Abstract
Background
The diagnosis of toxic alcohol poisoning is often based on clinical presentation and nonspecific surrogate laboratory studies due to limited testing availability. Fomepizole is the recommended antidote and often administered empirically. The objective of this study is to identify substances that mimic toxic alcohols and compare key clinical factors between toxic alcohol and non-toxic alcohol exposures when fomepizole was administered.
Methods
This study was a retrospective evaluation using the National Poison Data System from January 1, 2010 through December 31, 2021. Exposures were included if fomepizole was administered. Toxic alcohol exposures had ethylene glycol or methanol as a coded substance. For exposures not coded as a toxic alcohol, the first substance was described. Paracetamol (acetaminophen) exposures from 2020 and 2021 were excluded.
Results
Fomepizole was reportedly used 25,110 times over 12 years. Use increased from 1,955 in 2010 to 2,710 in 2021. Most administrations were for reported toxic alcohol poisoning (60 percent) but use in reported non-toxic alcohol poisoning was greater starting in 2020. Toxic alcohol exposures were older (43.3 versus 39.8 years; P < 0.001) and more likely male (65.7 percent versus 58.2 percent). Level of care was mostly a critical care unit (67.7 percent), which was less common in toxic alcohol (63.3 percent) than non-toxic alcohol exposures (74.2 percent). The most common non-toxic alcohol substances were ethanol (24.9 percent) or an unknown drug (17.5 percent). Acidosis, increased creatinine concentration, anion gap, and osmolal gap, and kidney failure were coded in a lower proportion of toxic alcohol exposures than non-toxic alcohol exposures (P < 0.001).
Discussion
The inability to provide rapid clinical confirmation of toxic alcohol poisoning results in the empiric administration of fomepizole to many patients who will ultimately have other diagnoses. Although fomepizole is relative well tolerated we estimated that this practice costs between $1.5 to $2.5 million. The major limitations of this work include the biases associated with retrospective record review, and the inability to confirm the exposures which may have resulted in allocation error.
Conclusion
Most fomepizole use was for a presumed toxic alcohol. This recently shifted to greater use in likely non-toxic alcohol poisoning. Key difference between the groups suggest fomepizole administration was likely due to the difficulty in diagnosis of toxic alcohol poisoning along with the efficacy and safety of fomepizole. Increased toxic alcohol laboratory testing availability could improve timely diagnosis, reserving fomepizole use for toxic alcohol poisoning.
Introduction
Toxic alcohols, primarily methanol and ethylene glycol, can result in life-threatening toxicity after ingestion [Citation1]. United States poison centers reported approximately 2,000 methanol and 6,000 ethylene glycol exposures in 2021 [Citation2]. Twenty-five percent and 30% were seen in healthcare facilities, respectively.
Fomepizole is the most used antidote for toxic alcohol poisoning in the United States [Citation2]. It competitively inhibits alcohol dehydrogenase, preventing conversion of methanol and ethylene glycol to toxic metabolites [Citation3]. It is associated with few adverse effects and allows for decreased intensive care unit utilization [Citation3, Citation4]. Use is often empiric because early initiation may decrease the severity of end organ toxicity and diagnosing toxic alcohol poisoning is challenging [Citation1, Citation5, Citation6]. Clinical presentation and readily available laboratory studies (e.g., basic metabolic panel, serum osmolality) are used to diagnose possible toxic alcohol poisoning. Additionally, rapid testing for methanol and ethylene glycol are not readily available at most clinical laboratories. Due to these diagnostic challenges, the risk of end organ toxicity in a patient with untreated toxic alcohol poisoning, and the safety profile of fomepizole, fomepizole is often administered to patients who have not ingested a toxic alcohol. The objective of this study is to identify substances that mimic toxic alcohols and compare key clinical factors between toxic alcohol and non-toxic alcohol exposures in which fomepizole was administered.
