique, according to published guidelines (Thijssen et al., Citation2011). Endothelial function was assessed since repeated IPC has been shown to enhance endothelial function (Jones et al., Citation2015) which may in turn enhance exercise performance via vascular adaptations such as increased muscle blood flow (Bailey et al., Citation2012). Participants laid in a supine position and were instructed to remain as still as possible during the test. A rapid inflation and deflation pneumatic cuff was positioned on the left thigh, just above the patella. To record an image of the left superficial femoral artery, a 10 MHz multi-frequency linear array probe connected to a high-resolution ultrasound machine (T3000; Terason Burlington, MA) was used. Images were captured above the occlusion cuff and distal from the artery bifurcation. To obtain the arterial diameters, the ultrasound parameters were adjusted to enhance the B-mode image of the lumen-arterial wall interface. Once a suitable image was detected, the probe was held in this position. Additionally, blood flow was assessed via Doppler ultrasound using the same machine with an insonation angle of 60 degrees and the sample volume placed in middle of the lumen, aligned with the blood flow. Following a 1-min baseline, the cuff was inflated to 220 mmHg for 5-min to induce local ischaemia. After cuff deflation, ultrasound recordings continued for a further 3-min. The same procedures were used for each endothelial function assessment, and all measures were completed by the same sonographer.
Data were analysed using Cardiovascular Suite (Version 2.8.1 Software, Quipu, Italy), an automated edge-detection and wall-tracking software. The software tracks the blood vessel walls and blood velocity trace via pixel density and frequency distribution algorithms and enables an optimal region of interest (ROI) to be selected from the initial frame of the B-mode image and Doppler waveform. For diameter analysis, the ROI was selected based on B-mode image quality and the clear distinction between the artery walls and lumen, whilst for blood velocity analysis, a second ROI was selected to encompass the Doppler waveform. Each frame was analysed, enabling synchronized arterial diameter, blood velocity, blood flow (arterial cross-sectional area × blood velocity), and shear rate (SR) data to be acquired. SR (4 × [blood velocity/arterial diameter]) was used as an estimation of shear stress due to the inability to measure blood viscosity. For the FMD assessment, within the software, each phase of the FMD protocol (baseline, ischaemia, cuff deflation) was selected by the researcher. Baseline arterial diameter and blood flow were determined as the mean of the data acquired 1-min prior to cuff inflation. Following cuff deflation, peak arterial diameter was automatically calculated, and FMD (%) was calculated as the percentage change in arterial diameter from baseline diameter ([peak arterial diameter-baseline arterial diameter]/baseline arterial diameter) × 100%). SR area under the curve (AUC) was automatically calculated from post cuff deflation until the point of peak arterial diameter.
Running-Based Anaerobic Sprint Test (RAST)
To assess anaerobic performance via RSA, participants completed the RAST (Zagatto et al., Citation2009). The RAST has been conducted in football populations due to the sport’s requirement to perform a high number of repeated sprints (Keir et al., Citation2013). Additionally, RSA has been recognized as an important physical fitness component for football players (Impellizzeri et al., Citation2005). The RAST was completed in an indoor Sports Barn. Indoor testing was adopted to remove the influence of differential outdoor conditions (e.g., wind and temperature) affecting sprint performance.
Each participant was required to complete six, maximal effort, 35 m sprints. Each sprint was separated by 10-s of rest before completing the next sprint. The time to complete each sprint was measured by photocell timing gates placed 1.0 m above ground level at the start and end of the 35 m distance (Witty System, Microgate, Italy). For each sprint, power was calculated as (body mass × distance2)/time3. Peak power was defined as the greatest power obtained from one of the six sprints, while mean power was calculated by obtaining the average power value from each of the six sprints. Fatigue index was calculated as: (maximal power – minimum power)/total time to complete all six sprints (Zagatto et al., Citation2009). During the RAST, heart rate was monitored using a telemetry system with a wireless chest strap (V800 GPS Sports Watch and H10 Heart Rate Sensor, Polar, Helsinki, Finland). Maximal heart rate was recorded following the culmination of the final sprint. In football players, the RAST has shown strong criterion validity for peak power and mean power (r = 0.70, p < 0.001; r = 0.60, p < 0.01, respectively) and strong reliability for peak power output and mean power output (ICC = 0.72; ICC = 0.88, respectively) (Burgess et al., Citation2016).
