ABSTRACT
Drought stress poorly impacts many morphological and physio-biochemical processes in plants. Pea (Pisum sativum L.) plants are highly nutritious crops destined for human consumption; however, their productivity is threatened under drought stress. Thiamine (vitamin B1) is well-known essential micronutrient, acting as a cofactor in key metabolic processes. Therefore, this study was designed to examine the protective effect of foliar application of thiamine (0, 250, and 500 ppm) on two varieties of pea plants under drought stress. Here, we conducted the pot experiment at the Government College Women University, Faisalabad, to investigate the physio-biochemical and morphological traits of two pea varieties (sarsabz and metior) grown under drought stress and thiamine treatment. Drought stress was applied to plants after germination period of 1 month. Results showed that root fresh and dry weight, shoot fresh and dry weight, number of pods, leaf area, total soluble sugars, total phenolics, total protein contents, catalase, peroxidase, and mineral ions were reduced against drought stress. However, the application of thiamine (both 250 and 500 ppm) overcome the stress and also enhances these parameters, and significantly increases the antioxidant activities (catalase and peroxidase). Moreover, the performance of sarsabz was better under control and drought stress conditions than metior variety. In conclusion, the exogenous application of thiamine enabled the plants to withstand drought stress conditions by regulating several physiological and biochemical mechanisms. In agriculture, it is a great latent to alleviate the antagonistic impact of drought stress on crops through the foliar application of thiamine.
1. Introduction
Water, an essential element of cell protoplasm, plays a crucial role in sustaining all life on earth; however, periodic climate shifting limits water accessibility, causing serious problems, e.g., the present water-threatened regimes in various areas of the biomass.Citation1–4 Water limitation is one of the most serious problems in many countries, particularly in PakistanCitation5. It adversely deteriorates the plant growth and agriculture yield production of many traditional crops by affecting physiological responses, including osmotic adjustment, photosynthesis, transpiration, and carbon metabolism.Citation6–8 In addition, physio-biochemical parameters such as enzyme activities, electrolyte leakage, hydrogen peroxide, lipid peroxidation, antioxidant machinery, proline content, and relative water content were adversely affected under drought stress.Citation9–12 Water status understanding plays an important role in securing the food production for rapidly overgrowing population by supporting sustainable agriculture production.Citation12,Citation13 Gene regulatory network revealed that 69 hub-genes, including independent and integrated ABA-dependent pathways controlling the sense of drought, senescence, stomatal closure, antibiotics pathways, thiamine metabolism, purine metabolism, uptake regulation and root growth.Citation14 Grain legumes are highly recognized due to their seeds with high nutritional value. To response the rising concerns about protein autonomy and food security, grain legume cultivation must be increased in up-coming years.Citation15 Pea (Pisum sativum L.) is among the most cultivated pulse corps across the world. Its economic value is particularly obtained from its nutritious seeds having low fat, and high dietary fibers, vitamins, essential minerals, proteins, carbohydrates, iron, zinc, and slow-digestible starch.Citation16–18 Agriculture productivity is facing environmental challenges, so like many other crops, pea is predominantly prone to abiotic stresses, especially more and severe episode of drought that can lead to huge yield lossesCitation19 that primarily arises from concentrated pollination, seed set, abortion of flower and pods, and shortened life cycle under drought.Citation20,Citation21 So, increasing the productivity of pea plants under uncertain conditions of drought stress is an important target.
A diverse range of plant growth-promoting agents accelerates the plant growth and developmental process, and helps plants to thrive under stressful conditions.Citation22–26 Recent studies exposed the dual role of vitamins besides their nutritional importance. Vitamins, especially ascorbic acid, and thiamine play influential roles in combating environmental stresses.Citation27 Plant-derived vitamins regulate plant metabolism, change the redox chemistry and act as enzymatic cofactors,Citation28 therefore enhancing the crop yield and resistance against biotic and abiotic stresses. Thiamine, a growth stimulant that acts as a cofactor in many dynamic plant mechanisms.Citation29 It also acts as an antioxidant to alleviate reactive oxygen species (ROS)Citation30 and is elaborated in energy-producing mechanisms such as Kreb’s and Calvin cycles.Citation31 Respiration and enzymatic activity are also linked with thiamine levels.Citation32 Moreover, growth, photosynthetic pigments, total sugars, proteins, antioxidant machinery and yield attributes are remarkably improved with the exogenous application of thiamine.Citation33,Citation34 Exogenous application of thiamine enhanced stress tolerance by activating stress-responsive genes and calcium signal transduction.Citation35 Furthermore, it also increased the amino acid content, total free amino acids, proline, soluble sugars, photosynthesis, polypeptides, polyehnol oxidase isozymes, peroxidase, growth, and yield attributes under drought stress.Citation36 Moreover, overexpression of thiamine thiazole synthase (needed for the thiamine biosynthesis) gene proved to be more sensitive to abscisic acid than wild plants with respect of both stomatal closure and activation of guard cell slow type anion channels that resulted in lower transpiration loss of water and enhanced drought tolerance.Citation37 Only a few focused studies are present that elucidate the inevitable role of thiamine under drought stress. The present study elaborates on the plausible role of thiamine foliar application in alleviating drought stress in two varieties of pea.
2. Materials and methods
2.1. Plant material and stress treatment
This research was performed at the research area of Government College Women University, Faisalabad (31.4504° N, 73.1350° E), Pakistan in 2019, to examine the positive role of the exogenous application of thiamine on two pea verities (metior and sarsabz) that were obtained from Ayyub Agricultural Research Institute (AARI), Faisalabad. Ninety-six pots of 8 kg soil capacity were filled with soil, and pots were arranged with completely randomized design (CRD). Four replicates per treatment were used during the experiment. Field capacity of the soil was maintained 50% and 100% after 20 days of germination throughout the experiment. Soil of pot was sandy loam having following properties: pH 7.8, electrical conductivity 2.16 dS m−1, organic matter 0.76%, total nitrogen 0.029%, available phosphorus 4.23 mg kg−1, and extractable potassium 314 mg kg−1. Three levels of thiamine solution were applied as 0, 250, and 500 ppm applied through foliar application after developing drought stress. EstefanCitation38 procedure was followed for analysis of site-soil physiochemical properties and nutrient status. Plants leaves were harvested after 3rd stage of BBCH growth scale (a scale which is used to identify the plant’s phenological and developmental stages) to investigate biochemical and growth-related attributes. Half set of experiments was harvested at the yield stage.
2.2. Measurement of growth attributes
Shoot length (cm), root length (cm), shoot fresh and dry weight (g), and root fresh and dry weight (g) were measured for four replicates per treatments. In contrast, numbers of pods per plant, pods length, and number of seeds per pod were measured at the maturity stage.
