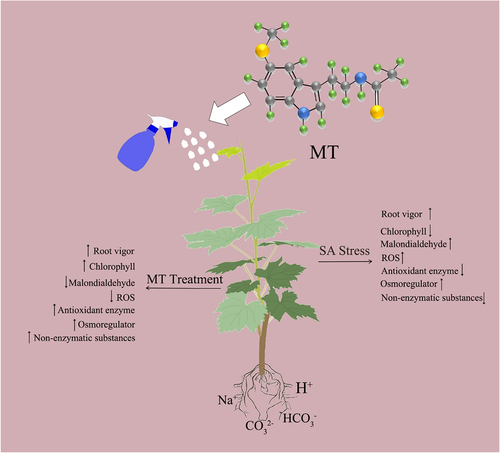
ABSTRACT
Saline and alkaline stress is one of the major abiotic stresses facing agricultural production, which severely inhibits the growth and yield of plant. The application of plant growth regulators can effectively prevent crop yield reduction caused by saline and alkaline stress. Exogenous melatonin (MT) can act as a signaling molecule involved in the regulation of a variety of physiological processes in plants, has been found to play a key role in enhancing the improvement of plant tolerance to abiotic stresses. However, the effects of exogenous MT on saline and alkaline tolerance of table grape seedlings and its mechanism have not been clarified. The aim of this study was to investigate the role of exogenous MT on morphological and physiological growth of table grape seedlings (Vitis vinifera L.) under saline and alkaline stress. The results showed that saline and alkaline stress resulted in yellowing and wilting of grape leaves and a decrease in chlorophyll content, whereas the application of exogenous MT alleviated the degradation of chlorophyll in grape seedling leaves caused by saline and alkaline stress and promoted the accumulation of soluble sugars and proline content. In addition, exogenous MT increased the activity of antioxidant enzymes, which resulted in the scavenging of reactive oxygen species (ROS) generated by saline and alkaline stress. In conclusion, exogenous MT was involved in the tolerance of grape seedlings to saline and alkaline stress, and enhanced the saline and alkaline resistance of grape seedlings to promote the growth and development of the grape industry in saline and alkaline areas.
1. Introduction
Plants are continuously exposed to adversity stresses such as drought, salinity, heavy metals, cold and heat throughout their life cycle. The detrimental effects of saline and alkaline soil on plant growth, development, and differentiation are among the many abiotic stressors that are particularly impotant.Citation1 The rise in soil-soluble salts is sometimes referred to as “soil salinization” since soil salinization and alkalization frequently occur simultaneously. Especially in arid and semi-arid regions, there is a gradual expansion of soil salinization due to over-fertilization and increase in irrigated areas.Citation2 Soil salinity has become one of the major environmental factors in the development of agricultural economies, which not only inhibits crop growth, development, and yield, but also causes soil degradation and deterioration of the ecological environment.Citation3,Citation4 Excessive soil salinity triggers saline and alkaline stress in plants, and saline stress and alkaline stress are two different types of stresses, saline stress leads to oxidative damage, ionic stress, and osmotic stress in plants, which manifests as inhibition of water uptake, root elongation and growth, and impaired leaves development, thus severely inhibits plant growth.Citation59 Whereas alkaline stress is caused by the Na2CO3 and NaHCO3 in the CO32- hydrolysis, on the basis of saline stress, causes an increase in soil pH, which severely interferes with cellular pH stability, showed damage to cell membrane structure, reduced root vigor. Besides, it also severely inhibited plant chlorophyll synthesis, and leads to a decrease in photosynthetic efficiency, which in turn affects the production of photosynthetic active products.Citation5 Currently, most of the research on saline and alkaline stress in plants focuses on plant growth inhibition, while the enhancement of plant resistance has not been sufficiently studied. Therefore, enhancing plant tolerance to saline and alkaline stress is essential to promote their normal growth and development.
The application of exogenous substances is a simple and effective method to increase the resistance of plants to abiotic stress, which plays an important role in regulating plant growth and tolerance to abiotic stress.Citation6 Melatonin (MT) is a naturally occurring small-molecule indoleamine hormone that can act as an exogenous substance to regulate plant growth and development, such as regulating photosynthesis, delaying leaves senescence, and promoting root development and so on. In animals, melatonin acts as a facilitator of dormancy, primarily by regulating circadian rhythms.Citation7 In plants, it also participates in responding to plant stress responses.Citation8 Plants can absorb external MT through their leaves and roots to enhance their tolerance to stress.Citation9 Research has shown that exogenous MT can increase the content of photosynthetic pigments in blueberries (Vaccinium spp.), improve the efficiency of PSII electron transfer, enhance photosynthesis, and thus enhance plant tolerance to saline and alkaline stress.Citation10 It can also increase the content of IAA, BR, and GA in the roots system of Gossypium spp, promote root growth, and alleviate the inhibitory effect of saline and alkaline stress on cotton root growth.Citation11 In addition, exogenous melatonin treatment inhibits chlorophyll degradation in rice leaves by upregulating chlorophyll synthesis-related genes, thereby delaying leaves senescence.Citation12 In summary, MT plays an important role in regulating plant resistance to adversity stress. However, there is a lack of clarity regarding the role of melatonin in regulating tolerance to saline and alkaline stress in table grapes.
Grapes (Vitis vinifera L.) are one of the most important economic crops that suffer from environmental limitations such as drought and saline and alkaline stress,Citation13 and also one of the world’s top five fruit trees, has the characteristics of high yield and strong adaptability, and also has an important economic value due to its rich nutrients and excellent quality loved by the majority of consumers.Citation14 In China, most of table grapes varieties are planted in arid and semi-arid regions such as Shanxi, Ningxia, Xinjiang and Gansu, which are prone to soil salinization due to low rainfall and high evaporation, thereby seriously restricts the development of Chinese grapes industry.Citation15 A large amount of salts can accumulate in saline and alkaline soils, affecting the growth of the grape roots system and thus causing yield loss and quality reduction.Citation16 Based on this, we need to take effective measures to mitigate the effects of on the growth of grapevine seedlings when facing adversities such as saline and alkaline stress, in order to enhance the resilience of grape seedlings. In recent years, in viticulture, domestic and foreign grape growers have mostly alleviated the inhibition caused by saline and alkaline stress on grape growth by selecting saline-tolerant rootstock varieties,Citation17 reducing evaporation to prolong irrigation,Citation18 and applying growth regulating substances.Citation19 However, the use of exogenous substances to improve the saline and alkaline tolerance of grape seedlings is less studied, especially the physiological mechanism of MT regulating in the growth of grape seedlings under saline and alkaline stress has rarely been reported. In this study, we investigated the response of seedlings of the table grape variety ‘Cuibao Seedless’ to exogenous MT under saline and alkaline stress by evaluating the growth of grape seedlings and physiological and biochemical indexes and other indexes, aiming to reveal the regulatory mechanism of exogenous MT on grape seedlings under saline and alkaline stress, and to provide technical methods and theoretical support for saline and alkaline land viticulture.
2. Materials and methods
2.1. Test material and locations
The experiment was conducted at the Horticultural Station of Shanxi Agricultural University, Taigu District, Jinzhong City, Shanxi Province (37°23′N 112°32′E, 830 m above sea level), which has an average annual temperature of 10°C, and maintains an annual rainfall of 400 mm-600 mm, with distinct seasons throughout the year. Follow-up index measurements were carried out in the Laboratory of Fruit Tree Germplasm Creation and Utilization, College of Horticulture, Shanxi Agricultural University.
In this experiment, ‘Cuibao seedless’, an annual branch with good growth potential, free of pests and diseases, and with full branch buds, was selected as the test material for cuttings, which belongs to the Eurasian species, whose parents are ‘Centennial seedless’ × ‘Guibao’.
