Abstract
The uncontrolled concentrations of heavy metals introduced into the water sources need an effective method for their removal. The dithiocarbamate (dtc) ligands are the most popular precipitating agents used for the removal of metals from wastewater. Presently, two novel dtc ligands, one without methylene linker (K2L1) and the other with methylene linker (K2L2) were used for the removal of eight heavy metals ions including Fe2+, Co2+, Ni2+, Cu2+, Cd2+, Ag+, Zn2+ and Pb2+ from the aqueous solution. These dtc ligands contain acyl (NH-C = O) and S, S (thiolate) functional groups. The results indicated that the metal removal efficiency of both ligands was almost 100% at pH 4 and 7 and free of the linker effect and acyl functional groups but depends on the S, S binding sites. It exhibited a distinct selectivity for the removal of metal ions (Pb2+ ∼ Cu2+ ∼ Ag1+ > Cd2+ > Co2+ > Ni2+ > Zn2+ > Fe2+). The developed methods have the potential for the removal of heavy metals and can be used for other thiophilic metals that are water-soluble.
HIGHLIGHTS
Water treatment, contaminated by heavy metal ions.
Synthesis and characterization of two novel dithiocarbamate ligands.
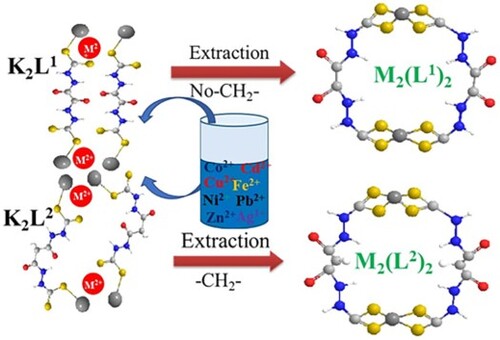
Effect of methylene (-CH2-) linker of dithiocarbamtes on heavy metals extraction.
pH and temperature effect on the extraction efficiency of ligands.
1. Introduction
Environmental pollution and toxicity of heavy metals cannot be underestimated. The toxicity of these metals is well known since World War 1 when the British army sprayed heavy metals that killed many peoples and was called a weapon of murder. The main sources of these metals are mostly anthropogenic and are discharged into the environment either directly or indirectly, reacting with water, air, and food sources [Citation1]. The main problem with these metals is their undegradable nature, thus having a large environmental, public health, and economic impact [Citation2]. These metals are used in a variety of consumer and basic engineering projects, including paper and pulp industries, leather tanning, plastic stabilizers, photographic materials, fertilizers, pigments, cosmetics, and batteries [Citation3,Citation4]. These heavy metals can cause serious health threats when comes in contact with the human body either physically or ingested and thus accumulated in the body of living organisms. Some of the heavy metals are essential and are an important part of food but if they exceed their maximum level, it becomes toxic to living organisms including human beings [Citation5].
Heavy metal poisoning can be acute or chronic, depending on how long you have been exposed to it. It can damage various internal organs, including the brain, lungs, liver, and kidneys, resulting in various types of disorders [Citation6]. Based on the experimental studies, the presence of metal ions has been found to interact with DNA and some nuclear protein and thus causing DNA damage that may disturb the cell cycle. The heavy metal-induced toxicity and carcinogenicity are due to many mechanistic aspects which are not clearly understood. However, it is assumed that these metals ions accumulate in the body and make complexes with amine (-NH2), the carboxylic acid (-COOH), and thiol (-SH) functional groups of proteins [Citation7,Citation8] and produce free radicals (RO) in the body which cause oxidative stress, and damages the biological components including enzymes, lipids, proteins, nucleic acids, and DNA and lead to cytotoxicity and genotoxicity in the cell [Citation4]. Ultimately the toxicity of heavy metals results in carcinogenicity and neurotoxicity. For that reason, the removal of such hazardous metals from water and wastewater is of prior concern in terms of the protection of public health and the environment [Citation9].
Different techniques are used for the removal of heavy metals from polluted water. These include the ion exchange process [Citation10], reverse osmosis, oxidation and reduction, solvent extraction, adsorption, bioremediation, electrochemical treatment technology, and membrane separation [Citation11]. These techniques may have good removal efficiency but also have some limitations such as high operational costs and always leaving secondary hazardous materials that are difficult to be treated. Therefore, finding new techniques and compounds with less expenditure is of great interest for the removal of heavy metals from water and wastewater. Dithiocarbamate (DTC) ligands have great importance in balancing the ecosystem due to their role in pollution control and are the most fertile research materials in this field [Citation12].
Dithiocarbamate (DTC) ligands are a type of dithiolate ligand and have a characteristics functional group (C=S) that can coordinate in several ways. The most important feature of dithiocarbamate ligands is the flow of π-electron from nitrogen towards the CS2 group through a delocalized π-orbital system and thus shifting the electron density towards the metal ion. Figure demonstrates the dithiocarbamate (a, b, and c) and thioureide (d) tautomeric forms. They have versatile binding abilities and form complexes with most transition metals [Citation13]. Apart from their usage in material and medical sciences [Citation14], the dithiocarbamate ligands are also efficiently used for the removal of heavy metals from wastewater. The ligands with S, S donor active sites [Citation15,Citation16], amides, and acyl functional groups have a strong chelating ability towards metal ions. Amongst the functional groups, the S2− act as a soft base and react quickly with the soft ions (metal ion). Importantly, the dithiocarbamates form insoluble and stable-coloured complexes in water which makes them an excellent ligand for the removal of heavy metals from polluted water [Citation17,Citation18]. Generally, the dithiocarbamates are synthesized from primary and tertiary amine and carbon disulphide under an alkaline environment. The amino compounds have the limitations of being volatile, very expensive and toxic [Citation19]. To address these problems, we have focused on the synthesis of dithiocarbamate ligands derived from easily available, simple precursors with low cost, non-toxic and that are specifically designed to remove various heavy metal ions from aqueous solutions or wastewater.
In this work, two novel dithiocarbamate ligands with S S donor sites that are slightly different from each other based on -CH2- linker (Schemes (a) and (b)) were prepared from acyl hydrazides which were widely available and non-toxic and readily available and carbon disulphide under alkaline environment. The structure of the ligands was confirmed by FT-IR, IHNMR and 13C NMR spectroscopy. The removal of the heavy metal ions (Fe2+, Co2+, Ni2+, Cu2+, