Abstract
Introduction
Patients with sedative overdose may have residual cognitive impairment at the time they are deemed medically cleared for discharge. Impairment could affect the performance of high-risk activities, including driving. The Trail Making Test is an alpha-numeric assessment that can be performed at the bedside to assess cognitive function. We examined whether there were differences in cognitive function when medically cleared between patients that overdosed on sedative and non-sedative drugs.
Methods
A prospective, observational study assessed cognitive function using the Trail Making Test between 2018 and 2021. Patients (16 years and greater) completed testing upon medical clearance if they spoke English and had no previous neurological injury. Continuous covariates were compared using t-tests or Mann–Whitney U tests and multiple linear regression; binary variables were modelled using logistic regression.
Results
Of 171 patients enrolled, 111 (65 per cent) had sedative overdose; they were older (median 32.1 versus 22.2 years) and more likely to be male (58.6 per cent versus 36.7 per cent). Benzodiazepines and paracetamol were the commonest drug overdoses. Patients with sedative overdose performed worse on Trail Making Test part A (37.0 versus 33.1 seconds, P = 0.017) and Trail Making Test part B (112.4 versus 81.5 seconds, P = 0.004). Multiple linear regression analysis indicated that patient age (P < 0.001, 1.7 seconds slower per year, 95 per cent confidence interval: 0.9–2.6 seconds) and perception of recovery (P = 0.006, 36.4 seconds slower if perceived not recovered, 95 per cent confidence interval: 10.8–62.0 seconds) were also associated with Trail Making Test part B times. Patients with sedative overdose were more likely to be admitted to the intensive care unit (Odds Ratio: 4.9, 95 percent confidence interval: 1.1–22.0; P = 0.04).
Discussion
Our results are broadly in keeping with previously published work, but include a wider range of drug overdose scenarios (polypharmacy and recreational drugs). While patients demonstrated some perception of their cognitive impairment, our model could not reliably be used to provide individual discharge advice. The study design did not allow us to prove causation of cognitive impairment, or to make comparison between the strength of an overdose to the trail making test time.
Conclusions
Trail Making Test results suggested that patients who had sedative drug overdoses may have significant cognitive deficits even when medically cleared. Risk of harm may be minimised with advice to avoid high-risk activities such as driving. More profound impacts seen on the Trail Making Test part B than A may mean higher-order thinking is more affected than simple cognitive function.
Introduction
Self-poisoning is a substantial healthcare issue worldwide: in 2020–2022, there were over 500,000 estimated yearly emergency presentations in the United States (US) [Citation1, Citation2] (with around 175,000 admissions for unintentional nonfatal overdose) [Citation3]. The United Kingdom (UK) reported 105,000 yearly hospital admissions (of which 23,963 were unintentional) [Citation4]. In Australia, there were 33,800 admissions from mid-2020–2021 (of which 10,800 were unintentional), 8.5%–10.6% required intensive care support such as mechanical ventilation (6.5%–8.3%). Most patients were discharged from the hospital with average admission time 2.3–3.0 days [Citation5, Citation6]. Younger (20–40 years) [Citation7, Citation8] and economically disadvantaged patients were overrepresented, and indigenous patients were more than three times as likely to die from unintentional overdose as non-indigenous people [Citation9].
The effect of self-poisoning agents varies and can be categorised into different classes, such as sedative or stimulant [Citation7, Citation9]. Similar pharmacological actions exist within these classes. Clinical effects, including cognitive function, could vary depending on co-morbidities and drug tolerance.
Patients who overdose on central nervous system (CNS) depressant drugs (sedatives) may have residual effects of sedation at the time of medical discharge that could affect their ability to safely perform routine or complex activities but are not detected on routine clinical examination. Cognitive testing that can be performed rapidly at the bedside may aid in further assessing residual cognitive impairment. The Trail Making Test part A and B is a potentially useful bedside test, as it requires only a pen and paper and can be completed by the patient under supervision in less than 10 min. Part A of the Trail Making Test is a numeric test that measures attention and visual scanning. Part B of the Trail Making Test is an alpha-numeric test that assesses task switching and executive function. It has been correlated with driving performance [Citation10–12] and demonstrated to be feasible to perform in the emergency department [Citation13].