2.2. Experimental design
‘Cuibao seedless’ branches were collected by winter pruning in November 2022, stored in sand, taken out in April 2023, the deflated buds were cut off, the branches were cut into segments containing two to three full buds, dipped into roots with rooting powder, and cuttings were taken in nutrient pots (10 cm × 10 cm) and then placed in plastic greenhouses for daily watering and management. The cuttings were then placed in a nutrient bowl (10 cm × 10 cm) and watered daily in a plastic greenhouse. When the grape seedlings grew to 4–5 leaves, they were transplanted and planted in 26 cm × 21 cm nutrient pots for potting experiments, and when the seedlings grew to about 10 leaves, the grape seedlings with basically the same growth were selected for the experiments. According to the NaCl:NaHCO3 = 1:1 concentration ratio of saline solution, grape seedlings were subjected to a mixture of 100 mmol/L saline and alkaline stress, and exogenously sprayed with different concentrations of melatonin (0, 50, 100, 150, 200), a total of five treatments were set up: M0: saline and alkaline + distilled water; M50: saline and alkaline +50 μmol/L MT; M100: saline and alkaline +100 μmol/L MT; M100: saline and alkaline +100 μmol/L MT; M150: saline and alkaline +150 μmol/L MT; M200: saline and alkaline +200 μmol/L MT, and 20 pots were set for each treatment, with a total of 100 pots. Melatonin was applied by foliar spraying, whichever was not dripping from the leaf tips, one day before the stress, and treated every 6 d. Dynamic sampling was used for the determination of physiological indexes.
2.3. Measurement indicators and methods
2.3.1. Measurement of plasma membrane permeability and reactive oxygen species content
Malondialdehyde content was determined by the thiobarbituric acid method,Citation20 in which grape leaves and roots were weighed and ground to homogenate in 50 mmol/L PBS (pH = 7.8), extracted with 0.5% thiobarbituric acid in boiling water for 10 min, cooled and centrifuged to determine the volume of supernatant liquid, which was colourimetrically measured at 450 nm, 532 nm and 600 nm.
Superoxide anion (O2−) was determined by the method of Yao et al.,Citation21 and grape leaves and roots were weighed and ground in 50 mmol/L PBS (pH = 7.8), centrifuged at 5000 r/min for 10 min, and the supernatant was added with PBS and 1 mmol/L hydroxylamine hydrochloride, and then mixed well in a water bath at 25°C for 20 min. After that, 17 mmol/L p-aminobenzenesulfonic acid solution and 7 mmo/L α-naphthylamine solution were added, mixed well, and then the color was measured at 530 nm after 30 min in a water bath at 30°C.
Hydrogen peroxide content was measured using Nanjing Jianjian Bioengineering Research Kit.
2.3.2. Determination of root vigour in grapevine seedlings
Root viability was determined using the triphenyltetrazolium chloride methodCitation22 by weighing the root system of grape seedlings and adding 1% TTC solution and 0.1 mol/L PBS (pH = 7.0), holding at 37°C for 1 h and then adding sulfuric acid to terminate the reaction. Root samples were taken, ground and fixed with ethyl acetate and measured at 485 nm.
2.3.3. Measurement of osmoregulatory substances
Proline content was determined by acid ninhydrin method,Citation23 grinding in 3% sulphosalicylic acid to homogenate, centrifugation at 10,000 r/min for 10 min, the supernatant was taken and added with acid ninhydrin and glacial acetic acid and mixed thoroughly, then the reaction mixture was placed in boiling water for 1 h. After terminating the reaction on ice, toluene was added and mixed thoroughly, and then colourimetrically at 520 nm.
Soluble protein content was determined by the G-250 methodCitation24 by adding a small amount of distilled water and grinding to homogenate, centrifuging at 5000 r/min for 10 min, taking the supernatant and adding the G-250 reagent to mix it thoroughly, and then leaving it to stand at room temperature for 2 min before colourimetry was carried out at 595 nm.
The soluble sugar content was determined by the anthrone-sulfuric acid methodCitation25 by grinding in a small amount of distilled water until homogenized, boiling water bath for 30 min, and filtering after cooling. The reaction system consisted of 0.5 mL of extract, 1.5 mL of distilled water, 0.5 mL of anthrone-ethyl acetate reagent and 5 mL of concentrated sulfuric acid. The reaction system was shaken well and then boiled in a boiling water bath for 1 min, and then cooled down to room temperature after removing and then colorimetrically measured at 630 nm.
2.3.4. Determination of chlorophyll content
Chlorophyll content was determined with reference to the method of Han et al.,Citation26 using anhydrous ethanol extraction in the dark until the leaves turned white, and then colourimetrically at 470 nm, 645 nm, and 669 nm, and the chlorophyll a (Chl a), chlorophyll b (Chl b), carotenoids (Car), and total chlorophyll (Chl a+b) contents were calculated according to the formulae, respectively.
2.3.5. Determination of antioxidant enzyme activity
Plant tissues were homogenized in 5 ml of 0.05 mol/L phosphate buffer (pH = 7.0), followed by centrifugation at 10,000 r/min for 15 min at 4°C. Supernatants were used for the determination of enzyme activities. Three biological replicates were included for all enzymes.
Superoxide dismutase was determined by the nitrogen blue tetrazolium method,Citation27 and the reaction system consisted of 1.5 mL PBS (0.05 mol/L, pH = 7.0), 0.3 mL methionine solution (130 mmol/L), 0.3 mL nitrotetrazolium blue chloride solution (750 μmol/L), 0.3 mL riboflavin solution (20 μmol/L), 0.3 mL EDTA-Na solution (100 μmol/L), 0.5 mL distilled water and 0.1 mL enzyme extract, the control tube was used without enzyme solution, and the reaction tube was placed under fluorescent light for 15 min, followed by colourimetry at 560 nm.
Peroxidase was determined by guaiacol method,Citation28 the reaction system consisted of 2.9 ml of PBS (0.05 mol/L, pH = 7.0), 1 ml of H2O2 (2%), 1 ml of guaiacol (0.05 mmol/L), and 0.1 ml of the enzyme extract, which was colourimetric at 470 nm.
2.3.6. Determination of non-enzymatic antioxidants
The ascorbic acid content was determined by the 2,6-dichlorophenol indophenol method.Citation29 The content of ascorbic acid was ground in a small amount of oxalic acid solution (20 g/L) until homogenized and fixed, and then filtered after 10 min of standing, and then the filtrate was put into a triangular flask and titrated with a calibrated 2,6-dichlorophenol indophenol solution until it reached the red color and did not fade within 15 s.Citation29
Glutathione content was determined by using a kit from Beijing Solarbio Technology Co.
2.4. Statistical analysis
Microsoft Excel 2016 was used for data collation and SPSS 20 was used for data processing and Pearson’s analysis of the data, Duncan’s multiple comparison Method was used to determine significant differences (p <0 .05) and GraphPad Prism 8, Adobe Photoshop 2020 were used for plotting.
3. Results
3.1. Effect of exogenous melatonin on the phenotype of grapevine seedlings under saline and alkaline stress
As can be seen from , the grape seedlings of M0 (CK) treat began to slow down their growth when subjected to saline and alkaline stress. With the continuation of saline and alkaline stress, the edges of the leaves began to yellow and wither. The application of exogenous melatonin can effectively alleviate the growth inhibition caused by saline and alkaline stress on grape seedlings. At 6 d of saline and alkaline stress, the treatment of leaves began to turn pale green. At 12 d of stress, the leaf margins of M50 start to yellow, yellowing was aggravated in M100 treatment, shrinking began to appear in the old leaves of M150, M200 treatment. After 18 d of stress, the yellow spots of M100 treatment began to turn brown, and the wilting and crumpling of M200 treatment leaves were obvious. In conclusion, melatonin was effective in alleviating the inhibition of seedling growth caused by saline and alkaline stress, but the physiological damage caused by saline and alkaline stress could not be reversed.