Prior to completing the RAST, participants completed the Fédération Internationale de Football Association (FIFA) 11+ warm-up protocol, a warm-up created by FIFA’s Medical and Research Centre (F-MARC) as a complete warm-up programme to prevent injuries in amateur football players (Bizzini & Dvorak, Citation2015). In addition to the FIFA 11+ warm-up, participants completed a series of short sprints of progressively increasing intensity prior to completing the RAST at maximal effort.
Blood lactate
Blood lactate was assessed at rest and immediately following the completion of the RAST via finger-prick sampling from the participant’s non-dominant hand. Following each finger prick, blood lactate was assessed using a portable lactate analyser (Lactate Pro 2 Analyser, Kyoto, Japan), with the sample collected using a lactate test strip obtained at a 90-degree angle from the blood sample.
Statistical analyses
Data were assessed for normality using the Shapiro–Wilk normality test. Data obtained from the RAST (peak power, mean power output, fatigue index), blood lactate, heart rate, blood pressure, FMD variables, sleep variables, and training load were analyzed using two-way repeated measures ANOVAs. Post-hoc analyses were completed with LSD adjustment. FMD data were also analyzed using an allometric approach that controls for changes in baseline diameter (Atkinson & Batterham, Citation2013). Peak arterial diameter and baseline arterial diameter were log transformed and the change in diameter calculated on the logged scale. Log-scaled values were then analyzed using an ANCOVA, with baseline arterial diameter as a covariate and the change in diameter as the dependent factor. Covariate-adjusted means were calculated and then back-transformed. Adjusted changes in diameter values were converted into a percentage change by subtracting 1 and then multiplying by 100 (Atkinson & Batterham, Citation2013). Data are presented as mean ± standard deviation (SD), from which, effect sizes (partial eta squared, η2) were calculated. These were considered as follows: small (η2 = 0.01), medium (η2 = 0.06), and large (η2 = 0.14) (Cohen, Citation1992). Data were analyzed using SPSS Statistics (Version 17, IBM SPSS Inc., Chicago, IL, USA), with significance accepted if p < 0.05.
Results
Descriptive statistics
Eight male academy football players completed the study and were included in analyses. Participants completed all 12 treatment sessions (6 IPC and 6 SHAM) for each intervention trial.
Running-based anaerobic sprint test performance
Mean power, peak power, maximal heart rate, blood lactate, and fatigue index data for each RAST performance are presented in . A significant interaction effect was observed for peak power output (p = 0.010, η2 = 0.639), with post hoc analyses revealing at POST the IPC trial had a higher peak power output compared to the SHAM trial (p = 0.026; ). A significant interaction effect was also observed for mean power output (p = 0.047, η2 = 0.453), with post hoc analyses revealing at POST the IPC trial had a higher mean power output compared to the SHAM trial (p = 0.019; ). Additionally, a significant interaction effect was observed for fatigue index (p = 0.013, η2 = 0.608), with post hoc analyses showing at POST the IPC trial had a higher fatigue index compared to the SHAM trial (p = 0.009; ). There was also a significant main effect for the condition for mean power output (p = 0.024, η2 = 0.540), with the IPC trial (525.3 ± 58.3) higher than the SHAM trial (469.9 ± 52.5) (). There were no other significant main effects for RAST performance measures (p > 0.05).
Figure 2. (A) peak power output and (b) mean power output before (PRE) and after (POST) the completion of the ischaemic preconditioning (IPC) and SHAM-controlled (SHAM) trials. Bars = mean, error bars = ±SD.
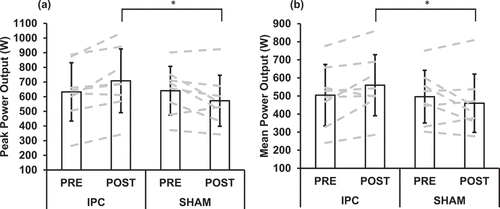
Table 1. Running-based anaerobic sprint test performance before (PRE) and after (POST) the completion of the ischaemic preconditioning (IPC) and SHAM-controlled (SHAM) trials (mean±sd).
Superficial femoral artery flow-mediated dilation
Results showed a significant interaction effect for relative FMD (p = 0.017, η2 = 0.577), with post hoc analyses revealing at POST the IPC trial had a higher FMD compared to the SHAM trial (p = 0.049; , ). This significant interaction effect was still observed when FMD data were analyzed using the allometric approach (p = 0.020, η2 = 0.185), with FMD still higher at POST in the IPC trial compared to the SHAM trial (p = 0.035; ). There were no significant main effects for any other FMD measurements (p > 0.05; ).