Two studies have examined the cognitive effects of deliberate self-poisoning with a single prescription medication. Dassanayake and colleagues [Citation14, Citation15] demonstrated that patients who overdosed on sedating medications displayed significant cognitive impairment at the time of discharge across a range of cognitive tests. Oxley and colleagues [Citation16] then showed that patients gradually improved upon repeated testing over 4 weeks of follow-up.
Data are lacking for other scenarios including unintentional overdose, non-prescription or multiple agents, which reflect the range of presentations to an emergency department. These patients may be at increased risk of harm to themselves or others due to misadventure or taking significant life decisions while cognitively impaired.
Objectives
Our primary objective was to compare cognitive function at the time of medical clearance between patients who overdosed on sedating and non-sedating drugs and model the possible contributing factors. Our hypothesis was that patients who overdosed on sedative drugs would show worse Trail Making Test time than those who overdosed on non-sedating drugs.
Methods
Setting
The study was carried out in the Clinical Toxicology Unit at Prince of Wales Hospital, a tertiary hospital in Sydney, Australia. All participants were admitted via presentation to the Emergency Department between 2018 and 2021 and reviewed by the toxicology team. Patients admitted under the toxicology team were entered into a database for research and clinical purposes. Ethics approval was granted by the South Eastern Sydney Human Research Ethics Committee (18/069 (LNR/18/POWH/141)) prior to the commencement of the study.
Patients 16 years old and older who were admitted following drug overdoses were prospectively screened for recruitment. Inclusion criteria comprised medical clearance for discharge by the toxicology team (Glasgow Coma Scale score of 15, normal vital signs, able to mobilise, eat, drink, pass urine) and adequate English proficiency to understand the test instructions. Patients were excluded if they lacked the dexterity, literacy, or numeracy to complete the test, if there was previous diagnosis of cognitive impairment, or they refused participation. Verbal consent (documented) and testing occurred shortly after the time of medical clearance.
Patients were determined to have taken an overdose based on history and overall clinical presentation (i.e., consistent with toxidrome, presence of medication packages). Patients were grouped into sedative or non-sedative overdose based on the known clinical or pharmacological effects of the drugs taken (see Appendix 1 for classifications), using information from multiple sources [Citation17–19]. When the dose taken of a sedative medication was known, patients were considered to have overdosed if they took ≥2 times the defined daily dose (with the exception of clozapine in drug naïve patients for whom even one dose could cause sedation) [Citation20]. Only a single presentation was included if a patient was admitted multiple times over the study period. Some patients required additional medical sedation for agitation or airway management during their admission. Patients were considered medically sedated only if they were administered at least the defined daily dose of a sedating drug.
Testing
The Trail Making Test parts A and B were administered in the patient’s individual bedspace by the toxicology team who were all trained by the investigators (BC and PS) to administer the test. Instructions were read to the patient, and testing material provided, from a pre-made pack to ensure consistency. Participants were instructed to connect irregularly spaced, sequential circled numbers and letters on a piece of paper using a pencil or pen. Part A involved numbers one through 25, whereas part B alternated between numbers (1 to 13) and letters (A to L; in the pattern “one, A, two, B” etc.) (Appendix 2). Mistakes were immediately indicated by the administrator and the patient recommenced from the last correct item, inflicting a time penalty for errors. The recorded test score was the time (in seconds) to complete the test. The test was terminated if not completed at 300 seconds (with resulting time recorded as 300 seconds). The patients completed a brief practice pattern prior to the timed assessment [Citation21].
Outcomes
The primary outcome measure was the time to complete the Trail Making Test (parts A and B). Secondary outcomes included length of stay and odds of intensive care unit (ICU) admission.