2.3. Measurement of biochemical attributes
2.3.1. Total chlorophyll and carotenoids contents
Plant total chlorophyll a, chlorophyll b, and carotenoid contents were measured according to Arnon.Citation39 Briefly, a 0.5 g of plant leaf samples were taken and ground in 10 mL of 80%. Then, the samples were centrifuged at 12,000 rpm for 12 min. Readings were taken at 663, 645, and 480 nm on a spectrophotometer. Davies and GoodwinCitation40 protocol was used to calculate the total carotenoids contents.
2.3.2. Total phenolic contents
Julkunen-TiittoCitation41 method was used to calculate the total phenolics content in plant leaves. Briefly, a 0.5 g plant leaf sample were ground in 10 mL of 80% solution of methanol. Samples were then centrifuged at 10,000 rpm for 10 min, then 0.25 mL of sample was mixed with 1.25 mL of Folin-Phenol Ciocalteus reagent. In last step, 1.25 mL of sodium carbonate was added, and vortex vigorously for 5–10 s. Then, absorbance of these samples was recorded at 750 nm.
2.3.3. Proline
Total proline content was measured according to Bates.Citation42 In brief, a 0.5 g plant leaf sample was taken and mixed with 3% sulfosalicylic acid. Then, running samples were centrifuged at 10,000 rpm for 10 min. For 2 mL of extract, 2 mL of acid ninhydrin were added. The resultant solution was mixed with 2 mL of glacial acetic acid, and incubated at 100°C for 1 h and then cooled in an ice bath. Then, 4 mL of toluene was added to each sample and the sample were vortexed for 1 min. These samples were then subjected to a spectrophotometer to measure the absorbance at 520 nm.
2.3.4. Total soluble sugars
Yemm and WillisCitation43 method was used to govern the total soluble sugar contents. Briefly, a 0.1 g plant fresh leaves were taken and then ground in 10 mL of 80% solution of ethanol. The resultant solution was then subjected to centrifugation at 10,000 rpm. Supernatant extract was filtered for estimation of total sugar content. A 0.1 mL of plant extract was taken in separate tubes and 3 mL of anthrone reagent was mixed in each test tube. Then, samples were heated in a water bath for 10 min after ice cooling, and optical density was recorded at 625 nm.
2.3.5. Total protein content
BradfordCitation44 method was utilized to evaluate the total soluble protein contents in plant leaves. Briefly, a 0.5 g of fresh plant leaves were ground in 10 mL of buffer solution. The resultant extract was then centrifuged at 10,000 rpm for 10 min. Then, 2 mL of Bradford reagent was poured into each test tubes. Then, 100 µL extract was added in these test tubes. Optical density was observed at 595 nm by using a spectrophotometer.
2.3.6. Antioxidant enzyme
A 0.5 g of fresh plant leaves were taken, then grind in 80 mL of 50 mM phosphate buffer having pH 7.8. After that the sample was centrifuged at 15,000 rpm for 10 min. Supernatants were used for the determination of catalase and peroxidase. Chance and MaehlyCitation45 method was to estimate plant catalase activity. It was determined by calculating the reduction in absorbance after every 20 s for 120 s at 240 nm. For 2.8 mL of phosphate buffer, 0.1 mL of H2O2 was added. Further, 0.1 mL of enzyme extract was then poured and slowly shaken well to mix it thoroughly and then observed in a spectrophotometer.
2.3.7. Peroxides
Peroxidase activity was recorded of leaf material by following the method of Chance and Maehly.Citation45 For 2.7 mL of phosphate buffer, 0.1 mL of guaiacol and 0.1 mL of enzyme extract was added. Optical density was measured at 470 nm after each 20 s for 120 s collectively.
2.3.8. Ions contents
WolfCitation46 method was used to determine the ions concentration of leaf material. A 0.15 g of dried samples were mixed with 3 mL of sulfuric acid and left overnight. Samples were then heated in a heat bath at 100°C for 30 min. Test tubes were then removed from the heating chamber. This procedure was repeated until the mixture became colorless. Volume of each test tube were then made up to 50 mL with distilled water. Readings of ions analysis sodium (Na+), calcium (Ca2+), and potassium (K+) were noted on flame photometer.
2.4. Statistical analysis
The data were statistically analyzed by using statistics 8.1 software (Analytical Software, Tallahassee, FL), and 3-way ANOVA was used to evaluate the significant differences between the treatments. Four replications were used in CRD factorial design with an alpha value 0.05. LSD test was used to compare the treatment means. R-studio software (Ross Ihaka and Robert Gentleman, USA) was used for correlation and PCA analysis. In PCA, spectral decomposition was examined for the covariances/correlations between different variables.
3. Results
3.1. Growth and yield attributes
3.1.1. Shoot length
Thiamine treatment at 500 ppm significantly increased shoot length irrespective of varietal differences under normal irrigation as well as drought stress. There was a significant interaction between variety and stress factor. In sarsabaz variety, 57.33% and 61.81% increase in shoot length was noted with thiamine spray (500) as compared to control, irrespective of stress treatments control under normal and drought stress conditions, respectively. In metior variety, thiamine spray (500) exhibited 59.80% and 60% increase in shoot length as compared to control irrespective of stress treatments control under normal and drought stress conditions, respectively. Moreover, higher shoot length was seen at 500 ppm of thiamine compared to 250 ppm. Drought stress significantly reduced the shoot length irrespective of varietal differences ().
Table 1. Effect of thiamine and drought stress on growth attributes of two pea varieties. Shoot length, root length, shoot fresh weight, shoot dry weight, root fresh weight, root dry weight, and number of leaves.
3.1.2. Root length
Thiamine treatment (500 ppm) had higher root lengths in plants under normal irrigation as well as drought stress. Interaction between stress and varieties was significant. In sarsabaz variety, thiamine foliar application at 500 ppm showed 54.92% upsurge in root length than control irrespective of stress treatments control conditions. In metior variety, higher dose of thiamine showed 56.28% increase in root length in plants than untreated plants irrespective of stress treatments control under normal conditions, while under drought stress conditions, 62.08% increase in root length was noted as compared to control irrespective of stress treatments control plants. Moreover, root length improved with an increasing dose of thiamine ().
3.1.3. Shoot fresh weight
Thiamine treatment at 500 ppm significantly improved the shoot fresh weights under normal irrigation and drought stress. Treatment and stress factors had more significant interaction between them. In sarsabaz variety, 500 ppm thiamine increased 69.54% and 70.96% shoot fresh weight than control irrespective of stress treatments control under normal and drought conditions, respectively. In metior variety, 500 ppm thiamine increased 64.28% and 73.33% shoot fresh weight than control irrespective of stress treatments control under normal and drought conditions, respectively. Moreover, higher shoot fresh weight was seen at 500 ppm of thiamine as compared with 250 ppm ().