Figure 3. Superficial femoral artery flow-mediated dilation (FMD) before (PRE) and after (POST) the completion of the ischaemic preconditioning (IPC) and SHAM-controlled (SHAM) trials. Bars = mean, error bars = ±SD.
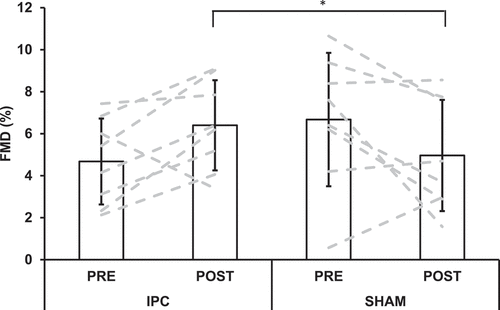
Table 2. Superficial femoral artery flow-mediated dilation (FMD) before (PRE) and after (POST) the completion of the ischaemic preconditioning (IPC) and SHAM-controlled (SHAM) trials (mean±sd).
Physiological measurements at rest
No significant main effects were observed for heart rate, systolic blood pressure, and diastolic blood pressure at rest (p > 0.05; ). A significant main effect for time was observed for resting blood lactate (p = 0.035, η2 = 0.533) with POST (1.23 ± 0.05) higher than PRE (1.14 ± 0.06). No significant condition or interaction effects were observed for resting blood lactate (p > 0.05; ).
Table 3. Resting physiological measurements taken before (PRE) and after (POST) the completion of the ischaemic preconditioning (IPC) and SHAM-controlled (SHAM) trials (mean±sd).
Sleep
Sleep parameters for the night before each laboratory testing visit are shown in . There was a significant main effect for condition for minutes in bed (p = 0.001, η2 = 0.847), with the IPC trial (545 ± 118 min) higher than the SHAM trial (464 ± 73 min). There were no other significant main effects for measures of sleep (p>0.05).
Table 4. Measures of sleep and training load before (PRE) and after (POST) the completion of the ischaemic preconditioning (IPC) and SHAM-controlled (SHAM) trials (mean±sd).
Training load
There were no significant main effects observed for session-RPE assessed prior to each laboratory testing visit (p > 0.05; ).
Discussion
The present study aimed to explore if a 2-week repeated IPC intervention could enhance anaerobic performance via improved RSA in male academy football players. Results demonstrate that the repeated IPC intervention enhanced peak and mean power output during the RAST. Furthermore, endothelial function significantly increased following the repeated IPC intervention, suggesting that endothelial function may be a mechanism explaining the improvement in peak and mean power. These findings suggest that a repeated IPC intervention could be used prior to training sessions and matches as an ergogenic aid to enhance anaerobic performance in male academy football players.
To the authors’ knowledge, this study is the first to explore the effects of IPC on academy football players’ anaerobic performance. The present study establishes that a 2-week repeated IPC intervention enhances anaerobic performance by improving peak power output and mean power output by 12% and 11%, respectively, during repeated sprints. This supports existing research which observed bilateral IPC enhanced peak power output in the first 3 out of a total of 12, 6-s cycling sprints (Patterson et al., Citation2015). However, these findings are in contrast with prior research investigating an acute, single dose of IPC and RSA (5 × 6 maximal effort sprints) in team sport athletes, including football players, which observed no performance enhancement (Gibson et al., Citation2013). However, this study administered IPC unilaterally, in comparison to the present study where IPC was conducted bilaterally. Importantly, it has been suggested that IPC applied bilaterally improves exercise performance to a greater magnitude than IPC applied unilaterally (Cocking et al., Citation2019). Furthermore, whilst less research has assessed the influence of IPC on running-based sprint performance, in such studies, they have only utilized a single dose of IPC and observed no performance enhancement (Gibson et al., Citation2013). The lack of an ergogenic effect following a single dose of IPC suggests that a repeated method of IPC, as adopted in the present study, is required to surpass a metabolic threshold where IPC can be effective (Salvador et al., Citation2016). Collectively, the use of bilateral and repeated IPC in the present study may therefore have provided a more optimal dose of IPC to enhance RSA. Although the repeated IPC intervention significantly improved peak and mean power output, the most advantageous dose of repeated IPC to obtain a performance enhancement remains unclear (Lindsay et al., Citation2017). Further research is recommended to determine the optimal repeated IPC dose that elicits the greatest performance enhancing effect for anaerobic performance.