Data collection and analysis
Demographic and clinical information were collected from the toxicology database, patient clinical records and the testing proforma. These included patient details (age, gender, education completed, medical comorbidities, psychiatric diagnoses, regular medications) and admission details (timing of overdose, type and quantity of drugs, alcohol co-ingestion, intent of overdose, duration of admission, whether the patient felt they had recovered from the overdose, medical interventions including sedation administered and ICU admission).
Demographic and clinical characteristics were compared between groups using independent samples t tests or Mann-Whitney U tests (continuous data) or Χ2 tests (categorical data). Data were checked for normality by measuring kurtosis and skewness, and visual check of histograms.
Multiple linear regression (backwards elimination with missing data treated pairwise) was used to create two models for the primary outcome. The first comprised variables that could contribute to cognitive impairment (type of overdose, age, gender, time since the overdose, suicide or self-harm intent, medical sedation administration, years of education, mental health diagnosis, or concurrent alcohol use with the overdose). It was noted that medically administered sedation was a possible confounder and consequently a single variable was created encompassing sedative overdose and/or medically administered sedation. The second analysis comprised possible predictors of impairment at the time of medical clearance (any sedative exposure variable, patient perception of recovery, age, gender, time since the overdose, suicide or self-harm intent, years of education, mental health diagnosis, or concurrent alcohol use with the overdose). The ICU admission rate was modelled using logistic regression. Adjusted R2 identifies the percentage of variance in the model explained by the independent variables and corrected for the number of independent variables entered. The F test compares the ratio of explained variance to unexplained variance and is used to determine whether the examined regression model as a whole, or the intercept-only model, is more strongly associated with the outcome. Data analysis was carried out using SPSS version 27 for Windows.
Results
Sample characteristics
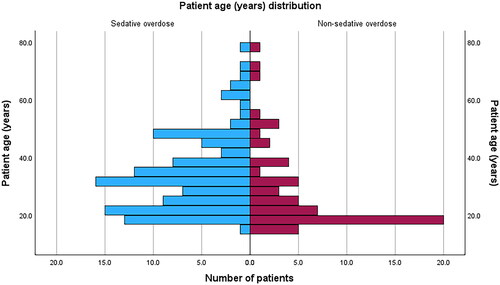
We enrolled 171 patients in the study, of whom 111 (65%) took sedative overdoses. Benzodiazepines, paracetamol, cocaine and metamfetamine were the commonest drug overdoses (Appendix 1). Patient characteristics are shown in . The patients in the sedative group were significantly older and more likely to be male than those in the non-sedative group. The differences in age arose largely from the non-sedative group comprising more people less than the age of 20 years, and the sedative group between 20 and 65 years (). Approximately 20% in each group required sedation during admission. The Trail Making Test part A times, time since exposure and length of stay were positively skewed.
Table 1. Characteristics of the sedative and non-sedative groups.
Sedative versus non-sedative
The sedative group was significantly slower than the non-sedative group to complete the Trail Making Test part B (median 112.4 seconds versus 81.5 seconds, P = 0.003) and part A (median 37.0 seconds versus 33.1 seconds, P = 0.017) (, and ), indicating cognitive impairment. Within the sedative group, those whose overdose included benzodiazepines were significantly slower than the others to complete Trail Making Test part B (median 137.8 seconds versus 91.0 seconds, P = 0.025) and Trail Making Test part A (median 43.0 seconds versus 35.7 seconds, P = 0.031) ().
Figure 2. Scatter plot demonstrating Trail Making Test part A time for patients with sedative versus non-sedative overdose. Horizontal bars represent the median and interquartile range for each group.
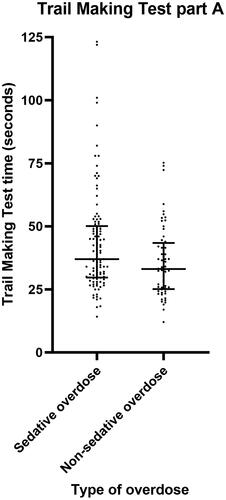
Figure 3. Scatter plot demonstrating Trail Making Test part B time for patients with sedative versus non-sedative overdose. Horizontal bars represent the median and interquartile range for each group.