3.1.4. Shoot dry weight
Thiamine treatment at 500 ppm in T3 had significantly increased shoot dry weights under normal irrigation and drought stress. There was more significant interaction between treatment and stress. However, stress, varieties and treatment had non-significant interactions between them. In sarsabz variety, 500 ppm thiamine increased 48.07% and 52.63% shoot dry weight as compared to control irrespective of stress treatments control under normal and drought conditions, respectively. In metior variety, 500 ppm thiamine increased 50% and 44.44% shoot dry weight than control irrespective of stress treatments control under normal and drought conditions, respectively. Moreover, higher shoot dry weight was seen at 500 ppm of thiamine as compared with 250 ppm ().
3.1.5. Root fresh weight
Thiamine treatment at 500 ppm had higher root fresh weights under normal irrigation as well as drought stress. There was a highly significant interaction between treatment and stress. Pea varieties and stress non-significantly affected root fresh weight. There was a noteworthy interaction among treatment and stress. Higher root fresh weight was seen in sarsabz than metior variety. Moreover, higher root fresh weight was seen at 500 ppm of thiamine as compared with 250 ppm. Drought stress significantly declinethe root fresh weight irrespective of varietal differences ().
3.1.6. Root dry weight
Thiamine treatment at 500 ppm had higher root dry weights under normal irrigation as well as drought stress. There was a highly significant interaction between treatment and stress. Pea varieties and stress non-significantly affected root dry weight. There was a significant interface among treatment and stress. Higher root dry weight was seen in sarsabz than metior variety. Moreover, higher root dry weight was seen at 500 ppm of thiamine as compared with 250 ppm. Drought stress significantly decreased the root dry weight irrespective of varietal differences ().
3.1.7. Number of leaves per plant
Thiamine treatment at 500 ppm had a higher number of leaves under normal irrigation and drought stress. There was a highly significant interaction between treatment and stress. Pea varieties and stress non-significantly affected number of leaves. Maximum number of leaves was seen in sarsabz as than metior variety with the application of thiamine at 500 ppm as compared with 250 ppm. Drought stress significantly declined the number of leaves per plant irrespective of varietal differences ().
3.1.8. Pod length
Thiamine treatment at 500 ppm significantly increased pod length under normal irrigation as well as drought stress. Treatment, stress, and varieties had non-significant interactions between them. In sarsabz variety, 53.99% and 52.79% increase in pod length was noticed with the application of 500 ppm thiamine as compared to control irrespective of stress treatments control under normal and stressful conditions, respectively. In metior variety, 52.66% and 68.38% increase in pod length was detected with the application of 500 ppm thiamine than control irrespective of stress treatments control under normal and stressful conditions, respectively. Maximum pod length was seen in sarsabz as than metior variety with the application of thiamine at 500 ppm as compared with 250 ppm. Drought stress significantly reduced the pod length irrespective of varietal differences ().
Table 2. Effect of thiamine and drought stress on yield-related attributes of two pea varieties. Pod length, number of pods per plant, seed fresh weight, and seed dry weight.
3.1.9. Number of pods per plant
Thiamine treatment at 500 ppm significantly increased number of pods per plant normal irrigation as well as drought stress. Treatment, stress, and varieties had highly significant interactions between them. In sarsabz variety, 37.5% and 43.4% increase in number of pods per plant was noticed with the application of 500 ppm thiamine than control irrespective of stress treatments control under normal and stressful conditions, respectively. In metior variety, 41.66% and 63.05% increase in this attribute was observed with the application of 500 ppm thiamine as compared to control irrespective of stress treatments control under normal and stressful conditions, respectively. Maximum number of pods per plant was seen in sarsabz as than metior variety with the application of thiamine at 500 ppm as compared with 250 ppm. Drought stress significantly reduced the number of pods per plant irrespective of varietal differences ().
3.1.10. Seed fresh weight
Thiamine treatment at 500 ppm significantly increased seed fresh weight under normal irrigation as well as drought stress. Treatment, stress, and varieties had non-significant interactions between them. In sarsabz variety, 68.82% and 64.28% increase in seed fresh weight was noticed with the application of 500 ppm thiamine as compared to control irrespective of stress treatments control under normal and stressful conditions, respectively. In metior variety, 69.03% and 50.22% increase in seed fresh weight was recorded with the application of 500 ppm thiamine as compared to control irrespective of stress treatments control under normal and stressful conditions, respectively. Maximum seed fresh weight was seen in sarsabz as than metior variety with the application of thiamine at 500 ppm as compared with 250 ppm. Drought stress significantly reduced the seed fresh weight irrespective of varietal differences ().
3.1.11. Seed dry weight
Thiamine treatment at 500 ppm had higher seed dry weight under normal irrigationand drought stress. There was highly significant interaction between treatment and stress. Pea varieties and stress significantly affected this attribute. Maximum seed dry weight was seen in sarsabz as than metior variety with the application of thiamine at 500 ppm as compared with 250 ppm. Drought stress significantly reduced the seed dry weight per plant irrespective of varietal differences ().
3.2. Biochemical attributes
3.2.1. Chlorophyll a content
Thiamin-treated plants had higher chlorophyll under normal irrigation as well as drought stress. A highly significant interaction was noticed among treatment and stress. Pea varieties and stress non-significantly affected this attribute. Maximum increase was seen in sarsabz as than metior variety with the application of thiamine at 500 ppm as compared with 250 ppm. Drought stress significantly reduced this attribute irrespective of varietal differences ().
3.2.2. Chlorophyll b content
Thiamine treatment at 500 ppm had higher chlorophyll b under normal irrigation as well as drought stress. A non-significant interaction was observed between treatment and stress. Pea varieties and stress non-significantly affected this attribute. Maximum increase was seen in sarsabz as than metior variety with the application of thiamine at 500 ppm as compared with 250 ppm. Drought stress significantly reduced this attribute irrespective of varietal differences ().
3.2.3. Carotenoids content
Foliar application of thiamine showed higher carotenoid contents under normal irrigation as well as drought stress. A non-significant interaction was recorded among treatment and stress. Pea varieties and stress non-significantly affected this attribute. Maximum increase was seen in sarsabz as than metior variety with the application of thiamine at 500 ppm as compared with 250 ppm. Drought stress significantly reduced this attribute irrespective of varietal differences ().
3.2.4. Total chlorophyll content
Foliar application of thiamine showed higher total chlorophyll contents under normal irrigation as well as drought stress. There was non-significant interaction between treatment and stress. Pea varieties and stress non-significantly affected this attribute. Maximum increase was seen in sarsabz as than metior variety with the application of thiamine at 500 ppm as compared with 250 ppm. Drought stress significantly reduced this attribute irrespective of varietal differences ().
3.2.5. Proline content
Thiamine exogenous application showed higher proline content under normal irrigation as well as drought stress. There was non-significant interaction between treatment and stress. Pea varieties and stress non-significantly affected this attribute. Maximum increase was seen in sarsabz as than metior variety with the application of thiamine at 500 ppm as compared with 250 ppm. Drought stress significantly increased this attribute irrespective of varietal differences ().