Figure 1. Effects of different melatonin concentrations on grape seedling morphology under saline and alkaline stress. M0, control; M50, saline and alkaline stress +50 μmol/L MT; M100, saline and alkaline stress +100 μmol/L MT; M150, saline and alkaline stress +150 μmol/L MT; M200, saline and alkaline stress +200 μmol/L MT. 6 d,12 d, and 18 d indicates processing time. The same as below. The scale in the diagram is 20 cm.

3.2. Effect of exogenous melatonin on the biomass of grape seedlings under saline and alkaline stress
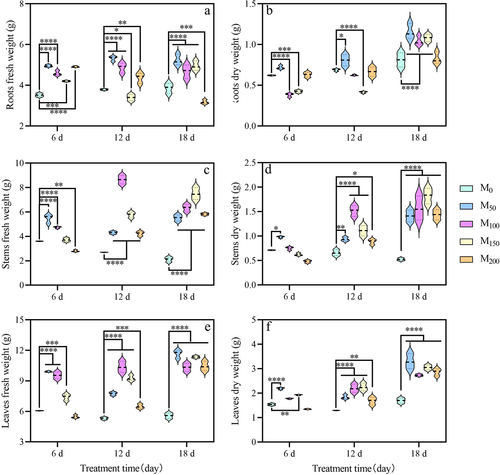
As can be seen from , under saline and alkaline stress, the biomass of roots, stems and leaves of grape seedlings was inhibited. Compared with the saline and alkaline treatment, the biomass of all grape seedlings under MT treatment showed a tendency to increase and then decrease. After 6 d of stress, the greatest increase in dry and fresh weights of roots, stems and leaves was observed under MT50 treatment. However, MT200 treatment showed a significant decrease in stems and leaves compared with CK. After 12 d of stress, the fresh weight of the root of grape seedlings was significantly accumulated in the M50 and M100 treatment. The dry weight followed the same trend. In the stems and leaves, the MT treatment significantly increased its dry and fresh weight. After 18 d of stress, the fresh weight and dry weight of root was decreased in the M200 treatment, and aboveground fresh weight increased significantly in the other treatment. The dry weight and fresh weight of stems and leaves significantly accumulated under melatonin treatment. In conclusion, the MT50 treatment was more effective in the first and middle stages of saline and alkaline stress. However, under continuous stress, MT150 was more effective.
Figure 2. Effects of different concentrations of melatonin on grape seedling biomass under saline and alkaline stress. (a) Roots fresh weight. (b) Roots dry weight. (c) Stems fresh weight. (d) Stems dry weight. (e) Leaves fresh weight. (f) Leaves dry weight. Values represent mean ± standard deviation (n = 3). The graph shows data as mean + standard error of three replicates, significant differences compared with M0 were detected using two-way ANOVA. * denotes significant difference at 0.05 level, **denotes significant difference at 0.01 level, ***denotes significant difference at 0.001 level, ****denotes significant difference at 0.0001 level and no significant difference among the rest of the treatments.
3.3. Effect of exogenous melatonin on photosynthetic pigments of grape seedlings under saline and alkaline stress
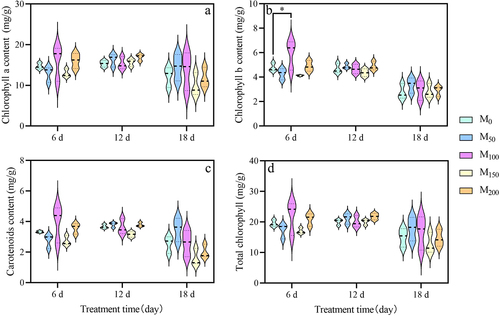
As can be seen in , the chlorophyll content of grape seedlings showed an overall trend of increasing and then decreasing with increasing treatment time. After 6 d of treatment, the M100 treatment showed a significant increase in chlorophyll b compared with the control (). After 12 d of treatment, the total chlorophyll content of M100 treatment was lower than that of the control (). After 18 d of treatment, photosynthetic pigment content was higher in M50 and M100 treatments than in the control, and decreased in M150 and M200 treatments compared with the control. In conclusion, the application of exogenous melatonin can effectively increase the chlorophyll content of grape seedling leaves, however, its effect may have a dose effect.
Figure 3. Effect of melatonin on photosynthetic pigments in grape leaves under saline and alkaline stress. (a) Chlorophyll a. (b) Chlorophyll b. (c) Carotenoids. (d) Total chlorophyll. Values represent mean ± standard deviation (n = 3). Significant differences compared with M0 were detected using two-way ANOVA. * denotes significant difference at 0.05 level, and no significant difference among the rest of the treatments.
3.4. Effect of exogenous melatonin on root vigor of grape seedlings under saline and alkaline stress
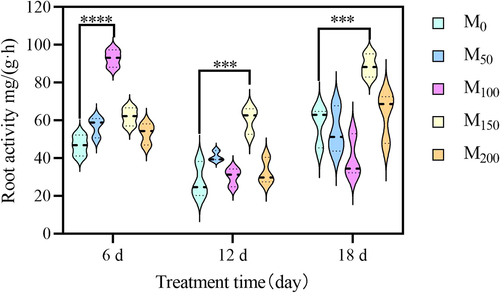
As can be seen from , with the continuous increase of the stress time, the root vigor of grape seedlings showed a trend of decreasing and then increasing. After 6 d of saline and alkaline stress, compared with M0, the root vigor of grape seedlings increased significantly by 98.6% in M100 treatment. At 12 d of stress, compared with M0, the root vigor of M150 treatment significantly increased 1.2 fold. At 18 d of stress, root vigor was significantly increased by 53.9% in M150 treatment compared with M0. At the initial stage of stress, the concentration of 100 μmol/L MT was effective. However, at the later stage of sustained stress, 150 μmol/L MT had a better mitigation effect.
Figure 4. Effect of melatonin on root activity of grape seedlings under saline and alkaline stress. Values represent mean ± standard deviation (n = 3). Significant differences compared with M0 were detected using two-way ANOVA. ***denotes significant difference at 0.001 level, ****denotes significant difference at 0.0001 level and no significant difference among the rest of the treatments.
3.5. Effect of exogenous melatonin on reactive oxygen species and plasma membrane permeability in grape seedlings under saline and alkaline stress
As shown in , after spraying exogenous melatonin, the MDA content of grape seedlings under saline and alkaline stress decreased continuously in the leaves. In the roots, it was risen and then decreased. After 6 d of stress, compared with the M0 (CK) treatment, the MDA content of grape leaves in M50, M100, M150 and M200 treatments decreased by 34.21%, 35.71%, 41.64% and 32.90%. After 12 d of stress, compared with M0 (CK) treatment, the MDA content of leaves was significantly decreased by 25.68% and 33.32%in M150 and M200 treatments. In the root, the changes in MDA content did not form a significant difference. After 18 d of stress, compared with M0 (CK) treatment, the MDA content of grape leaves was reduced by 32.15% in M100 treatment compared with the M0 treatment. In the root, the MDA content of the M100 treatments was reduced by 24.59%. In conclusion, the MDA content was significantly higher after saline and alkaline treatment, indicating water loss and osmotic imbalance in grape seedlings under saline and alkaline conditions.
Figure 5. Effect of exogenous melatonin on MDA and reactive oxygen species in grape seedlings under saline and alkaline stress. (a) Leaves MDA content. (b) root MDA content. (c) Leaves H2O2 content. (d) Root H2O2 content. (e) Leaves O2−content. (f) Root O2− content. Values represent mean ± standard deviation (n = 3). Significant differences compared with M0 were detected using two-way ANOVA. * denotes significant difference at 0.05 level, **denotes significant difference at 0.01 level, *** denotes significant difference at 0.001 level, ****denotes significant difference at 0.0001 level and no significant difference among the rest of the treatments.