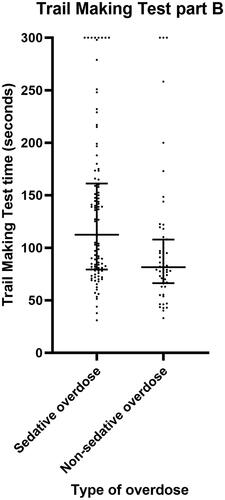
Table 2. Comparison of trail making test parts A and B, and duration of admission, between the sedative and non-sedative groups.
Table 3. Comparison of Trail Making Test parts A and B between those whose overdose included benzodiazepines or did not, within the sedative group.
Factors which were identified by multiple regression to significantly contribute to Trail Making Test part B times included age (P < 0.001) and overdose type (sedative group was 24.0 seconds slower, P = 0.032) (). Factors which best predicted Trail Making Test part B results at the time of medical clearance included age (1.7 seconds slower per year, P < 0.001), patient perception of recovery (36.4 seconds slower if perceived not recovered, P = 0.006) and exposure to sedative drugs (via poisoning and/or medically administered sedation: 32.5 seconds slower if patient had exposure to sedation, P = 0.017) (). The adjusted R2 was 0.207 (F3, 108 = 10.659, P < 0.001). Patient gender, time since overdose, presence of suicide or self-harm intent, years of education, major mental health diagnosis, medical sedation, or concurrent alcohol use were not significant in any model.
Table 4. Final regression models of sedative versus non-sedative overdose for trail Making Test part B time. Model created using multiple linear regression refined by backwards elimination method with missing data treated pairwise.
Age was the only significant predictive factor for Trail Making Test part A following multiple linear regression analysis (0.5 seconds slower per year, 95% confidence interval [CI] 0.3–0.7 seconds, P < 0.001). The adjusted R2 was 0.135 (F1, 169 = 27.280, P < 0.001).
Length of stay
There was no significant difference in length of stay (P = 0.9) between the sedative (median 14.2 h, interquartile range [IQR] 8.4–21 h, range 1.5–100.2 h) and non-sedative groups (median 13.4 h, IQR 10.4–20.6 h, range 2.2–124.9 h). The only significantly associated factors in multiple regression models were administration of medical sedation (11.2 h longer, P < 0.001) and presence of a mental health diagnosis (5.6 h longer, P = 0.016).
Intensive care unit admission
The odds of admission to the ICU for a patient who overdosed on sedative medications (16 patients, 11 of whom were intubated) were 4.9 times those of patients with non-sedative overdose (two patients, one of whom was intubated) (P = 0.04; 95% CI 1.1–22.0).
Limitations
The nature of our cross-sectional study means that we cannot prove causation as the pre-exposure Trail Making Test time could not be measured for our study population, but there is a plausible mechanism for both sedative overdose and medical sedation to cause cognitive impairment. Bias may have been introduced because only patients who agreed to participate in the study that were discharged during working hours were included. A similar patient cohort (non-sedative) was used as the control to balance these and unmeasurable variables.
Determining an exposure level at which an overdose is clinically significant is fraught with difficulty. The defined daily dose makes assumptions [Citation20] about patient tolerance whereas many of the patients in this study were drug naïve or on much lower doses for a different indication. Ultimately, we included a clozapine naïve patient who overdosed on 200 mg (defined daily dose 300 mg) in the sedative group. It is possible that some patients who were sedated with other antipsychotics were unnecessarily excluded. Due to the inclusion in our study of patients who overdosed on multiple and/or illicit drugs, we also could not calculate or did not know the dose ingested relative to the defined daily dose for approximately half the patient cohort. Consequently, we did not include the strength of the overdose (relative to defined daily dose) in the multiple regression analysis. We have included as Appendix 3 a scatterplot which does not support a correlation between the defined daily dose and Trail making Test part B time for known sedative overdoses but lacks sufficient patient numbers for legitimate statistical analysis.