Figure 2. Effect of thiamine and drought stress on secondary metabolites and antioxidant activities of two pea varieties: (a) proline contents, (b) phenolic contents, (c) protein contents, (d) soluble sugars, (e) peroxidase, (f) catalase. The alphabets indicate the significant (P < 0.05) difference among the treatments.

3.2.6. Total phenolics
Foliar application of thiamine showed higher total phenolics contents under normal irrigation as well as drought stress. There was a significant interaction between treatment and stress. Pea varieties and stress non-significantly affected this attribute. Maximum increase was seen in sarsabz as than metior variety with the application of thiamine at 500 ppm as compared with 250 ppm. Drought stress significantly reduced this attribute irrespective of varietal differences ().
3.2.7. Total protein content
Thiamine treatment at 500 ppm significantly increased total protein content normal irrigation as well as drought stress. Treatment, stress and varieties had significant interactions between them. In sarsabz variety, 62.06% and 52.27% increase in total protein content was observed with the application of 500 ppm thiamine as compared to control, irrespective of stress treatments control under normal and stressful conditions, respectively. In metior variety, 62.29% and 62.83% increase in this attribute were observed with the application of 500 ppm thiamine as compared to control irrespective of stress treatments control under normal and stressful conditions, respectively. Maximum total protein content was seen in sarsabz as than metior variety with the application of thiamine at 500 ppm as compared with 250 ppm. Drought stress significantly reduced the total protein content irrespective of varietal differences ().
3.2.8. Total soluble sugar content
Thiamine treatment at 500 ppm significantly increased total soluble sugar content normal irrigation as well as drought stress. Treatment, stress, and varieties had non-significant interactions between them. In sarsabz variety, 57.41% and 56.47% increase in total soluble sugar content was observed with the application of 500 ppm thiamine as compared to control irrespective of stress treatments control under normal and stressful conditions, respectively. In metior variety, 58.82% and 57.60% increase in this attribute was observed with the application of 500 ppm thiamine as compared to control irrespective of stress treatments control under normal and stressful conditions, respectively. Maximum total soluble sugar content was seen in sarsabz as than metior variety with the application of thiamine at 500 ppm as compared with 250 ppm. Drought stress significantly reduced the total soluble sugar content irrespective of varietal differences ().
3.2.9. Peroxidase activity
Thiamine exogenous application showed higher peroxidase activity under normal irrigation as well as drought stress. There was non-significant interaction between treatment and stress. Pea varieties and stress non-significantly affected this attribute. Maximum increase was seen in sarsabz as than metior variety with the application of thiamine at 500 ppm as compared with 250 ppm. Drought stress significantly reduced this attribute irrespective of varietal differences ().
3.2.10. Catalase activity
Foliar application of thiamine showed higher catalase activity under normal irrigation as well as drought stress. A non-significant interaction was observed between treatment and stress. Pea varieties and stress non-significantly affected this attribute. Maximum increase was seen in sarsabz as than metior variety with the application of thiamine at 500 ppm as compared with 250 ppm. Drought stress significantly reduced this attribute irrespective of varietal differences ().
3.3. Ions contents
3.3.1. Potassium content
Thiamine exogenous application showed higher potassium content under normal irrigation as well as drought stress. A non-significant interaction was noticed between treatment and stress. Pea varieties and stress non-significantly affected this attribute. Maximum increase was seen in sarsabz as than metior variety with the application of thiamine at 500 ppm as compared with 250 ppm. Drought stress significantly reduced this attribute irrespective of varietal differences ().
3.3.2. Calcium content
Foliar application showed higher potassium content under normal irrigation as well as drought stress. There was a significant interaction between treatment and stress. Interactions between varieties, stress, and treatments was non-significant. Pea varieties and stress non-significantly affected this attribute. Maximum increase was seen in sarsabz as than metior variety with the application of thiamine at 500 ppm as compared with 250 ppm. Drought stress significantly reduced this attribute irrespective of varietal differences ().
3.3.3. Sodium content
Foliar application showed higher sodium content under normal irrigation as well as drought stress. There was a significant interaction between treatment and stress. Interactions between varieties, stress and treatments was significant. Pea varieties and stress non-significantly affected this attribute. Maximum increase was seen in sarsabz as than metior variety with the application of thiamine at 500 ppm as compared with 250 ppm. Drought stress significantly reduced this attribute irrespective of varietal differences ().
3.3.4. PCA and correlation
First, PCA was performed by using the data of all growth and yield-related variables and the second PCA for biochemical attributes, including ion analysis. The first PCA component (PC1) depicted most of the inertia of the data (96.8% of total variance) and showed close relation with all variables (). The PC2 explained 1.9% of the variance of the population, and was mainly driven by the number of pods per plant and shoot dry weight (). The PC1 and PC2 accounted for 87.4% and 5.8% of the total variance and was mainly driven by proline and sodium content. Pearson correlation showed a positive linear correlation among all growth, yield and biochemical attributes, except a negative correlation with sodium and proline content (). Heatmap matrix showed that the performance of sarsabz variety with the application of 500 ppm of thiamine was more effective in improving all the parameters (growth, yield, and biochemical) under normal conditions as compared to control irrespective of stress treatments other treatments. Intriguingly, the performance of sarsabz variety was best under control and stressful conditions as compared to control irrespective of stress treatments metior. The metior variety showed negative relation with all parameters under no-spray treatment at drought stress condition ().
Figure 4. Principal component analysis based on two pea varieties grown under the influence of two thiamine doses and drought stress. First PCA (a) indicates the growth and yield attributes, while second for biochemical attributes (b). The positions of the different variables, includes root length (RL), number of pods per plant (NPP), number of leaves (NOL), pod length (podL), root fresh weight (RFW), root dry weight (RDW), shoot fresh weight (SFW), shoot dry weight (SDW), number of seeds per plant (NSPP), seed fresh weight (SeFW), seed dry weight (SeDW), catalase (CAT), peroxidase (POD), sodium (Na), potassium (K), calcium (Ca), soluble sugars (SS), and phenolics (phenol). As indicated at the bottom left of each circle, (a) gathers 96.8% of the total variance, whereas (b), 87.4% of the total variance.

Figure 5. Pearson correlation among different variables in two pea varieties with an application of thiamine under drought stress. The positions of the different variables, includes root length (RL), number of pods per plant (NPP), number of leaves (NOL), pod length (podL), root fresh weight (RFW), root dry weight (RDW), shoot fresh weight (SFW), shoot dry weight (SDW), number of seeds per plant (NSPP), seed fresh weight (SeFW), seed dry weight (SeDW), catalase (CAT), peroxidase (POD), sodium (Na), potassium (K), calcium (Ca), soluble sugars (SS), and phenolics (phenol).