Whilst mean data demonstrate enhanced performance following the IPC intervention, examination of individual responses highlights variation in the magnitude and direction of this change in performance. The potential of IPC responder and non-responder phenotypes has been suggested, due to factors such as fitness or training status (Incognito et al., Citation2016). Fitness parameters can vary between football playing positions (Boone et al., Citation2012) and the sample in this study included players from all outfield positions. As such, this may, in part, explain the range of individual responses observed. Future research should, therefore, examine if there is a specific phenotype that is most likely to respond to IPC.
A 2-week IPC protocol consisting of six sessions (4 × 5 min) was administered as previous literature demonstrated the efficacy of a 2-week period to improve endothelial function (Jones et al., Citation2015). The present study corroborates this finding by also observing enhanced endothelial function utilizing a 2-week protocol. The observed improvement in endothelial function following repeated IPC may explain the increase in peak and mean power output. Previous research has demonstrated enhancements in skeletal muscle oxidative capacity and microvascular blood flow following a repeated IPC intervention (Jeffries et al., Citation2019). Improved blood flow during high intensity exercise can result in enhanced exercise performance by means of improved vasodilation which supports greater blood flow to enter the exercising muscles (Segal, Citation2005). It is, therefore, possible that the increase in endothelial function in the present study allowed for greater skeletal muscle blood flow which contributed towards the improvement in peak and mean power output, and future research should explore this further. Additionally, no significant differences in session-RPE or parameters of sleep were observed between the IPC or SHAM conditions. As such, acute differences in training load and sleep cannot be attributed to the observed improvements in peak and mean power output. Measuring sleep and training load strengthened this study by controlling for external factors that could influence performance and supports recommendations for greater pre-study restrictions and control measures in IPC and exercise performance research (Incognito et al., Citation2016).
Practical applications
The results from this study have potential practical implications for academy football players, coaches, and support staff. The study was conducted during the mid-season, indicating it was feasible to administer the intervention without interfering with their usual playing and training schedules. Furthermore, IPC carries no risk of fatigue or high exertion that can be associated with physical training methods. Coaches and support staff could, therefore, look to implement IPC in the days leading up to training and matches, without compromising subsequent performance due to fatigue. The repeated administration of IPC may further add to the practicality of the intervention as it could allow IPC to be implemented as a loading phase in the days leading up matches, rather than being limited to the acute period prior to a match, which may interfere with other match-day related preparations. Finally, IPC can also be viewed as a cost-effective method to enhance performance, increasing its accessibility to non-elite players and coaches who may have fewer financial resources.
Limitations and future directions
The present study had limitations that should be acknowledged. It was not possible to design the study to be double-blinded; therefore, the participants knew whether they were receiving the high- or low-cuff inflation treatment condition. However, the participants were not informed which treatment condition researchers predicted would enhance performance to reduce any placebo effect. The study only analyzed the effect of IPC on anaerobic performance in a small sample of male academy football players, fully powered studies are, therefore, needed to confirm or refute initial findings. Furthermore, results are not generalizable to other populations, such as females or other sports. Importantly, sex-based differences in the response to IPC are suggested (Paradis-Deschênes et al., Citation2017). Future research should aim to explore the effects of repeated IPC on anaerobic performance in a larger sample of male and female athletes from different team-based sports. Moreover, the repeated sprint distance of 35 m in the RAST is not representative of the typical sprint distance in football matches, which can range between 16.6 m − 20.3 m, depending on playing position (Ade et al., Citation2016). Future research should, therefore, look to assess anaerobic performance following the administration of IPC using ecologically valid assessments specific to the physical match demands of football. The IPC intervention was only 2-weeks duration; therefore, future research should explore the effects of longer repeated IPC sessions on anaerobic performance to assess if extended intervention periods lead to greater enhancements in performance. Additionally, although research has investigated repeated IPC protocols administered over a period of several days to several weeks (Lindsay et al., Citation2017; Foster et al., Citation2014; Jones et al., Citation2015) minimal research has assessed the effects of multiple IPC sessions per day on exercise performance. Applying multiple IPC sessions over a day could be more feasible and time efficient for athletes. Finally, whilst this study provides initial insight into the mechanisms explaining the performance enhancing effect of IPC, these should be further explored.