Discussion
Implications
The findings of this study shed light on the residual cognitive effects of sedative drug overdose, extending previous research to include unintentional and mixed drug overdoses – a group representing around a third of hospital admissions for self-poisoning in Australia [Citation5, Citation6]. We have also considered additional predictive factors of impairment including patient perception of their recovery.
We observed that individuals who had overdosed on sedative drugs exhibited significant cognitive impairment at the time of medical clearance, indicated by prolonged Trail Making Test part B completion times compared to non-sedative overdose patients (112.2 seconds versus 81.5 seconds). This impairment suggests potential risks associated with their ability to safely perform routine activities, such as driving [Citation22], or making important decisions. Amongst those who overdosed on sedative drugs, people who used benzodiazepines may be more severely impaired. The significant difference between sedative and non-sedative groups in part B but not part A of the Trail Making Test implies that residual impairment is greater for more complex cognitive tasks, which may reflect more gradual recovery of higher order cognition. Better recovery of Trail Making Test part A times is in keeping with the gross assessment of coordination inherent in current medical clearance practices. Residual impairment should be considered at the time of discharge, for example the Therapeutic Guidelines recommends patients admitted due to sedative poisoning avoid tasks requiring substantial levels of concentration (e.g., driving) or making important decisions until at least three days post-discharge [Citation23].
Patients demonstrated insight into their impairment evidenced by an association between a patient’s perception of recovery from overdose and Trail Making Test part B time. The strongest multiple regression model that predicted impairment included whether the patient was exposed to any substantial sedation (overdose or medically administered sedation) in addition to the patient’s age and perception of recovery. However, the adjusted R2 for this model was only 0.207 and hence the association was not strong enough to advise patients they will safely perform their normal activities once they feel recovered.
Age was strongly associated with Trail Making Test times in our study and was highly significant in multiple regression models. While there is a known association between age and Trail Making Test times, the effect on normative data is more strongly seen over the age of 65 years (only 7 patients in this study). The proportion of the Trail Making Test part B time attributed to age (around 1.7 seconds per year) was possibly overestimated in our study (when compared with normative data where an average increase of 1.35 seconds per year was seen to the age of 79 years) [Citation24]. Although the overdose type (sedative versus non-sedative) remained significant after adjusting for age, this may have diminished the significance of other predictors.
Previous studies
Our findings reinforce the results of previous studies [Citation14, Citation16] that reported poorer performance in cognitive testing at the time of discharge by patients who had self-poisoned on sedative drugs. In addition, we have expanded the applicability of the findings to clinical practice by testing a patient cohort that encompasses a broader range of hospital-treated poisonings.
The Trail Making Test times in our study were substantially slower than earlier studies assessing cognitive function after self-poisoning [Citation14] and when compared to age-matched normative data [Citation24]. The Trail Making Test part A performance in our study was slower than normative data by 15.4 seconds for our sedative group and 9.9 seconds for the non-sedative group. The Trail Making Test part B performance was also slower by 76.1 seconds for our sedative group and 45.4 seconds for the non-sedative group. Several patient cohort details could account for the differences when comparing between studies of patients after poisoning. Our study reported a shorter time from exposure to testing (85% of our sedative group were tested within 36.2 h of exposure compared to 48 h) [Citation14], a slightly older maximum patient age, and included participants with illicit drug overdose. Previous Australian data has shown that patients who presented with illicit drug poisoning had greater acuity and shorter length of stay than those with intentional medication overdose [Citation25]. There may also have been other unreported cohort differences such as regular recreational drug use.
The previous study by Dassanayake and colleagues [Citation14] indicated no significant differences in outcomes between subtypes of sedative medications. Statistical comparison was not reported in this study because the poisoning agents were too heterogenous to achieve required numbers within each subgroup.
Secondary outcomes
Potential differences in hospital resource use were compared between sedative and non-sedative overdose patients. While most overdose patients of any type had a brief length of stay (75% discharged within 21 h), some patients required resource-intensive interventions in the ICU to support cardiorespiratory function. We had expected that patients who overdosed on sedative medications could have higher care requirements due to the mechanism of action of these drugs causing depression of the aforementioned respiratory and cardiovascular systems and this was supported by our data.