Figure 6. Heatmap matrix among different variables in two wheat varities with application of thiamine under drought stress. V1 and V2 indicates sarsabz and metior varieties, respectively, while S1 and S2 for control and normal conditions. T1, T2, and T3 for control, 250 ppm and 500 ppm thiamine doses. The positions of the different variables, includes root length (RL), number of pods per plant (NPP), number of leaves (NOL), pod length (podL), root fresh weight (RFW), root dry weight (RDW), shoot fresh weight (SFW), shoot dry weight (SDW), number of seeds per plant (NSPP), seed fresh weight (SeFW), seed dry weight (SeDW), catalase (CAT), peroxidase (POD), sodium (Na), potassium (K), calcium (Ca), soluble sugars (SS), and phenolics (phenol).

4. Discussion
Drought stress is frequently increasing in the milieu of changing climate and is a foremost constraint for agriculture production. The capacity of plants to sustain their higher productivity under drought stress depends on their capability to endure the drought stress with subsequent recovery.Citation2,Citation47,Citation48 This study designed at dynamically assessing the drought response of two pea varieties, namely sarsabz and metior, with the application of thiamine as growth promoting agent. Drought stress led to a remarkable reduction in shoot length, root length, shoot fresh and dry weight, and root fresh and dry weight and number of leaves as shown in . Our results are in corroborated with the findings of Arafa et al.,Citation19 they found that drought stress severely reduced the leaf area plant height and number of leaves per plant as compared with control plants. These outcomes are in agreement with the findings of some investigators in diverse plant species under drought stress, for example, faba bean,Citation49,Citation50 sugar beat,Citation51 barleyCitation52 and flax plants.Citation53 According to Mohammadi Alagoz et al.,Citation54 frequent and intense drought events impact agricultural productionby challenging water status, resulting in lower plant growth and development. However, thiamine applications 250 and 500 ppm effectively stimulate all the growth-related attributes under drought stress. Aminifard et al.Citation55 also deduced that thiamine application at the rate of 250, 500 and 700 ppm increased the growth and yield-related attributes in fenugreek and coriander. A similar increase was also confirmed in . In sarsabz variety, 37.5% and 43.4% increase in number of pods per plant was observed with the application of 500 ppm thiamine as compared to control irrespective of stress treatments control irrespective of stress treatments, respectively. In metior variety, 41.66% and 63.05% increase in number of pods was observed with the application of 500 ppm thiamine as compared to control irrespective of stress treatments control under normal and stressful conditions, respectively. However, drought stress adversely affected the yield attributes of pea irrespective of varietal differences. Arafa et al.Citation19 also noted remarkable reduction in number of flowers per plant, pod length and number of pods per plant in drought exposed pea. Similar trend of reduction was seen in the present trial. As reviewed by Khan et al.,Citation56 the accretion of plant growth-promoting agents cope with the stress condition; through contributing detoxification of ROS, osmoregulation and pH adjustment, so higher growth and yield performance was ascertained with the foliar spray of thiamine.
Moreover, drought stress poorly exacerbated the photosynthetic system, and deteriorated the accumulation of assessor pigments such as chlorophyll a, chlorophyll b, and carotenoids (). The reduced photosynthetic efficiency was due to the stomatal closure and downregulation of the photosynthetic pathway and Calvin cycle genes.Citation57 However, the application of 500 ppm of thiamine efficiently improved the photosynthetic pigments, especially sarsabz as compared with metior varieties, as confirmed by Aminifard et al.,Citation55 they found a similar trend with the foliar application of 500 and 700 ppm of thiamine. ABA seems to take part in the genes activation, encoding enzymes that function in thiamine,Citation58 which triggered the regulatory pathways to improve physiological responses, which may be the reason of improved photosynthetic activities with thiamine application. The reason for the reduction of chlorophyll pigments is may be associated with the degradation of chlorophyll directly in response to drought stressCitation59. Besides, chlorophyll b was more severely affected under drought stress than the other pigments, the reason is that chlorophyll b is more sensitive to osmotic stress as compared to chlorophyll b.Citation60 Drought stress caused a severe reduction in biochemical accumulation such as soluble sugars, protein and phenolics contents and antioxidant machinery (). Reduction in photosynthetic assimilates may be the main reason for lower production of these biomolecules,Citation57 because lower ATP is available for the synthesis of these biochemicals. Besharati et al.Citation61 also identified a lower accumulation of total soluble phenolics under severe drought stress, due to diminished photosynthetic activities. Lowest proline and protein content was found when thiamine was not applied, a similar trend was confirmed by Mehrasa et al.,Citation62 while working on white bean under drought stress. However, thiamine application, especially at the dose of 500 ppm, significantly improved the secondary metabolites and antioxidant production, especially in sarsabz as compared with metior varieties as confirmed by Hosseinifard et al.Citation63 Thiamine acts as signaling molecule under biotic and abiotic stress, and modulates the plant metabolism according to its environment.Citation64 Vitamins could be considered as a compound of bioregulators or phytohormone precursors that, in a tiny amount, retain a beneficial effect on plant growth, development that could effect on the energy metabolic pathways and antioxidant productionCitation65–67 as confirmed in this study. So, all essential physiological process, secondary metabolites and nutrients are more and less dependent on the availability of vitamins.Citation68 Similarly, in this study, higher dependence of nutrient uptake was seen on thiamine availability. These results are corroborated with the findings of Arafa et al.Citation19, while working on pea as a model plant. However, lower nutrient accumulation was recorded under drought stress as shown in . The lower uptake of the nutrients are due to the osmotic effect of drought stress.Citation54 The PCA and pearson correlation matrix showed strong relation with sodium and proline (), that might be due to the metabolic reshuffle during stress and osmotic effect of drought stress. Heatmap matrix showed positive correlation of sarsabz variety with almost all the above mentioned parameters as compared with metior variety.
5. Conclusion
Drought stress is an predictable factor that happens in multiple environments deprived of recognizing borders and no clear warnings, thereby hindering plant biomass and quality of agriculture production. However, foliar application of thiamine, especially 500 ppm, significantly improved photosynthetic attributes, soluble sugars, proline, pehnolics, antioxidants, and mineral uptake that resulted in higher growth and productivity irrespective of varietal differences (); however, the performance of sarsabz variety was much better than metior variety. The beans are rich source of proteins and are mostly preferred due to their high nutritional profile. Thiamine application further enhances protein accretion up to 52–62.83%. The vitamin thiamine application offers a rich source for understanding many aspects of plants primary and secondary metabolism. Exogenous application of thiamine opens new avenues to boost the abiotic resistance mechanisms, especially against drought stress that could overcome the economic burden of poor productivity and treat and rehabilitate severely malnourished problems under climate variability.