Methods
We examined human exposures reported to the National Poison Data System (NPDS) from January 1, 2010 through December 31, 2021 if at least one of the treatments coded included fomepizole either as “performed” or “recommended and performed”. The NPDS is owned and operated by America’s Poison Centers and a detailed description is available from Gummin and colleagues [Citation2]. In brief, cases come from calls to regional poison centers from patients, caregivers, healthcare professionals, and others. Calls are managed and coded in real time by Specialists in Poison Information (typically pharmacists or nurses with additional training in toxicology). For all calls, a free-text note is written, and the call is coded by the specialist. In most instances, all information is provided verbally from the bedside team to the specialist. Substances are coded based on reported history and clinical information. Clinical effects are coded based on standard definitions.
An exposure was considered a toxic alcohol if either ethylene glycol (generic codes 051260 or 051221) or methanol (generic codes 051222, 031140 or 031220) was coded as any of the substances. For exposures not coded as a toxic alcohol, the first substance was described unless ethanol was co-ingested. In these instances, the exposure was considered ethanol because concomitant administration of fomepizole to a patient who has consumed ethanol (another inhibitor of alcohol dehydrogenase and treatment option for toxic alcohol poisoning) is often redundant and of special interest for the study.
We examined the reason for exposure, demographics, outcomes, level of care, co-ingested ethanol, and key clinical effects and interventions between the toxic alcohol and non-toxic alcohol groups. The reasons for exposure were grouped into intentional, unintentional, and unknown. They were also separated into suspected self-harm or not suspected self-harm. The described level of care is the highest level of admission for the healthcare visit (i.e., if a patient is admitted to the intensive care unit, then downgraded, the coded level of care is intensive care unit). Continuous data were analyzed with Student’s t-tests and categorical data were compared with chi-square with odds ratios for the difference of two independent proportions. Statistical significance was set at P < 0.05. After initial review of the data, we noted that paracetamol alone, but not paracetamol in combination, exposures increased significantly in 2020 and 2021 from about 40 per year to 155 and 461, respectively. These 616 exposures from 2020 and 2021 were excluded from comparisons as it was impossible to determine if this use was intentional to treat paracetamol poisoning or due to suspicion of a toxic alcohol. Additional exposures were excluded if it was unclear if the product was a toxic alcohol (e.g., “automotive product: other” or “automotive product: unknown” that did not have further description).
This study was determined to be Not Human Subject Research by the Institutional Review Board at the University of Maryland Baltimore.
Results
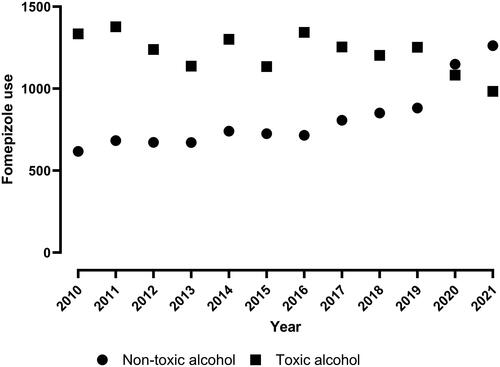
There were 25,110 reports of fomepizole administration from 2010 through 2021. We excluded 616 paracetamol exposures from 2020 and 2021 and 83 exposures for which it was unclear if the product was a toxic alcohol for a final exposure number of 24,411 that received fomepizole (see Supplemental Figure S1 for number of paracetamol exposures over time). Fomepizole use averaged 2,041 exposures per year and increased from 1,955 in 2010 to 2,249 in 2021. In 2010, there were 617 non-toxic alcohol exposures and 1,331 toxic alcohol exposures (). In 2021, there were 1,262 non-toxic alcohol and 983 toxic alcohol exposures. The mean age was 41.6 years (standard deviation 17.0) and the population was 63.3% male (). Approximately 66% of all exposures were single-substance exposures. Self-harm attempts were more likely in females (49.3% versus 47.3%; odds ratio 0.724 [95% confidence interval: 0.69–0.76]). Most fomepizole use was for a reported toxic alcohol (14,636; 60.0%). Reported toxic alcohol exposures were older (43.6 versus 39.8 years; P < 0.001) and a greater proportion were male (65.7% versus 59.6%). Level of care was mostly a critical care unit (67.7%), but this was less common in toxic alcohol exposures compared with non-toxic alcohol exposures (63.3% versus 74.7%; P < 0.001). The initial call was less likely to originate from a healthcare facility in toxic alcohol versus non-toxic alcohol exposures (93.3% versus 79.2%; P < 0.001).