Conclusion
This study demonstrates that a 2-week IPC intervention can enhance peak and mean power output in male academy football players. Moreover, endothelial function was significantly improved following the repeated IPC intervention, providing a potential mechanistic explanation for the observed enhancement in performance. These findings present IPC as a potential intervention strategy for coaches and support staff to carry out prior to matches and training to enhance anaerobic performance. Future research should look to investigate the effect of repeated IPC in a more diverse and larger sample to confirm these initial findings.
Acknowledgements
N/A
Disclosure statement
No potential conflict of interest was reported by the author(s).
Data availability statement
The data that support the findings of this study are available from the corresponding author, ESS, upon reasonable request.
Additional information
Funding
References
- Ade, J., Fitzpatrick, J., & Bradley, P. (2016). High-intensity efforts in elite soccer matches and associated movement patterns, technical skills and tactical actions. Information for position-specific training drills. Journal of Sports Sciences, 34(24), 2205–2214. https://doi.org/10.1080/02640414.2016.1217343
- Atkinson, G., & Batterham, A. (2013). Allometric scaling of diameter change in the original flow-mediated dilation protocol. Atherosclerosis, 226(2), 425–42734. https://doi.org/10.1016/j.atherosclerosis.2012.11.027
- Bailey, T., Jones, H., Gregson, W., Atkinson, G., Cable, N., & Thijssen, D. (2012). Effect of ischemic preconditioning on lactate accumulation and running performance. Medicine and Science in Sports & Exercise, 44(11), 2084–2089. https://doi.org/10.1249/MSS.0b013e318262cb17
- Bishop, D., Girard, O., & Mendez-Villanueva, A. (2011). Repeated-sprint ability – part II. Sports Medicine, 41(9), 741–756. https://doi.org/10.2165/11590560-000000000-00000
- Bizzini, M., & Dvorak, J. (2015). FIFA 11+: An effective programme to prevent football injuries in various player groups worldwide—a narrative review. British Journal of Sports Medicine, 49(9), 577–579. https://doi.org/10.1136/bjsports-2015-094765
- Boone, J., Vaeyens, R., Steyaert, A., Bossche, L. V., & Bourgois, J. (2012). Physical fitness of elite Belgian soccer players by player position. The Journal of Strength & Conditioning Research, 26(8), 2051–2057. https://doi.org/10.1519/JSC.0b013e318239f84f
- Burgess, K., Holt, T., Munro, S., & Swinton, P. (2016). Reliability and validity of the running anaerobic sprint test (RAST) in soccer players. Journal of Trainology, 5(2), 24–29. https://doi.org/10.17338/trainology.5.2_24
- Carney, C., Buysse, D., Ancoli-Israel, S., Edinger, J., Krystal, A., Lichstein, K., & Morin, C. (2012). The consensus sleep diary: Standardizing prospective sleep self-monitoring. Sleep, 35(2), 287–302. https://doi.org/10.5665/sleep.1642
- Caru, M., Levesque, A., Lalonde, F., & Curnier, D. (2019). An overview of ischemic preconditioning in exercise performance: A systematic review. Journal of Sport and Health Science, 8(4), 355–369. https://doi.org/10.1016/j.jshs.2019.01.008
- Cellini, N., Buman, M., McDevitt, E., Ricker, A., & Mednick, S. (2013). Direct comparison of two actigraphy devices with polysomnographically recorded naps in healthy young adults. Chronobiology International, 30(5), 691–698. https://doi.org/10.3109/07420528.2013.782312
- Cheng, C., Kuo, Y., Hsu, W., Chen, C., & Pan, C. (2021). Local and remote ischemic preconditioning improves sprint interval exercise performance in team sport athletes. International Journal of Environmental Research and Public Health, 18(20), 10653. https://doi.org/10.3390/ijerph182010653
- Cocking, S., Jones, H., Cable, N., & Thijssen, D. (2019). Enhancing Sports performance through ischemic preconditioning: Moderating factors and potential mechanisms. The Science of Hormesis in Health and Longevity, 213–222.
- Cocking, S., Landman, T., Benson, M., Lord, R., Jones, H., Gaze, D., Thijssen, D., & George, K. (2017). The impact of remote ischemic preconditioning on cardiac biomarker and functional response to endurance exercise. Scandinavian Journal of Medicine & Science in Sports, 27(10), 1061–1069. https://doi.org/10.1111/sms.12724
- Cocking, S., Wilson, M., Nichols, D., Cable, N., Green, D., Thijssen, D., & Jones, H. (2018). Is there an optimal ischemic-preconditioning dose to improve cycling performance? International Journal of Sports Physiology and Performance, 13(3), 274–282. https://doi.org/10.1123/ijspp.2017-0114
- Cohen, J. (1992). Statistical power analysis. Current Directions in Psychological Science, 1(3), 98–101. https://doi.org/10.1111/1467-8721.ep10768783
- de Groot, P., Thijssen, D., Sanchez, M., Ellenkamp, R., & Hopman, M. (2010). Ischemic preconditioning improves maximal performance in humans. European Journal of Applied Physiology, 108(1), 141–146. https://doi.org/10.1007/s00421-009-1195-2
- Foster, C., Florhaug, J., Franklin, J., Gottschall, L., Hrovatin, L., Parker, S., Doleshal, P., & Dodge, C. (2001). A new approach to monitoring exercise training. The Journal of Strength & Conditioning Research, 15(1), 109–115. https://doi.org/10.1519/00124278-200102000-00019
- Foster, G., Giri, P., Rogers, D., Larson, S., & Anholm, J. (2014). Ischemic preconditioning improves oxygen saturation and attenuates hypoxic pulmonary vasoconstriction at high altitude. High Altitude Medicine and Biology, 15(2), 155–161. https://doi.org/10.1089/ham.2013.1137
- Garcia, C., da Mota, G., Leicht, A., & Marocolo, M. (2017). Ischemic preconditioning and acute recovery of performance in rugby union players. Sports Medicine International Open, 1(3), 107–112. https://doi.org/10.1055/s-0043-111082
- Gibson, N., White, J., Neish, M., & Murray, A. (2013). Effect of ischemic preconditioning on land-based sprinting in team-sport athletes. International Journal of Sport Physiology and Performance, 8(6), 671–676. https://doi.org/10.1123/ijspp.8.6.671
- Gualtieri, A., Rampinini, E., Sassi, R., & Beato, M. (2020). Workload monitoring in top-level soccer players during congested fixture periods. International Journal of Sports Medicine, 41(10), 677–681. https://doi.org/10.1055/a-1171-1865
- Haddad, M., Stylianides, G., Djaoui, L., Dellal, A., & Chamari, K. (2017). Session-RPE method for training load monitoring: Validity, ecological usefulness, and influencing factors. Frontiers in Neuroscience, 11, 612. https://doi.org/10.3389/fnins.2017.00612
- Hausenloy, D., & Yellon, D. (2009). Preconditioning and postconditioning: Underlying mechanisms and clinical application. Atherosclerosis, 204(2), 334–341. https://doi.org/10.1016/j.atherosclerosis.2008.10.029
- Impellizzeri, F. M., Rampinini, E., & Marcora, S. M. (2005). Physiological assessment of aerobic training in soccer. Journal of Sports Sciences, 23(6), 583–592. https://doi.org/10.1080/02640410400021278
- Incognito, A., Burr, J., & Millar, P. (2016). The effects of ischemic preconditioning on human exercise performance. Sports Medicine, 46(4), 531–544. https://doi.org/10.1007/s40279-015-0433-5
- Jeffries, O., Evans, D. T., Waldron, M., Coussens, A., & Patterson, S. (2019). Seven-day ischaemic preconditioning improves muscle efficiency during cycling. Journal of Sports Sciences, 37(24), 2798–2805. https://doi.org/10.1080/02640414.2019.1664537
- Jones, H., Nyakayiru, J., Bailey, T., Green, D., Cable, N., Sprung, V., Hopkins, N., & Thijssen, D. (2015). Impact of eight weeks of repeated ischaemic preconditioning on brachial artery and cutaneous microcirculatory function in healthy males. European Journal of Preventive Cardiology, 22(8), 1083–1087. https://doi.org/10.1177/2047487314547657
- Kawada, T. (2008). Agreement rates for sleep/wake judgments obtained via accelerometer and sleep diary: A comparison. Behavior Research Methods, 40(4), 1026–1029. https://doi.org/10.3758/BRM.40.4.1026