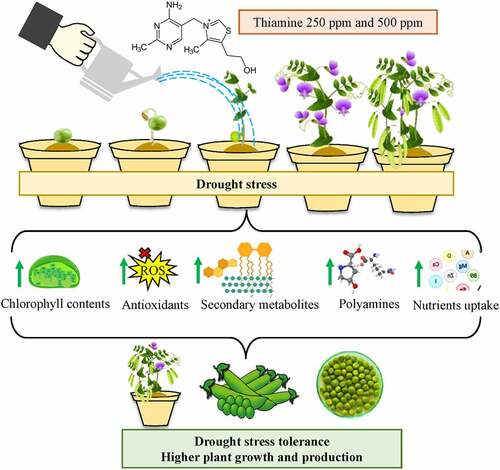
Figure 7. Mechanism of thiamine-induced drought stress tolerance in pea plants. In short, thiamine application improves drought tolerance by increasing several biochemical attributes such as chlorophyll contents, secondary metabolites, polyamines, and nutrient contents. Whereas it also helps ROS scavenging by increasing antioxidant enzyme activities. Upwards green means increased/upregulated, while downward green arrow mean decreased/downregulated.

Authors contribution
The authors confirm their contribution to the paper as follows: study conception and design: A.K.; data collection: H.Z. and N.Z.; analysis and interpretation of results: M.B.H., A.R., N.Z., A.R., and S.Z.; draft manuscript preparation, proofreading and editing: A.K., A.F, N.Z, A.R, P.V.V.P., and I.D. All authors reviewed the results and approved the final version of the manuscript.
Disclosure statement
No potential conflict of interest was reported by the authors.
Data availability statement
The data reported in this study will be available from the corresponding author on a reasonable request.
Correction Statement
This article has been corrected with minor changes. These changes do not impact the academic content of the article.
Additional information
Funding
References
- Wahab A, Abdi G, Saleem MH, Ali B, Ullah S, Shah W, Mumtaz S, Yasin G, Muresan CC, Marc RA. Plants’ physio-biochemical and phyto-hormonal responses to alleviate the adverse effects of drought stress: a comprehensive review. Plants. 2022;11(13):1620. doi:10.3390/plants11131620.
- Farooq MS, Uzaiir M, Raza A, Habib M, Xu Y, Yousuf M, Yang SH, Ramzan Khan M. Uncovering the research gaps to alleviate the negative impacts of climate change on food security: a review. Front Plant Sci. 2022;13:927535. doi:10.3389/fpls.2022.927535.
- Pereira, L. S. Water, agriculture and food: challenges and issues. Water Resources Management, 2017;31(10),2985–13. doi:10.1007/s11269-017-1664-z.
- Stamm MD, Enders LS, Donze-Reiner TJ, Baxendale FP, Siegfried BD, Heng-Moss TM. Transcriptional response of soybean to thiamethoxam seed treatment in the presence and absence of drought stress. BMC Genom. 2014;15:1–13. doi:10.1186/1471-2164-15-1055.
- Waseem M, Rehman ZU, Sabetta F, Ahmad I, Ahmad M, Sabri MMS. Evaluation of the predictive performance of regional and global ground motion predictive equations for shallow active regions in Pakistan. Sustainability. 2022;14:8152. doi:10.3390/su14138152.
- Mehmood M, Khan I, Chattha M, Hussain S, Ahmad N, Aslam M, Hafeez MB, Hussan M, Hassan MU, Nawaz M, Iqbal MM, Hussain F. Thiourea application protects maize from drought stress by regulating growth and physiological traits. Pak J Sci. 2021;73:355. http://pjosr.com/index.php/pjs/article/view/684
- Hafeez MB, Zahra N, Zahra K, Raza A, Khan A, Shaukat K, Khan S. Brassinosteroids: molecular and physiological responses in plant growth and abiotic stresses. Plant Stress. 2021;2:100029. doi:10.1016/j.stress.2021.100029.
- Zhang W, Wang J, Xu L, Wang A, Huang L, Du H, Qiu L, Oelmüller R. Drought stress responses in maize are diminished by Piriformospora indica. Plant Signal Behav. 2018;13:e1414121. doi:10.1080/15592324.2017.1414121.
- Ghani MI, Saleem S, Rather SA, Rehmani MS, Alamri S, Rajput VD, Kalaji HM, Saleem N, Sial TA, Liu M. Foliar application of zinc oxide nanoparticles: an effective strategy to mitigate drought stress in cucumber seedling by modulating antioxidant defense system and osmolytes accumulation. Chemosphere. 2022;289:133202. doi:10.1016/j.chemosphere.2021.133202.
- Iftikhar I, Anwar-Ul-Haq M, Akhtar J, Maqsood M. Exogenous osmolytes supplementation improves the physiological characteristics, antioxidant enzymatic activity and lipid peroxidation alleviation in drought-stressed soybean. Pak J Agri Sci. 2022;59:43–53. doi:10.21162/PAKJAS/22.923.
- Saddiq MS, Wang X, Iqbal S, Hafeez MB, Khan S, Raza A, Iqbal J, Maqbool MM, Fiaz S, Qazi MA, et al. Effect of water stress on grain yield and physiological characters of quinoa genotypes. Agronomy. 2021;11(10):1934. doi:10.3390/agronomy11101934.
- Raza A, Mubarik MS, Sharif R, Habib M, Jabeen W, Zhang C, Chen H, Chen Z-H, Siddique KHM, Zhuang W, et al. Developing drought-smart, ready-to-grow future crops. Plant Genome. 2022. e20279. doi:10.1002/tpg2.20279
- Penna D, Geris J, Hopp L, Scandellari F. Water sources for root water uptake: using stable isotopes of hydrogen and oxygen as a research tool in agricultural and agroforestry systems. Agric Ecosyst Environ. 2020;291:106790. doi:10.1016/j.agee.2019.106790.
- Iquebal MA, Sharma P, Jasrotia RS, Jaiswal S, Kaur A, Saroha M, Angadi UB, Sheoran S, Singh R, Singh GP, et al. Rnaseq analysis reveals drought-responsive molecular pathways with candidate genes and putative molecular markers in root tissue of wheat. Sci Rep, 2019;9:1–18. doi:10.1038/s41598-019-49915-2.
- Morin A, Maurousset L, Vriet C, Lemoine R, Doidy J, Pourtau N. Carbon fluxes and environmental interactions during legume development, with a specific focus on Pisum sativum. Physiol Plant. 2022;174:e13729. doi:10.1111/ppl.13729.
- Hacisalihoglu G, Beisel NS, Settles AM, Gupta D. Characterization of pea seed nutritional value within a diverse population of Pisum sativum. PloS One. 2021;16(11):e0259565. doi:10.1371/journal.pone.0259565.
- Khan R, Manzoor N, Zia A, Ahmad I, Ullah A, Shah SM, et al. Exogenous application of chitosan and humic acid effects on plant growth and yield of pea (Pisum sativum). Inter J Biosci. 2018;12:43–50.