Table 1. Patient overview, reason for exposure, level of care, and caller site.
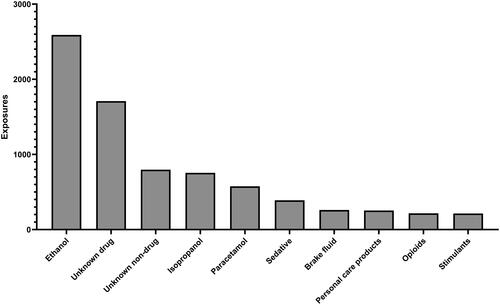
The most common non-toxic alcohol substances were ethanol (2,590; 24.9%), unknown drug (1,709; 17.5%), unknown non-drug (797; 7.7%), and isopropyl alcohol (755; 7.3%) (). Ethanol exposure, either alone or in combination, was more common in non-toxic alcohol exposures versus toxic alcohol exposures (37.8% versus 16.1%; P < 0.0001). Self-harm as the reason for exposure was more common in toxic alcohol exposures (65.9%) than non-toxic alcohol exposures (35.2%) (odds ratio 3.73 [95% confidence interval: 3.53–3.93]). Acidosis, increased creatinine concentration, anion gap, and elevated osmolal gap were coded in a lower proportion of toxic alcohol exposures than non-toxic alcohol exposures (). Hemodialysis was performed more frequently in toxic alcohol exposures versus non-toxic alcohol exposures (34.7% versus 19.4%; P < 0.001). Both vasopressors and sodium bicarbonate were administered less frequently in toxic alcohol versus non-toxic alcohol exposures (P < 0.001) ().
Table 2. Key laboratory abnormalities reported.
Table 3. Key interventions performed.
Discussion
From 2010 through 2019, fomepizole use was primarily for a presumed toxic alcohol, but in 2020 and 2021, use for non-toxic alcohol was greater. Over this period, 60% of all exposures in which fomepizole was used were coded as a toxic alcohol. Approximately 25% of non-toxic alcohol expsoures were coded as unknown. This is not surprising as there are several clinical presentations similar to toxic alcohol poisoning including kidney failure, alcoholic ketoacidosis, shock, diabetic ketoacidosis, mannitol administration, and isopropanol ingestion [Citation1, Citation7]. Some of the substances involved included isopropanol, sedatives, personal care products, and opioids. This may have been due to critical illness and kidney failure mimicking the toxic alcohol. We suspect the high proportion of “unknown” drugs and non-drugs are likely tied to diseases without generic codes. These include diabetic ketoacidosis, alcoholic ketoacidosis, kidney failure, sepsis, and others.
The diagnosis of toxic alcohol poisoning is based on history, physical examination, and laboratory findings. The gold standard for diagnosis is gas chromatography tandem mass spectrometry [Citation8]. Most clinical laboratories in the United States do not test for toxic alcohols, therefore, clinicians use a combination of history and surrogate markers including elevated osmolal gap, elevated anion gap, presence of oxalate crystals in the urine, and others for diagnosis [Citation1, Citation9]. As previously mentioned, many disease states mimic a non-toxic alcohol ingestion. These disease states are often reflected in the laboratory changes described in . Importantly, fomepizole is often administered empirically because it is effective and well tolerated with few adverse effects [Citation10]. Furthermore, it treats one of the possible causes of metabolic acidosis allowing for time to evaluate other causes.