The association of length of stay with medically administered sedation is not a surprising result. Patients who are more severely affected by poisoning may require a longer period of treatment. Such treatment will often require sedation either as a pharmacological antagonist to the effects of overdose or to facilitate further required procedures (e.g., intubation). In this study, half the total number of patients sedated were admitted to ICU indicating a more critically unwell cohort.
It is of note that the only other factor associated with length of stay was a diagnosis of a mental health disorder, implying social factors or mental health assessment could play a part in delaying hospital discharges.
Future research
Future studies could investigate the effects of in-hospital sedation administered to patients for behavioural or symptom control (compared to anaesthetic or procedural sedation doses). Greater numbers of patients will be required to determine if there is a difference in residual cognitive impairment with different subtypes of sedative drugs or following stimulant overdose.
Conclusion
Our study has extended previous research to include recreational drugs and unintentional overdose, finding sedative overdose is associated with residual cognitive impairment at the time of discharge. These findings emphasize the need for clinicians to be vigilant for ongoing cognitive impairment at the time of discharge, even if the administered sedative was considered relatively minor, and that current screening practice is poor at detecting higher-order cognitive impairment. While patients demonstrated some insight into their impairment it was not enough to safely make discharge decisions. Appropriate guidance following sedative poisoning should be provided to patients to avoid tasks requiring substantial levels of concentration (e.g., driving) or making important decisions until at least 3 days post-discharge, especially those whose occupations may have them engaging in risky activities.
Acknowledgements
We would like to thank the staff of the Prince of Wales Hospital, University of New South Wales Schools of Clinical Medicine and Population Health.
Disclosure statement
No potential conflict of interest was reported by the authors.
Data availability statement
The participants of this study did not give written consent for their data to be shared publicly, so due to the sensitive nature of the research supporting data is not available.
Additional information
Funding
References
- Cairns C, Kang K. National Hospital Ambulatory Medical Care Survey: 2020 emergency department summary tables: Centres for Disease Control and Prevention; 2020 [cited 2023 September 27]. Available from https://www.cdc.gov/nchs/data/nhamcs/web_tables/2020-nhamcs-ed-web-tables-508.pdf.
- Substance Abuse and Mental Health Services Administration. Drug Abuse Warning Network (DAWN): Non-Fatal Overdoses Short Report. 2023 [cited 2023 September 28]. Available from https://www.samhsa.gov/data/report/dawn-non-fatal-overdoses.
- Centers for Disease Control and Prevention. Drug Overdose Surveillance and Epidemiology (DOSE) System: Nonfatal Overdose Emergency Department and Inpatient Hospitalization Discharge Data Atlanta, GA: US Department of Health and Human Services, CDC; 2023 [cited 2023 September 28]. Available from https://www.cdc.gov/drugoverdose/nonfatal/dose/discharge/dashboard/index.html.
- NHS Digital Secondary Care Analytical Team. Hospital Admitted Patient Care Activity, 2021–22 UK: NHS Digital; 2022 [cited 2023 September 27]. Available from https://files.digital.nhs.uk/3C/F3A925/hosp-epis-stat-admi-ext-caus-2021-22-tab.xlsx.
- Australian Institute of Health and Welfare. Intentional self-harm hospitalisations by method. Suicide & Self-Harm Monitoring. Canberra: AIHW; 2022.
- Australian Institute of Health and Welfare. Accidental poisoning. Canberra: AIHW; 2022.
- Australian Institute of Health and Welfare. Alcohol, tobacco & other drugs in Australia. Canberra: AIHW; 2023.
- Chrzanowska A, Man N, Akhurst J, et al. Trends in drug-related hospitalisations in Australia, 1999–2021. Sydney: National Drug and Alcohol Research Centre, UNSW Sydney; 2022.
- Penington Institute. Australia’s annual overdose report 2023. Melbourne, Australia: Penington Institute; 2023.
- Mathias JL, Lucas LK. Cognitive predictors of unsafe driving in older drivers: a meta-analysis. Int Psychogeriatr. 2009;21(4):637–653. doi: 10.1017/S1041610209009119.