- Tan C, Zhang L, Duan X, Chai X, Huang R, Kang Y, Yang X. Effects of exogenous sucrose and selenium on plant growth, quality, and sugar metabolism of pea sprouts. J Sci Food Agric. 2022;102:2855–2863. doi:10.1002/jsfa.11626.
- Arafa SA, Attia KA, Niedbała G, Piekutowska M, Alamery S, Abdelaal K, Alateeq TK, Ali M, Elkelish A, Attallah SY. Seed Priming boost adaptation in pea plants under drought stress. Plants. 2021;10:2201. doi:10.3390/plants10102201.
- Kumari VV, Roy A, Vijayan R, Banerjee P, Verma VC, Nalia A, Pramanik M, Mukherjee B, Ghosh A, Reja MH, et al. Drought and heat stress in cool-season food legumes in sub-tropical regions: consequences, adaptation, and mitigation strategies. Plants, 2021;10:1038. doi:10.3390/plants10061038.
- Sukhova E, Yudina L, Akinchits E, Vodeneev V, Sukhov V. Influence of electrical signals on pea leaf reflectance in the 400–800-nm range. Plant Signal Behav. 2019;14:1610301. doi:10.1080/15592324.2019.1610301.
- Mubarik MS, Khan SH, Sajjad M, Raza A, Hafeez MB, Yasmeen T, Rizwan M, Ali S, Arif MS. A manipulative interplay between positive and negative regulators of phytohormones: a way forward for improving drought tolerance in plants. Physiol Plant. 2021;172:1269–1290. doi:10.1111/ppl.13325.
- Granaz KS, Baksh G, Zahra N, Hafeez MB, Raza A, Raza A, Samad A, Nizar M, Wahid A. Foliar application of thiourea, salicylic acid, and kinetin alleviate salinity stress in maize grown under etiolated and de-etiolated conditions. Discover Food. 2022;2:1–14. doi:10.1007/s44187-022-00027-3.
- Raza A, Salehi H, Rahman MA, Zahid Z, Haghjou MM, Najafi-Kakavand S, Charagh S, Osman HS, Albaqami M, Zhuang Y, et al. Plant hormones and neurotransmitter interactions mediate antioxidant defenses under induced oxidative stress in plants. Front Plant Sci, 2022;13:961872. doi:10.3389/fpls.2022.961872.
- Raza A, Charagh S, García-Caparrós P, Rahman MA, Ogwugwa VH, Saeed F, Jin W. Melatonin-mediated temperature stress tolerance in plants. GM Crops & Food. 2022;13(1):196–217. doi:10.1080/21645698.2022.2106111.
- Raza A, Tabassum J, Mubarik M, Anwar S, Zahra N, Sharif Y, Hafeez MB, Zhang C, Corpas FJ, Chen H, et al. Hydrogen sulfide: an emerging component against abiotic stress in plants. Plant Biol, 2022;24:540–558. doi:10.1111/plb.13368.
- Alam, S.S., Akhi, A.H., Alam, F., Hasanuzzaman, M. and Rohman, M., 2022. 22 Enhancement of Plant Productivity and Stress Tolerance by the Application of an Exogenous Supply of Vitamins. In Biostimulants for Crop Production and Sustainable Agriculture. (pp. 348–371). GB: CABI. UK. DOI:10.1079/9781789248098.0022.
- Asensi-Fabado MA, Munné-Bosch S. Vitamins in plants: occurrence, biosynthesis and antioxidant function. Trend Plant Sci. 2010;15:582–592. doi:10.1016/j.tplants.2010.07.003.
- Colinas M, Fitzpatrick TB. Natures balancing act: examining biosynthesis de novo, recycling and processing damaged vitamin B metabolites. Curr Opin Plant Biol. 2015;25:98–106. doi:10.1016/j.pbi.2015.05.001.
- Subki A, Abidin AAZ, Yusof ZNB. The role of thiamine in plants and current perspectives in crop improvement. B Group Vitamins-Current Uses and Perspectives. 2018;5:33–44.
- Du Q, Wang H, Xie J. Thiamin (vitamin B1) biosynthesis and regulation: a rich source of antimicrobial drug targets? Int J Biol Sci. 2011;7:41. doi:10.7150/ijbs.7.41.
- Fitzpatrick TB, Chapman LM. The importance of thiamine (vitamin B1) in plant health: from crop yield to biofortification. J Biol Chem. 2020;295:12002–12013. doi:10.1074/jbc.REV120.010918.
- Fallahi H-R, Aminifard MH, Jorkesh A. Effects of thiamine spraying on biochemical and morphological traits of basil plants under greenhouse conditions. J Hortic Postharvest Res. 2018;1:27–36.
- El-Metwally IM, Sadak MS. Physiological role of thiamine and weed control treatments on faba bean and associated weeds grown under salt affected soil. Bullet Natl Res Centre. 2019;43:1–16. doi:10.1186/s42269-019-0142-6.
- Li W, Mi X, Jin X, Zhang D, Zhu G, Shang X, Zhang D, Guo W. Thiamine functions as a key activator for modulating plant health and broad-spectrum tolerance in cotton. Plant J. 2022;111:374–390. doi:10.1111/tpj.15793.
- Sadak MS, El-Bassiouny H, Mahfouz S, El-Enany M, Elewa T. Use of thiamine, pyridoxine and biostimulant for better yield of wheat plants under water stress: growth, osmoregulations, antioxidantive defense and protein pattern. Egypt J Chem. 2022. doi:10.21608/ejchem.2022.160140.6898.
- C-L L, Wang M, X-M W, Chen D-H, H-J L, Shen J-L, Qiao Z, Zhang W. THI1, a thiamine thiazole synthase, interacts with Ca2±dependent protein kinase CPK33 and modulates the S-type anion channels and stomatal closure in Arabidopsis. Plant Physiol. 2016;170:1090–1104. doi:10.1104/pp.15.01649.
- Estefan G, Sommer R, Ryan J. Methods of soil, plant, and water analysis: a manual for the West Asia and North Africa region. International Center for Agricultural Research in the Dry Areas (ICARDA). 2013;3:20–135.
- Arnon A. Method of extraction of chlorophyll in the plants. Agron J. 1967;23:112–121.
- Davies B, Goodwin T. Chemistry and biochemistry of plant pigments. Carotenoids. 1976;2:38–165.
- Julkunen-Tiitto R. Phenolic constituents in the leaves of northern willows: methods for the analysis of certain phenolics. J Agric Food Chem. 1985;33:213–217. doi:10.1021/jf00062a013.
- Bates J. Tell it how it is: few school leavers understand what nursing is all about. Nursing Standard. 2004;18:25–26. doi:10.7748/ns.18.19.25.s40.
- Yemm E, Willis A. The estimation of carbohydrates in plant extracts by anthrone. Biochem J. 1954;57:508. doi:10.1042/bj0570508.