The site of the caller being more likely a healthcare facility in non-toxic alcohol exposures (93.5%) versus toxic alcohol exposures (79.2%) was also an interesting finding. Taken in context with clinical experience, we suspect that these exposures are patients who are critically ill and have multiple laboratory derangements. This highlights the difficulty in toxic alcohol diagnosis, especially when patients do not present early in the course of illness. When a toxic alcohol is suspected, it is generally safer to administer a dose of fomepizole pending further evaluation, however, the abundance of use in non-toxic alcohol exposures warrants a need for better available diagnostics to differentiate non-toxic alcohol and toxic alcohol exposure.
In contrast to administration in patients with undifferentiated acidosis, administration of fomepizole to patients who reportedly ingested ethanol is both redundant and potentially harmful. Ethanol is a potential antidote for toxic alcohol poisoning due to preferential alcohol dehydrogenase binding over other alcohols. Fomepizole as a non-competitive inhibitor of alcohol dehydrogenase, also inhibits the metabolism of ethanol reducing elimination by 40–50%, prolonging ethanol intoxication [Citation11]. The presence of ethanol was suggestive of alcoholic ketoacidosis over a toxic alcohol in one study and strongly suggestive that toxic alcohol was not the correct diagnosis in another, especially when the concentration of ethanol exceeds the therapeutic target for toxic alcohol treatment (i.e., >1,000 mg/L; 21.7 mmol/L) [Citation9, Citation12].
There are potential cost-savings with the ability to rapidly test for toxic alcohol concentrations. Fomepizole is available as a generic medication, however, the average wholesale price in the United States is between $1,200 and $2,000 per 1g vial [Citation13]. A rapid and readily available test would prevent unnecessary fomepizole administration. As the number of doses is not provided in NPDS data, drug cost alone for an initial fomepizole dose of all non-toxic alcohol exposures in 2021 would be between $1.5 and $2.5 million. Although the potential national savings are high, many small hospitals with fewer exposures may not benefit as much from rapidly available diagnostics because of low utilization.
There are notable limitations with this research. There is no mechanism in the database to identify if the exposure was confirmed. It is retrospective and utilizes a database with fixed variable coding. We selectively excluded paracetamol exposures from 2020 to 2021. This was due to the significant increase noted in those years (Supplemental Figure S1). We removed these because we assume most of this use was intentional to target paracetamol poisoning rather than empiric for possible toxic alcohol poisoning. It is possible we removed exposures in which fomepizole was administered empirically for possible toxic alcohol poisoning, but given results of previous years, it is unlikely this is a large number that would significantly impact the interpretation of our study. We also excluded 83 exposures that were coded as an unknown automotive product. It is possible these contained a toxic alcohol, but the coding for these was ambiguous enough that they could have been included in either group. We were not able to review free-text notes to determine if a toxic alcohol was suspected or confirmed. It is possible that some of the toxic alcohol coded exposures were actually non-toxic alcohol and vice versa, specifically the large number of “unknowns.” It is also possible that subtle laboratory changes and symptoms were not coded. We focused on symptoms that are most affected by toxic alcohol poisoning and could aid in the diagnosis. Poison centers should be changing the coded substance as exposures evolve, for example if the initial history or concern was a toxic alcohol, but serum concentrations fail to detect a toxic alcohol, the substance should be changed to another substance and vice versa. There is no way to verify if these changes were made.
Conclusion
Most fomepizole use reported to United States Poison Centers from 2010 through 2021 was for a presumed toxic alcohol. In recent years, there has been an increase in use for what is coded as non-toxic alcohol poisoning. In exposures for which no toxic alcohol was coded, 26% were unknown and 25% were ethanol. Key difference between the groups suggests that the non-toxic alcohol exposures were critically ill and treated empirically due to the difficulty in rapid definitive diagnosis, efficacy and safety of fomepizole, and delay in toxic alcohol testing results. Increased toxic alcohol laboratory testing availability could improve timely diagnosis and reserve fomepizole use only for toxic alcohol poisoning.
Supplemental Material
Download MS Word (26.1 KB)Acknowledgement
America’s Poison Centers maintains the National Poison Data System (NPDS), which houses de-identified records of self-reported information from callers to the country’s Poison Centers. The NPDS data do not reflect the entirety of United States exposures and incidences related to any substance(s). Exposures do not necessarily represent a poisoning or over-dose and America’s Poison Centers is not able to completely verify the accuracy of every report. The NPDS data do not necessarily reflect the opinions of America’s Poison Centers.