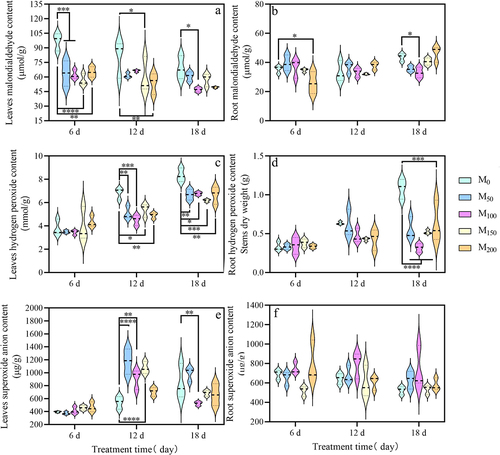
As shown in , the H2O2 content in the leaves and roots of grape seedlings showed an increasing trend with the continuous increase of saline and alkaline stress time. After 6 d of stress, compared with the M0 (CK), the H2O2 content in leaves and roots did not show significant difference. After 12 d of stress, compared with M0 (CK), the H2O2 content of leaves in the M50, M100, M150, and M200 was decreased 25.30%, 33.56%, 20.82%, and 28.02%. After 18 d of stress, the H2O2 content of grape leaves and roots were reduced. Compared with the M0 (CK) treatment, the H2O2 content of leaves in the M50, M100, M150, and M200 were reduced by 19.86%, 18.75%, 25.93%, and 20.06%. And the H2O2 content of the roots was reduced by 48.57%, 70.51%, and 51.85% 41.61%.
As shown in , the O2− content of grape seedlings under saline and alkaline stress increased continuously with the prolongation of treatment time, while the roots were decreased continuously. After 6 d of stress, compared with the M0 (CK) treatment, the O2− content of leaves and roots did not show a significant increase or decrease. After 12 d of stress, compared with the CK, the O2− content of leaves increased significantly by 1.17 times, 68.55%, 97.82% and 30.96%. After 18 d of stress, the O2− content of leaves were significantly reduced by 37.75% in the M100 treatment.
3.6. Effect of exogenous melatonin on the antioxidant system of grape seedlings under saline and alkaline stress
As shown in , the SOD activity of leaves and roots of grape seedlings under saline and alkaline stress showed a tendency to first decrease and then increase with treatment time. After 6 d of stress, compared with M0 treatment, the SOD activity in leaves of M150 treatment was increased by 7.03%. In the root, compared with CK, the SOD activity of all treatments increased by 21.55%, 16.54%, 10.89%, and 8.62%. After 12 d of stress, in leaves, compared with control, the SOD activity was increased significantly by 29.50% in M50 treatment. In roots, the SOD activity increased by 9.63% in M200 treatment. Meanwhile, there were decreased by 4.82%, 4.82% and 8.05% in other treatments. After 18 d of stress, the SOD activity increased in all melatonin treatments. In the roots, the SOD activity of M150 treatment was decreased by 1.12% compared with the control.
Figure 6. Effect of exogenous melatonin on antioxidant enzymes in grape seedlings under saline and alkaline stress. (a) Leaves superoxide dismutase activity. (b) Leaves peroxidase activity. (c) Roots superoxide dismutase activity. (d) Roots peroxidase activity. Values represent mean ± standard deviation (n = 3). Significant differences compared with M0 were detected using two-way ANOVA. * denotes significant difference at 0.05 level, **** denotes significant difference at 0.0001 level and no significant difference among the rest of the treatments.
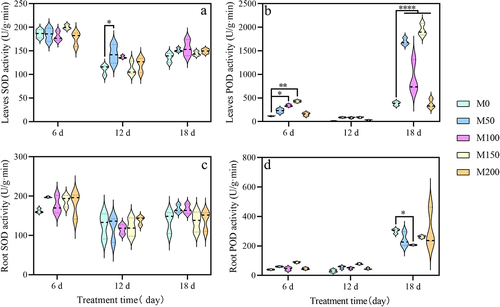
As shown in , the POD activity of leaves and roots of grape seedlings under saline and alkaline stress showed a trend of first decrease and then rapid increase with treatment time. After 6 d of stress, compared with M0, the POD activity of leaves significantly increased by 1.93 times, 2.71 times in M100, M150 treatment. In the roots, its POD activity enhanced 50.42%, 1.22 times in M50, M150 treatment. After 12 d of stress, compared with the control, all melatonin treatments in grape seedling leaves and roots were not to form significant differences. After 18 d of stress, compared with the control, the POD activity of leaves under M50, M100, and M150 treatments were increased significantly by 3.44, 1.44, and 4.08 times. In the roots, the MT100 treatment was significantly reduced compared to the control.
3.7. Effect of exogenous melatonin on osmoregulatory substances in grape seedlings under saline and alkaline stress
As shown in , under saline and alkaline stress, the soluble sugar of grape seedling leaves decreased firstly and then increased with the treatment time. While in the roots, the trend was firstly increasing and then decreasing. After 6 d of stress, compared with the control, the leaves soluble sugar content of each treatment was significantly reduced by 38.46%, 45.79%, 54.33%, and 21.41%. In the roots, the soluble sugar content of M150 and M200 treatments was significantly increased by 17.18% and 66.24%. And the M50 and M100 treatments were significantly reduced by 10.80%, 13.34%. After 12 d of stress, in the leaves, compared with the control, the soluble sugar content was increased significantly by 43.68% in M200 treatment. And it was decreased significantly by 37.38%, 31.69%, and 22.19% in M50, M100, and M150 treatments. In the roots, compared with the control, the soluble sugar content was increased significantly by 3.21% and 6.42% in M50 and M200 treatments. While it was significantly decreased by 21.10% and 1.91% in M100 and M150 treatments. After 18 d of stress, the soluble sugar content of leaves of each treatment was significantly reduced by 14.68%, 23.74%, 38.63%, and 34.31% compared with the control. In the roots, the soluble sugar content of M50 and M200 treatments was significantly increased by 49.81% and 2.40%. While the soluble sugar content was significantly reduced by 51.90% and 19.63% in M100 and M150 treatments.
Figure 7. Effect of exogenous melatonin on osmoregulatory substances in grape seedlings under saline stress. (a) Leaves soluble sugar content. (b) Root soluble sugar content. (c) Leaves soluble protein content. (d) Root soluble protein content. (e) Leaves proline content. (f) Root proline content. Values represent mean ± standard deviation (n = 3).
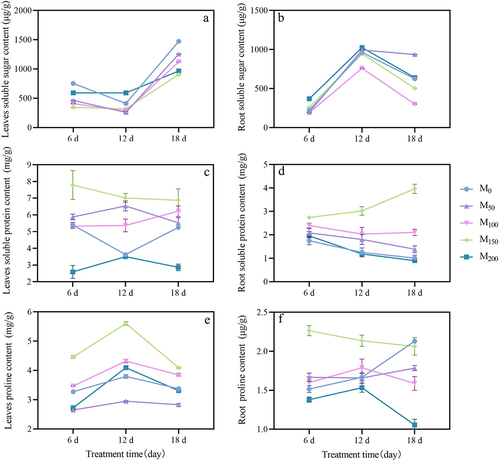
As shown in , the soluble protein content in the root of grape seedlings under saline and alkaline stress showed a trend of decreasing and then increasing with the treatment time, and it was showed fluctuating changes in the leaves. After 6 d of stress, compared with the M0, the leaves soluble protein increased significantly by 44.16% in the M150 treatment. In the root, the soluble protein content of the M50, M100, and M150 treatments increased significantly by 18.98%, 36.99%, and 56.64%. After 12 d of stress, compared with the control, the leaves soluble protein content increased by 79.85%, 47.82%, and 92.98% significantly in the M50, M100, and M150 treatments. In the root, compared with the M0 treatment, except for the M200 treatment, the other treatments were increased significantly by 43.97%, 62.48%, and 1.41 times. After 18 d of stress, compared with the control, the soluble protein content of leaves and roots in M100 and M150 treatments increased significantly by 18.51%, 30.49%, 1.08 times, and 2.92 times, respectively.