- Bradford MM. A rapid and sensitive method for the quantification of microgram quantities of protein using the principle of protein dye-binding. Anal Biochem. 1976;72:248–254. doi:10.1016/0003-2697(76)90527-3.
- Chance B, Maehly A. Assay of catalases and peroxidases. Methods Enzymol. 1955;2:764–775.
- Wolf B. An improved universal extracting solution and its use for diagnosing soil fertility. Comm Soil Sci Plant Anal. 1982;13:1005–1033. doi:10.1080/00103628209367331.
- Couchoud M, Salon C, Girodet S, Jeudy C, Vernoud V, Prudent M. Pea efficiency of post-drought recovery relies on the strategy to fine-tune nitrogen nutrition. Front Plant Sci. 2020;11:204. doi:10.3389/fpls.2020.00204.
- Nataraja KN, Dhanyalakshmi K, Govind G, Oelmüller R. Activation of drought tolerant traits in crops: endophytes as elicitors. Plant Signal Behav. 2022;17:2120300. doi:10.1080/15592324.2022.2120300.
- Abdelaal K, AlKahtani M, Attia K, Hafez Y, Király L, Künstler A. The role of plant growth-promoting bacteria in alleviating the adverse effects of drought on plants. Biology. 2021;10:520. doi:10.3390/biology10060520.
- Kasim WA, Nessem AA, Gaber A. Effect of seed priming with aqueous extracts of carrot roots, garlic cloves or ascorbic acid on the yield of Vicia faba grown under drought stress. Pak J Bot. 2019;51:1979–1985. doi:10.30848/PJB2019-6(41).
- AlKahtani MD, Hafez YM, Attia K, Rashwan E, Husnain LA, AlGwaiz HI, Abdelaal KAA. Evaluation of silicon and proline application on the oxidative machinery in drought-stressed sugar beet. Antioxidants. 2021;10(3):398. doi:10.3390/antiox10030398.
- Abdelaal KA, Attia KA, Alamery SF, El-Afry MM, Ghazy AI, Tantawy DS, Al-Doss AA, El-Shawy ESE, Abu-Elsaoud A, Hafez YM. Exogenous application of proline and salicylic acid can mitigate the injurious impacts of drought stress on barley plants associated with physiological and histological characters. Sustainability. 2020;12:1736. doi:10.3390/su12051736.
- Rashwan E, Alsohim AS, El-Gammaal A, Hafez Y, Abdelaal KA. Foliar application of nano zink-oxide can alleviate the harmful effects of water deficit on some flax cultivars under drought conditions. Fresenius Environ Bull. 2020;29:8889–8904.
- Mohammadi Alagoz S, Zahra N, Hajiaghaei Kamrani M, Asgari Lajayer B, Nobaharan K, Astatkie T, Siddique KHM, Farooq M. Role of Root Hydraulics in Plant Drought Tolerance. J Plant Growth Regul. 2022;1–16. doi:10.1007/s00344-022-10807-x.
- Aminifard MH, Jorkesh A, Fallahi H-R, Alipoor K. Foliar application of thiamin stimulates the growth, yield and biochemical compounds production of coriander and fenugreek. J Hortic Res. 2018; 26. 10.2478/johr-2018-0009.
- Khan N, Ali S, Zandi P, Mehmood A, Ullah S, Ikram M, Ismail I, Shahid MA, Babar MA. Role of sugars, amino acids and organic acids in improving plant abiotic stress tolerance. Pak J Bot. 2020;52:355–363. doi:10.30848/PJB2020-2(24).
- Pandey K, Kumar RS, Prasad P, Pande V, Trivedi PK, Shirke PA. Coordinated regulation of photosynthesis and sugar metabolism in guar increases tolerance to drought. Environ Exp Bot. 2022;194:104701. doi:10.1016/j.envexpbot.2021.104701.
- Rapala-Kozik M, Wolak N, Kujda M, Banas AK. The upregulation of thiamine (vitamin B1) biosynthesis in Arabidopsis thaliana seedlings under salt and osmotic stress conditions is mediated by abscisic acid at the early stages of this stress response. BMC Plant Biol. 2012;12:1–14. doi:10.1186/1471-2229-12-2.
- Yang X, Lu M, Wang Y, Wang Y, Liu Z, Chen S. Response mechanism of plants to drought stress. Horticulturae. 2021;7:50. doi:10.3390/horticulturae7030050.
- Shin YK, Bhandari SR, Lee JG. Monitoring of salinity, temperature, and drought stress in grafted watermelon seedlings using chlorophyll fluorescence. Front Plant Sci. 2021;12:2913. doi:10.3389/fpls.2021.786309.
- Besharati J, Shirmardi M, Meftahizadeh H, Ardakani MD, Ghorbanpour M. Changes in growth and quality performance of Roselle (Hibiscus sabdariffa L.) in response to soil amendments with hydrogel and compost under drought stress. S Afr J Bot. 2022;145:334–347. doi:10.1016/j.sajb.2021.03.018.
- Mehrasa H, Farnia A, Kenarsari MJ, Nakhjavan S. Endophytic bacteria and SA application improve growth, biochemical properties, and nutrient uptake in White Beans under drought stress. Journal of Soil Sci Plant Nutr. 2022;1–12. doi:10.1007/s42729-022-00884-y.
- EL Sayed NI, Abd-ELhady WM, Selim EM. Increased Resistance to Salt Stress of Duranta plumieri Irrigated with Seawater by Using Thiamin, Humic Acid and Salicylic acid. Journal of Plant Production. 2017;8(5):617–27. DOI:10.21608/JPP.2017.40492.
- Goyer A. Thiamine in plants: aspects of its metabolism and functions. Phytochemistry. 2010;71(14–15):1615–1624. doi:10.1016/j.phytochem.2010.06.022.
- Dawood M, El-Metwally I, Abdelhamid M. Physiological response of lupine and associated weeds grown at salt-affected soil to α‑tocopherol and hoeing treatments. Gesunde Pflanzen. 2016;68:117–127. doi:10.1007/s10343-016-0367-3.
- Mostafa MR, Mervat SS, Safaa R-L, Ebtihal MAE, Magdi TA. Exogenous α-tocopherol has a beneficial effect on Glycine max (L.) plants irrigated with diluted sea water. J Hortic Sci Biotechnol. 2015;90:195–202. doi:10.1080/14620316.2015.11513172.
- Orabi SA, Abdelhamid MT. Protective role of α-tocopherol on two Vicia faba cultivars against seawater-induced lipid peroxidation by enhancing capacity of anti-oxidative system. J Saudi Soc Agric Sci. 2016;15:145–154. doi:10.1016/j.jssas.2014.09.001.
- Abdelhamid MT, Sadak MS, Schmidhalter U, El-Saady AK. Interactive effects of salinity stress and nicotinamide on physiological and biochemical parameters of faba bean plant. Acta Biol Colomb. 2013;18:499–509.