Data sharing
The data that support the findings of this study are available from the National Poison Data System. Restrictions apply to the availability of these data.
Disclosure statement
No potential conflict of interest was reported by the authors.
References
- Kraut JA, Mullins ME. Toxic alcohols. N Engl J Med. 2018;378(3):270–280. doi: 10.1056/NEJMra1615295.
- Gummin DD, Mowry JB, Beuhler MC, et al. 2021 Annual report of the National Poison Data System © (NPDS) from America’s Poison Centers: 39th annual report. Clin Toxicol (Phila). 2022;60(12):1381–1643. doi: 10.1080/15563650.2022.2132768.
- Brent J. Fomepizole for ethylene glycol and methanol poisoning. N Engl J Med. 2009;360(21):2216–2223. doi: 10.1056/NEJMct0806112.
- Minhaj FS, Leonard JB. Evaluation of level of care for toxic alcohol ingestions receiving fomepizole. Am J Emerg Med. 2021;50:795–796. doi: 10.1016/j.ajem.2021.03.016.
- McQuade DJ, Dargan PI, Wood DM. Challenges in the diagnosis of ethylene glycol poisoning. Ann Clin Biochem. 2014;51(Pt 2):167–178. doi: 10.1177/0004563213506697.
- Filip AB, Farnsworth CW, Mullins ME, et al. Accuracy of a glycerol dehydrogenase assay for ethylene glycol detection. J Med Toxicol. 2023;19(4):362–367. doi: 10.1007/s13181-023-00967-x.
- Krasowski MD, Wilcoxon RM, Miron J. A retrospective analysis of glycol and toxic alcohol ingestion: utility of anion and osmolal gaps. BMC Clin Pathol. 2012;12(1):1. doi: 10.1186/1472-6890-12-1.
- Wu AHB, McKay C, Broussard LA, et al. National academy of clinical biochemistry laboratory medicine practice guidelines: recommendations for the use of laboratory tests to support poisoned patients who present to the emergency department. Clin Chem. 2003;49(3):357–379. doi: 10.1373/49.3.357.
- Cohen ET, Su MK, Biary R, et al. Distinguishing between toxic alcohol ingestion vs alcoholic ketoacidosis: how can we tell the difference? Clin Toxicol (Phila). 2021;59(8):715–720. doi: 10.1080/15563650.2020.1865542.
- Riana R, Hélène B, Marie-Pierre B, et al. Analysis of fomepizole safety based on a 16-year post-marketing experience in France. Clin Toxicol. 2020;58(7):742–747. doi: 10.1080/15563650.2019.1676899.
- Jacobsen D, Sebastian CS, Dies DF, et al. Kinetic interactions between 4-methylpyrazole and ethanol in healthy humans. Alcohol Clin Exp Res. 1996;20(5):804–809. doi: 10.1111/j.1530-0277.1996.tb05255.x.
- Minhaj F, Leonard J, Seung H, et al. Determination of risk factors associated with toxic alcohol ingestion. Clin Toxicol. 2021;59:1095.
- Fomepizole. RED BOOK search results – MICROMEDEX [Internet]. [cited 2023 Sep 22]. Available from: https://www.micromedexsolutions.com/micromedex2/librarian/CS/A0792B/ND_PR/evidencexpert/ND_P/evidencexpert/DUPLICATIONSHIELDSYNC/695EF1/ND_PG/evidencexpert/ND_B/evidencexpert/ND_AppProduct/evidencexpert/ND_T/evidencexpert/PFActionId/redbook.ShowProductSearchResults?SearchTerm=fomepizole&searchType=redbookGenericName&searchTermId=924408&searchContent=REDBOOK&searchFilterAD=filterADActive&searchFilterRepackager=filterExcludeRepackager&searchPattern=%5Efomepizole.