- Ball KK, Roenker DL, Wadley VG, et al. Can high‐risk older drivers be identified through performance‐based measures in a department of motor vehicles setting? J Am Geriatr Soc. 2006;54(1):77–84. doi: 10.1111/j.1532-5415.2005.00568.x.
- Edwards JD, Leonard KM, Lunsman M, et al. Acceptability and validity of older driver screening with the DrivingHealth ® inventory. Accid Anal Prev. 2008;40(3):1157–1163. doi: 10.1016/j.aap.2007.12.008.
- Sun J, Stewart P, Chiew A, et al. Association between shift work and cognitive performance on the trail making test in emergency department health officers. Emerg Med Australas. 2021;33(4):711–717. doi: 10.1111/1742-6723.13753.
- Dassanayake TL, Michie PT, Jones A, et al. Cognitive impairment in patients clinically recovered from central nervous system depressant drug overdose. J Clin Psychopharmacol. 2012;32(4):503–510. Aug doi: 10.1097/JCP.0b013e31825d6ddb.
- Dassanayake TL, Michie PT, Jones AL, et al. Cognitive skills underlying driving in patients discharged following self-poisoning with central nervous system depressant drugs. Traffic Inj Prev. 2012;13(5):450–457. Sep doi: 10.1080/15389588.2012.671983.
- Oxley SO, Dassanayake TL, Carter GL, et al. Neurocognitive recovery after hospital-treated deliberate self-poisoning with central nervous system depressant drugs: a longitudinal cohort study. J Clin Psychopharmacol. 2015;35(6):672–680. doi: 10.1097/JCP.0000000000000417.
- New Zealand National Poisons Centre. Toxinz. University of Otago, Dunedin, New Zealand: New Zealand National Poisons Centre; 2023 [cited 2023 October 4]. Available from https://www.toxinz.com/.
- Health Communications Network. MIMS online: HCN; 2023 [cited 2023 October 4].
- Little M, Pascu O, Murray L. Toxicology handbook. Chatswood, NSW: Elsevier; 2015.
- WHO Collaborating Centre for Drug Statistics Methodology. 2023. ATC classification index with DDDs, 2023 Oslo, Norway. [cited 2023 September 6]. Available from https://www.whocc.no/atc_ddd_index/.
- Bowie CR, Harvey PD. Administration and interpretation of the trail making test. Nat Protoc. 2006;1(5):2277–2281. doi: 10.1038/nprot.2006.390.
- Dassanayake TL, Jones AL, Michie PT, et al. Risk of road traffic accidents in patients discharged following treatment for psychotropic drug overdose: a self-controlled case series study in Australia. CNS Drugs. 2012;26(3):269–276. doi: 10.2165/11599790-000000000-00000.
- Therapeutic guidelines. Therapeutic guidelines North Melbourne, Vic: Therapeutic Guidelines Limited; [cited 2023 October 4]. Available from https://tgldcdp.tg.org.au/etgcomplete.
- Tombaugh TN. Trail making test a and B: normative data stratified by age and education. Arch Clin Neuropsychol. 2004;19(2):203–214. doi: 10.1016/S0887-6177(03)00039-8.
- Buykx P, Dietze P, Ritter A, et al. Characteristics of medication overdose presentations to the ED: how do they differ from illicit drug overdose and self-harm cases? Emerg Med J. 2010;27(7):499–503. doi: 10.1136/emj.2009.075549.
Appendices Appendix 1.
Poisoning agents included in the study with categorisation as sedative, stimulant or neither, defined daily dose, and number of patients who were poisoned with each.
Appendix 2.