As shown in , the proline content of grape seedlings under saline and alkaline stress showed a trend of increasing and then decreasing with treatment time, while the roots did not change synchronously. After 6 d of stress, compared with the control, the proline content of leaves of M100 and M150 treatments were increased significantly by 6.00% and 36.37%, while M50 and M200 treatments decreased slightly. In the roots, it was increased significantly by 9.96% and 49.40% in M50 and M150 treatments. After 12 d of stress, the proline content of M150 treatment was significantly increased by 47.73% and 28.11% compared with the control in both leaves and roots. After 18 d of stress, compared with the control, the proline content of M100 and M150 treatments were significantly increased by 14.01% and 21.16%, while the M50 and M200 treatments were significantly decreased by 16.20% and 2.04%. In the roots, the proline content of all treatments was decreased.
3.8. Effect of exogenous melatonin on GSH and AsA in grape seedlings under saline and alkaline stress
As shown in , under saline and alkaline stress, the AsA content of leaves under low melatonin treatment showed a trend of increasing and then decreasing, while other treatments showed a trend of decreasing and then increasing. In the roots, its content showed a trend of increasing and then decreasing. After 6 d of stress, compared with the control, the AsA content of leaves under M50 and M200 treatments increased significantly by 2.57 and 6.10 times. In the roots, the content was increased significantly under M100 treatment by 1.62 times, while the rest of the treatments were lower than the control, but no significant difference was formed. After 12 d of stress, the AsA content of leaves under all treatments was significantly reduced compared with the control, with a decrease of 29.96%, 86.31%, 87.73%, and 93.68%. In the roots, compared with the control, the content of AsA was increased in M150 and M200 treatments. After 18 d of stress, compared with the control, the AsA content in leaves was significantly reduced by 40.75% and 73.12% in M50 and M150 treatments. In the roots, it was significantly decreased by 63.01% in M150 treatment.
Figure 8. Effect of exogenous melatonin on ascorbic acid and glutathione in grape seedlings under saline and alkaline stress. (a) Leaves ascorbic acid content. (b) Roots ascorbic acid content. (c) Leaves glutathione content. (d) Roots glutathione content. Values represent mean ± standard deviation (n = 3).
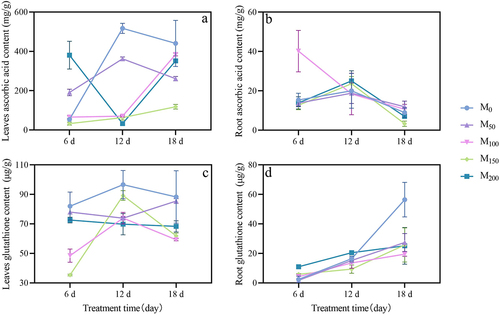
As shown in , under saline and alkaline stress, the GSH content of grape seedling leaves showed a general trend of increasing and then decreasing with treatment time, while the roots showed an increasing trend. After 6 d of stress, the GSH content of all treatments had different degrees of decline in the leaves. In the roots, compared with the control, its content was increased significantly under M150 and M200 treatments by 1.42 times and 3.50 times. After 12 d of stress, compared with the control, in the leaves, the GSH content were decreased in all treatments. In the roots, the GSH content was increased by 24.69% in the M200 treatment, and decreased in the rest of the treatments. After 18 d of stress, the GSH contents in leaves of M100, M150, and M200 treatments were significantly reduced compared with the control. While in roots its was decreased.
4. Discussion
Melatonin can act as a signaling molecule to promote resistant plant growth as one of the phytohormones with the ability to regulate plant response to adversity stress.Citation30 Saline and alkaline stress is one of the most important abiotic stresses limiting plant growth and constraining agricultural production worldwide.Citation31 As a major environmental factor causing plant yield reduction, saline and alkaline stress significantly inhibits the growth and physiological homeostasis of grape seedlings.Citation32 In the present study, grape seedlings under saline and alkaline stress morphologically showed yellowing and wilting and growth retardation (). The application of exogenous melatonin effectively alleviated these conditions. However, the grape seedlings under MT200 treatment showed serious yellowing and even caused old leaves to fall off. Hence, we speculated that there might be a similar effect to that of growth hormone, which promotes growth at low concentrations and inhibits plant growth at high concentrations, a conclusion corroborated by the study of Ren et al.Citation33 Studies on tea tree showed that leaves damage under salt stress was mainly at the leaf margins, whereas alkaline stress was at the veins,Citation34 which is similar to our findings. This suggests that saline and alkaline stress cause more damage to plants than a single stress.
In addition, we found that exogenous melatonin application effectively promoted the increase of grape seedling biomass (). Plant biomass, as one of the important indicators of response to adversity stress, can visually reflect plant growth.Citation35 Studies have shown that different melatonin concentrations have different effects on tomato biomass under stress conditions.Citation36 Our study on grapes was similar to its results in that in the pre-stress period, the dry fresh weight of grape roots and leaves was significantly increased under M50, M100, and M150 treatments. And all of them reached the maximum accumulation in the M50 treatment. While under M200 treatment, this indexes were all significantly reduced. In the middle of the stress period, the dry fresh weight of grape seedlings was significantly accumulated in the M150 treatment. In the late stage of stress, it was M150 treatment. This suggests that 200 μM MT concentration may have a synergistic effect with saline and alkaline stress, then resulting in a decrease in grape seedling biomass.
Photosynthesis is one of the most important processes for green plants to obtain energy, however, adversity stress greatly affects the structure of chloroplasts and thus photosynthesis.Citation37 As chloroplasts are important carriers for green plants to absorb light energy and convert it into energy for themselves, the content of photosynthetic pigments inside them is essential for the normal functioning of photosynthesis. In this study, appropriate melatonin concentration could effectively reduce the chlorophyll content caused by saline and alkaline stress. In comparison, 50 μmol/L MT was particularly effective in the middle and late stages of the stress. This suggests that high melatonin concentrations may exacerbate the loss of photosynthetic pigments in leaves of grapevine seedlings subjected to saline and alkaline stress. We speculate that saline and alkaline stress may block photosynthetic pigment synthesis, which may be alleviated by low melatonin concentrations,Citation38 while high melatonin concentrations exacerbate chlorophyll breakdown.Citation39
The normal growth and development of the root system is crucial for the plant, and it is the main source of nutrient absorption. Therefore, root vigor can be used as a major indicator to evaluate the physiological metabolism of the root system.Citation40 In order to maintain the energy and materials required for growth and development of grape seedlings under saline and alkaline stress, the roots needs to absorb water and nutrients. However, the application of exogenous substances can effectively enhance root vigor to ensure the normal metabolism of the plant.Citation41,Citation42 The results of this study showed that the root vigor of all treatments increased compared with the control after spraying different concentrations of exogenous melatonin solution, but the root vigor of grapevine seedlings in 200 μM MT solution was decreased, which may be attributed to the inhibition of grape root growth due to the high concentration of melatonin solution, which was similar to the results of Duan et al.Citation11
Under normal conditions, biological membranes are selectively permeable to maintain a stable intracellular environment.Citation43 When plants are subjected to adversity stress, the plant body’s own antioxidant mechanism is disrupted, resulting in the accumulation of large amounts of reactive oxygen species (ROS), which leads to changes in the permeability of the cell membrane.Citation44 After the cell membrane is disrupted, excessive accumulation of ROS leads to an increase in MDA content. We found that: the MDA content of leaves were decreased continuously under saline and alkaline stress, while in roots were not synchronized with leaves changes. Moreover, the M200 treatment was higher than the control in the late stage of stress, and it was hypothesized that this concentration might be responsible for the inhibition of grape growth, which is similar to the study of Song et al.Citation45
Saline and alkaline stress increases reactive oxygen species (ROS) content and causes sustained cellular damage and even cellular death.Citation46 Hydrogen peroxide (H2O2) is an important signaling molecule in response to plant stress response.Citation47 The results of this study showed that saline and alkaline stress caused a continuous increase in H2O2 and O2− content in grape leaves and roots, and this content decreased after MT treatment (). This is consistent with the results of Dong et al.Citation48 It has been shown in several studies that SOD and POD in plants act synergistically to reduce the large amount of reactive oxygen species accumulated by adversity stress, thus alleviating the harm caused by oxidative damage.Citation49 Interestingly, SOD catalyses the decomposition of reactive oxygen species into hydrogen peroxide, which can be decomposed into water and oxygen by POD.Citation50 We found that SOD activity was high in the early stage of stress, and its tendency to decrease and then increase with the continuation of stress time was consistent with the results of Ahmad et al.,Citation51 which confirmed the theory that SOD can catalyze the decomposition of ROS. In the late stage of stress, POD acted to convert the hydrogen peroxide after ROS decomposition in the early stage into water and oxygen to be excreted from the cells, thus reducing the large amount of ROS produced by saline and alkaline stress.