- Keir, D., Thériault, F., & Serresse, O. (2013). Evaluation of the running-based anaerobic sprint test as a measure of repeated sprint ability in collegiate-level soccer players. The Journal of Strength & Conditioning Research, 27(6), 1671–1678. https://doi.org/10.1519/JSC.0b013e31827367ba
- Lindsay, A., Petersen, C., Blackwell, G., Ferguson, H., Parker, G., Steyn, N., & Gieseg, S. (2017). The effect of 1 week of repeated ischaemic leg preconditioning on simulated keirin cycling performance: A randomised trial. BMJ Open Sport and Exercise Medicine, 3(1), 1–8. https://doi.org/10.1136/bmjsem-2017-000229
- Loukogeorgakis, S., Williams, R., Panagiotidou, A., Kolvekar, S., Donald, A., Cole, T., Yellon, D., Deanfield, J., & MacAllister, R. (2007). Transient limb ischemia induces remote preconditioning and remote postconditioning in humans by a KATP channel-dependent mechanism. Circulation, 116(12), 1386–1395. https://doi.org/10.1161/CIRCULATIONAHA.106.653782
- Murry, C., Jennings, R., & Reimer, K. (1996). Preconditioning with ischemia: A delay of lethal cell injury in ischemic myocardium. Circulation, 74(5), 1124–1136. https://doi.org/10.1161/01.CIR.74.5.1124
- Paradis-Deschênes, P., Joanisse, D., & Billaut, F. (2017). Sex-specific impact of ischemic preconditioning on tissue oxygenation and maximal concentric force. Frontiers in Physiology, 7, 674. https://doi.org/10.3389/fphys.2016.00674
- Patterson, S., Bezodis, N., Glaister, M., & Pattison, J. (2015). The effect of ischemic preconditioning on repeated sprint cycling performance. Medicine and Science in Sports and Exercise, 47(8), 1652–1658. https://doi.org/10.1249/MSS.0000000000000576
- Rampinini, E., Sassi, A., Morelli, A., Mazzoni, S., Fanchini, M., & Coutts, A. (2009). Repeated-sprint ability in professional and amateur soccer players. Applied Physiology, Nutrition, and Metabolism, 34(6), 1048–1054. https://doi.org/10.1139/H09-111
- Sadeh, A., & Acebo, C. (2002). The role of actigraphy in sleep medicine. Sleep Medicine Reviews, 6(2), 113–124. https://doi.org/10.1053/smrv.2001.0182
- Sadeh, A., Sharkey, M., & Carskadon, M. A. (1994). Activity-based sleep-wake identification: An empirical test of methodological issues. Sleep, 17(3), 201–207. https://doi.org/10.1093/sleep/17.3.201
- Salvador, A., de Aguiar, R., Lisbôa, F., Pereira, K., de Cruz, R., & Caputo, F. (2016). Ischemic preconditioning and exercise performance: A systematic review and meta-analysis. International Journal of Sports Physiology and Performance, 11(1), 4–14. https://doi.org/10.1123/ijspp.2015-0204
- Segal, S. (2005). Regulation of blood flow in the microcirculation. Microcirculation, 12(1), 33–45. https://doi.org/10.1080/10739680590895028
- Thijssen, D., Black, M., Pyke, K., Padilla, J., Atkinson, G., Harris, R., Parker, B., Widlansky, M., Tschakovsky, M., & Green, D. (2011). Assessment of flow-mediated dilation in humans: A methodological and physiological guideline. American Journal of Physiology-Heart and Circulatory Physiology, 300(1), H2–H12. https://doi.org/10.1152/ajpheart.00471.2010
- Walsh, N., Halson, S., Sargent, C., Roach, G., Nédélec, M., Gupta, L., Leeder, J., Fullagar, H. H., Coutts, A., Edwards, B., Pullinger, S., Robertson, C. M., Burniston, J. G., Lastella, M., Le Meur, Y., Hausswirth, C., Bender, A. M., Grandner, M. A., & Samuels, C. H. (2021). Sleep and the athlete: Narrative review and 2021 expert consensus recommendations. British Journal of Sports Medicine, 55(7), 356–368. https://doi.org/10.1136/bjsports-2020-102025
- Zagatto, A., Beck, W., & Gobatto, C. (2009). Validity of the running anaerobic sprint test for assessing anaerobic power and predicting short-distance performances. The Journal of Strength & Conditioning Research, 23(6), 1820–1827. https://doi.org/10.1519/JSC.0b013e3181b3df32