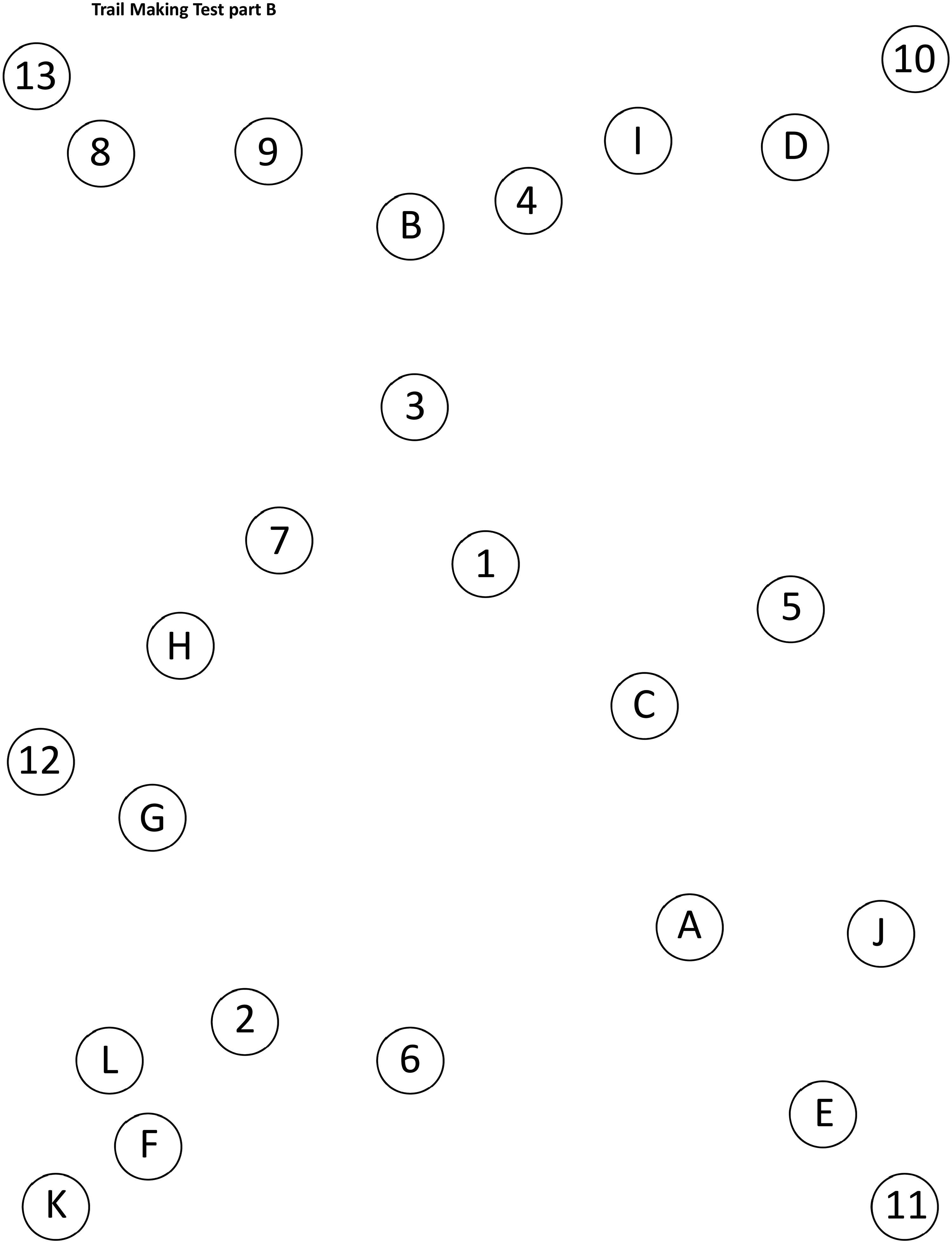
Example of Trail Making Test part A and part B patterns.
Trail Making Test part A
Trail Making Test part B
Appendix 3.
Scatterplot showing correlation between the Trail Making Test part B time and the sedative dose ingested (as a multiple of the defined daily dose). No substantial correlation was seen (R2=0.047). There were only 39 cases that could be entered into the scatterplot and hence no formal statistical analysis was undertaken. Most cases needed to be excluded as either the dose ingested was unknown (e.g., of a recreational drug), was of multiple drugs preventing calculation of defined daily dose, or there was no defined daily dose available (e.g. for ketamine).