ROS scavenging pathways in plants include water-water, ascorbic acid-glutathione (AsA-GSH), etc.Citation34 AsA and GSH are two important antioxidants in plants, which have important roles in ROS scavenging and maintenance of redox balance in plant cells.Citation52 Studies have shown that the application of exogenous substances significantly increases GSH and AsA content and can scavenge excess reactive oxygen species to maintain cellular integrity.Citation53,Citation54 After application of exogenous melatonin, the AsA-GSH content showed fluctuating changes. Lu et al. also concluded that cantaloupe promoted an increase in AsA content after ozone treatment.Citation55 It was hypothesized that there might be a correlation between AsA and GSH, which act synergistically to scavenge the large amount of reactive oxygen species (ROS) accumulated by plants during adversity stress, in order to maintain the integrity of the cell membrane structure.Citation56 However, the scavenging of ROS by AsA and GSH was inhibited by high concentrations of melatonin, suggesting that appropriate melatonin can promote the increase of AsA and GSH contents under adversity stress, while too high a concentration would be counterproductive.
When plants are subjected to adversity stress, osmotic stress is induced, which subsequently leads to ion toxicity and oxidative stress.Citation31 In contrast, exogenous melatonin promotes the accumulation of osmoregulatory substances and maintains cellular osmotic balance.Citation57 Proline, as an osmoregulator, exists in the free state in plants with high hydration capacity and helps to maintain cellular stability.Citation58,Citation59 After exogenous application of melatonin, both lower concentrations of melatonin significantly increased the concentration of soluble protein (SP) in grape leaves and roots, while higher concentrations (200 μM) decreased the SP concentration. The M100 and M150 treatments were significantly increased the concentration of proline (Pro) in grape leaves, and it was lower than the control in the roots. The above results suggest that exogenous melatonin acts as a signaling molecule and may influence the synthesis of soluble sugars.Citation39
In summary, the physiological mechanism of exogenous melatonin involved in regulating the growth of grape seedlings to saline and alkaline stress is relatively complex. We produced a simple mechanism diagram by analysing the morphological, physiological and biochemical indexes of grape seedlings, with a view to elucidating the promotion of exogenous melatonin on the growth of grape seedlings under saline stress. As shown in , the application of exogenous melatonin promoted the increase of antioxidant enzymes (SOD, POD) activity, the increase of AsA and GSH content, and the accumulation of osmoregulatory substances, which led to the decrease of MDA and ROS content in order to alleviate the saline stress suffered by grape seedlings.
5. Conclusion
In this study, the effect of exogenous melatonin on the growth and development of grape seedlings under saline and alkaline stress was investigated by foliar spraying. The results showed that exogenous melatonin was alleviated the inhibitory effect of saline and alkaline stress on grape growth. After exogenous application of melatonin, grapevine seedling biomass were accumulated significantly and promoted the growth of new grape leaves. At the same time, it mitigated the damage caused by saline stress on grape growth by increasing the concentration of osmoregulatory substances and the activity of antioxidant enzymes. However, we found that exogenous melatonin has a dose effect in alleviating the stress response to saline and alkaline stress, and too high a concentration will inhibit the growth of grapes. Therefore, in production, we need to further explore the appropriate concentration for different varieties.
Disclosure statement
No potential conflict of interest was reported by the author(s).
Correction Statement
This article has been corrected with minor changes. These changes do not impact the academic content of the article.
Additional information
Funding
References
- Jin X, Zhao K, Hu J, Gailing O, Zhou LD, Du SH, Han YZ, Wang SJ. PagMYB73A enhances poplar salt tolerance by facilitating adventitious roots elongation and stomata density. For Res. 2024;4:e003. doi:10.48130/forres-0023-0032.
- Yang YR, Cao YP, Li ZX, Zhukova A, Yang ST, Wang JL, Tang ZH, Cao YH, Zhang YF, Wang DL. Interactive effects of exogenous melatonin and rhizophagus intraradices on saline-alkaline stress tolerance in Leymus chinensis. Mycorrhiza. 2020;30(2–3):357–14. doi:10.1007/s00572-020-00942-2.
- Briones-May Y, Quijano-Medina T, Pérez-Niño B, Benrey B, Turlings TCJ, Bustos-Seguta C, Abdala-Roberts L. Soil salinization disrupts plant–plant signaling effects on extra-floral nectar induction in wild cotton. Oecologia. 2023;202(2):313–323. doi:10.1007/s00442-023-05395-w.
- Ma CQ, Bian CJ, Liu WJ, Sun ZJ, Xi XL, Guo DM, Liu XL, Tian YK, Wang CH, Zheng XD. Strigolactone alleviates the salinity-alkalinity stress of Malus hupehensis seedlings. Front Plant Sci. 2022;13:901782–901797. doi:10.3389/fpls.2022.901782.
- Li YX, Zhang CL, Xu XY, Chen YQ, Xiang DJ, Liu P. Transcriptomics-assisted mining of salt-tolerant genes in ricinus communis. Zhiwu Yichuan Ziyuan Xuebao. 2023b;24:1778–1793.
- Wang Q, Xu WW, Ren CZ, Zhan C, Wang CL, Li JW, Ren QY, Liang XT, Wei LM, Xiang DB. et al. Physiological and biochemical mechanisms of exogenous melatonin regulation of saline-alkali tolerance in oats. Agronomy. 2023;13(5):1327–1346. doi:10.3390/agronomy13051327.
- Beilby MJ, Turi CE, Baker TC, Tymm FJ, Murch SJ. Circadian changes in endogenous concentrations of indole-3-acetic acid, melatonin, serotonin, abscisic acid and jasmonic acid in characeae (Chara australis Brown). Plant Signal Behav. 2015;10(11):e1082697. doi:10.1080/15592324.2015.1082697.
- Nawaz MA, Huang Y, Bie ZL, Ahmed W, Reiter RJ, Niu ML, Hameed S. Corrigendum: melatonin: current status and future perspectives in plant science. Front Plant Sci. 2016;7:714–715. doi:10.3389/fpls.2016.00714.
- Silalert P, Pattanagul W. Foliar application of melatonin alleviates the effects of drought stress in rice (Oryza sativa L.) seedlings. Not Bot Horti Agrobot Cluj Napoca. 2021;49(3):12417–12429. doi:10.15835/nbha49312417.
- Jia WF, Wei XQ, Ma JH, Wang LX, Li LY, Li JY, Wang Y, Wu L. Exogenous application of melatonin improves the growth and physiological properties of blueberry seedlings under salt stress. Biotechnol & Biotechnol Equip. 2023;37(1):2202781–2202795. doi:10.1080/13102818.2023.2202781.
- Duan WJ, Lu B, Liu LT, Meng YJ, Ma XY, Li J, Zhang K, Sun HC, Zhang YJ, Dong HZ. et al. Effects of exogenous melatonin on root physiology, transcriptome and metabolome of cotton seedlings under salt stress. Int J Mol Sci. 2022;23(16):9456–9483. doi:10.3390/ijms23169456.
- Liang CZ, Zheng GY, Li WZ, Wang YQ, Hu B, Wang HR, Wu HK, Qian YW, Zhu XG, Tan DX. et al. Melatonin delays leaf senescence and enhances salt stress tolerance in rice. J Pineal Res. 2015;59(1):91–101. doi:10.1111/jpi.12243.
- Montanaro G, Briglia N, Petrozza A, Carlomagno A, Rustioni L, Cellini F, Nuzzo V. Image-based sensing of salt stress in grapevine. OENO One. 2024;58(1). doi: 10.20870/oeno-one.2024.58.1.7757.
- Jiao XB, Ji W. Progress in the mechanisms of cold tolerance in grape. Sino-Overseas Grapevine & Wine. 2019;(5):63–68.
- Wang J, Zhou GS. The climatic suitability and climatic impact factors affecting the wine grapes (Vitis vinifera L.) planting distribution in China. Sheng Tai Xue Bao. 2021;41(6):2418–2427. doi:10.5846/stxb201903180507.
- Lu X, Ma L, Zhang CC, Yan HK, Bao JY, Gong MS, Wang WH, Li S, Ma SY, Chen BH. Grapevine (Vitis vinifera) responses to salt stress and alkali stress: transcriptional and metabolic profiling. BMC Plant Biol. 2022;22(1):528–549. doi:10.1186/s12870-022-03907-z.
- Wang R, Shi XM, Zhang YX, Wu X, Wang N, Chen ZP, Wang ZP. Relationships between salt tolerance and ion absorption,photosynthetic characteristics of different grape rootstocks under NaCl stress. Agric Res in the Arid Areas. 2023;41:114–126.
- Zhou-Tsang A, Wu Y, Henderson SW, Walker AR, Borneman AR, Walker RR, Gilliham M. Grapevine salt tolerance. Aust J Grape Wine Res. 2021;27(2):149–168. doi:10.1111/ajgw.12487.
- Karimi R, Salimi F. Iron-chlorosis tolerance screening of 12 commercial grapevine (Vitis vinifera L.) cultivars based on phytochemical indices. Sci Hortic (Amsterdam). 2021;283:110111–110122. doi:10.1016/j.scienta.2021.110111.
- Duan BB, Li L, Chen GQ, Su-Zhou CX, Li YS, Merkeryan H, Liu W, Liu X. 1-aminocyclopropane-1-carboxylate deaminase-producing plant growth-promoting rhizobacteria improve drought stress tolerance in grapevine (Vitis vinifera L.). Front Plant Sci. 2021;12:706990–707005. doi:10.3389/fpls.2021.706990.
- Yao YC, Wei PP, Li M, Zhang KC, Fan GZ. Effect of hydrogen sulfide on different forms of calcium and superoxide anion production in phellinus baumii. Edible Fungi Of China. 2020;39:106–111+115.
- Huang HX, Cao Y, Xin KJ, Liang RH, Chen YT, Qi JJ. Dynamic responses of root vigor, lipid peroxidation and antioxidant enzymes in Artemisia selengensis to long-term drought and re-watering. Aquat Ecol. 2023;57(2):321–335. doi:10.1007/s10452-023-10012-2.
- Subramanian S, Mitkus E, Souleimanov A, Smith DL. Lipo-chitooligosaccharide and thuricin 17 act as plant growth promoters and alleviate drought stress in Arabidopsis thaliana. Front Microbiol. 2023;14:1184158–1184175. doi:10.3389/fmicb.2023.1184158.
- Peng JS, Zhu SL, Lin X, Wan X, Zhang Q, Njie A, Luo DC, Long YH, Fan R, Dong XQ. Evaluation of preharvest melatonin on soft rot and quality of kiwifruit based on principal component analysis foods. Foods. 2023;12(7):1414–1429. doi:10.3390/foods12071414.
- Wei JS, Liu JY, Wu SY, Wang XY, Lu W, Zheng XL. Physicochemical properties of leaves from seven Ficus species and its effects on the growth and development of phauda flammans. Huan Jing Kun Chong Xue Bao. 2024:1–23. http://kns.cnki.net/kcms/detail/44.1640.Q.20231221.1109.002.html.
- Han RT, Han LX, Zhang GX. Efffect of drought stress on photosynthetic characteristics and leaf structure of Chionanthus virginicus seedlings. J Plant Resour and Environ. 2023;32:61–70.
- Faghih S, Ghobadi C, Zarei A. Response of strawberry plant cv. ‘Camarosa’ to salicylic acid and methyl jasmonate application under salt stress condition. J Plant Growth Regul. 2017;36(3):651–659. doi:10.1007/s00344-017-9666-x.
- Elmongy MS, Abd El-Baset MM. Melatonin application induced physiological and molecular changes in carnation (dianthus caryophyllus L.) under heat stress. Horticulturae. 2024;10(2):122–137. doi:10.3390/horticulturae10020122.
- Qin C, Li D, Zhao HD, Chen QM, Fu MR. Effects of exogenous ethylene treatment on the peel browning and shelf life quality of Huangguan pears. TCSAE. 2022;38:312–318.
- Feng BS, Kang DC, Sun J, Leng P, Liu LX, Wang L, Ma C, Liu YG. Research on melatonin in fruits and vegetables and the mechanism of exogenous melatonin on postharvest preservation. Food Biosci. 2022;50(PB):1–15. doi:10.1016/j.fbio.2022.102196.
- Sun YM, Zhao N, Sun HJ, Xu S, Lu YW, Xi HJ, Guo ZF, Shi HF. Transcriptome profiling reveals molecular responses to salt stress in common vetch (Vicia sativa L.). Plants. 2024;13(5):714. doi:10.3390/plants13050714.
- Zhu LX, Li AC, Sun HC, Li P, Liu XQ, Guo CC, Zhang YJ, Zhang K, Bai ZY, Dong HZ. et al. The effect of exogenous melatonin on root growth and lifespan and seed cotton yield under drought stress. Ind Crops Prod. 2023;204(Part B):117344–117356. doi:10.1016/j.indcrop.2023.117344.
- Ren S, Rutto L, Katuuramu D, Oono Y. Melatonin acts synergistically with auxin to promote lateral root development through fine tuning auxin transport in Arabidopsis thaliana. PLoS One. 2019;14(8):e0221687. doi:10.1371/journal.pone.0221687.
- Wan SQ, Zhang YG, Liu L, Xiao YZ, He JY, Zhang YH, Wang WD, Yu YB. Comparative effects of salt and alkali stress on photosynthesis and antioxidant system in tea plant (camellia sinensis). Plant Growth Regul. 2024. doi:10.1007/s10725-023-01115-9.
- Li CY, Chen CY, Mao HT, Zhang HY, Chen YE. Effect of exogenous melatonin on the growth and photosystem activity of wheat under drought stress. Mai Lei Zuo Wu Xue Bao. 2022;42:846–856.
- Lv YY, Zhao YT, He YS, Wang JM, Zheng YX, Chen XL, Huang FY, Liu JN, Yu L. Synergistic effects of gamma-aminobutyric acid and melatonin on seed germination and cadmium tolerance in tomato. Plant Signal Behav. 2023;18(1):2216001–2216012. doi:10.1080/15592324.2023.2216001.
- Yuan XL, An J, Zheng T, Liu WJ. Exogenous melatonin improves salt tolerance mainly by regulating the antioxidant system in cyanobacterium nostoc flagelliforme. Peer J. 2022;10:e14479. doi:10.7717/peerj.14479.
- Zhao YT, Wang QW, Gu D, Huang FY, Liu JN, Yu L, Yu XY. Melatonin, a phytohormone for enhancing the accumulation of high-value metabolites and stress tolerance in microalgae: applications, mechanisms, and challenges. Bioresour Technol. 2024;393:130093–130102. doi:10.1016/j.biortech.2023.130093.
- Zhou D, Li M, Wang XJ, Li HY, Li ZH, Li QW. Effects of exogenous melatonin on the growth and physiological characteristics of ginkgo biloba L. under salinity stress conditions. Horticulturae. 2024;10(1):89–110. doi:10.3390/horticulturae10010089.
- Zhu LX, Liu LT, Sun HC, Zhang YJ, Liu XW, Wang N, Chen J, Zhang K, Bai ZY, Wang GY. et al. The responses of lateral roots and root hairs to nitrogen stress in cotton based on daily root measurements. J Agron Crop Sci. 2022;208(1):89–105. doi:10.1111/jac.12525.
- Nie MG, Ning N, Chen J, Zhang YZ, Li SS, Zheng L, Zhang HP. Melatonin enhances salt tolerance in sorghum by modulating photosynthetic performance, osmoregulation, antioxidant defense, and ion homeostasis. Open Life Sci. 2023;18(1):1–17. doi:10.1515/biol-2022-0734.
- Zhang H, Zhao D, Tang ZY, Zhang Y, Zhang K, Dong JG, Wang FR. Exogenous brassinosteroids promotes root growth, enhances stress tolerance, and increases yield in maize. Plant Signal Behav. 2022;17(1):e2095139. doi:10.1080/15592324.2022.2095139.
- Zhao MX, Li JJ, Shi XN, Malik MS, Quan Y, Guo DH, Wang L, Wang SP. Effects of exogenous plant regulators on growth and development of “kyoho” grape under salt alkali stress. Front Plant Sci. 2023;14:1274684–127698. doi:10.3389/fpls.2023.1274684.
- Wang Q, Liang XT, Xiang DB, Xu WW, Wang CL, Zhan C, Ren CZ, Wei LM, Zhang SQ, Zhang L. et al. The physiological mechanism of melatonin enhancing the tolerance of oat seedlings under saline-alkali stress. Agronomy. 2023;13(9):2343–2365. doi:10.3390/agronomy13092343.
- Song RX, Ritonga FN, Yu HY, Ding CJ, Zhao XY. Effects of exogenous antioxidant melatonin on physiological and biochemical characteristics of populus cathayana × canadansis ‘xin lin 1’ under salt and alkaline stress. Forests. 2022;13(8):1283–1298. doi:10.3390/f13081283.
- Jiang D, Lu B, Liu LT, Duan WJ, Meng YJ, Li J, Zhang K, Sun HC, Hang YJ, Dong HZ. et al. Exogenous melatonin improves the salt tolerance of cotton by removing active oxygen and protecting photosynthetic organs. BMC Plant Biol. 2021;21(1):1–19. doi:10.1186/s12870-021-03082-7.
- Gao WY, Zhang YJ, Feng Z, Bai QQ, He JJ, Wang YJ. Effects of melatonin on antioxidant capacity in naked oat seedlings under drought stress. Molecules. 2018;23(7):1580–1594. doi:10.3390/molecules23071580.
- Dong B, Da FF, Chen YL, Ding XC. Melatonin treatment maintains the quality of fresh-cut gastrodia elata under low-temperature conditions by regulating reactive oxygen species metabolism and phenylpropanoid pathway. Int J Mol Sci. 2023;24(18):14284–14298. doi:10.3390/ijms241814284.
- Liang D, Gao F, Ni ZY, Lin LJ, Deng QX, Tang Y, Wang X, Luo X, Xia H. Melatonin improves heat tolerance in kiwifruit seedlings through promoting antioxidant enzymatic activity and glutathione s-transferase transcription. Molecule. 2018;23(3):584–595. doi:10.3390/molecules23030584.
- Kaouthar F, Ameny FK, Yosra K, Walid S, Ali G, Faiçal B. Responses of transgenic Arabidopsis plants and recombinant yeast cells expressing a novel durum wheat manganese superoxide dismutase TdMnSOD to various abiotic stresses. J Plant Physiol. 2016;198:56–68. doi:10.1016/j.jplph.2016.03.019.
- Ahmad S, Su WN, Kamran M, Ahmad I, Meng XP, Wu XR, Javed T, Han QF. Foliar application of melatonin delay leaf senescence in maize by improving the antioxidant defense system and enhancing photosynthetic capacity under semi-arid regions. Protoplasma. 2020;257(4):1079–1092. doi:10.1007/s00709-020-01491-3.
- Bashri G, Prasad MS. Exogenous IAA differentially affects growth, oxidative stress and antioxidants system in Cd stressed Trigonella foenum-graecum L. seedlings: toxicity alleviation by up-regulation of ascorbate-glutathione cycle. Ecotox Environ Safe. 2016;132:329–338. doi:10.1016/j.ecoenv.2016.06.015.
- Jiang ZZ, Zhu HG, Zhu HY, Tao YZ, Liu CZ, Liu JQ, Yang FQ, Li M. Exogenous ABA enhances the antioxidant defense system of maize by regulating the AsA-GSH cycle under drought stress. Sustainability. 2022;14(5):3071–3085. doi:10.3390/su14053071.
- Njie A, Dong XQ, Liu QG, Lu CY, Pan XJ, Zhang WE. Melatonin treatment inhibits mango fruit (cv. ‘Guiqi’) softening by maintaining cell wall and reactive oxygen metabolisms during cold storage. Postharvest Biol Technol. 2023;205:112500–112512. doi:10.1016/j.postharvbio.2023.112500.
- Lu XH, Zhang HJ, Zhang N, Dong CH, Ji HP, Yu JZ, Ban ZJ, Yan RX, Zhang T, Chen CK. et al. Effects of ozone treatment on gene profiling involved in ASA-GSH cycle in postharvest cantaloupe. Sci Hortic (Amsterdam). 2023;312:111843. doi:10.1016/j.scienta.2023.111843.
- Tan XL, Zhao YT, Shan W, Kuang JF, Lu WJ, Su XG, Tao NG, Lakshmanan P, Chen JY. Melatonin delays leaf senescence of postharvest Chinese flowering cabbage through ROS homeostasis. Food Res Int. 2020;138(PB):109790–109801. doi:10.1016/j.foodres.2020.109790.
- Ou C, Cheng WH, Wang ZL, Yao XM, Yang SM. Exogenous melatonin enhances Cd stress tolerance in platycladus orientalis seedlings by improving mineral nutrient uptake and oxidative stress. Ecotoxicol. Environ. Saf. 2023;252(1):114619.
- Liu GS, Zhang YX, Yun Z, Hu MJ, Liu JL, Jiang YM, Zhang ZK. Melatonin enhances cold tolerance by regulating energy and proline metabolism in litchi fruit. Foods. 2020;9(4):454–467. doi:10.3390/foods9040454.
- Li J, Yang YQ. How do plants maintain pH and ion homeostasis under saline-alkali stress? Front Plant Sci. 2023a;14:1217193–1217203. doi:10.3389/fpls.2023.1217193.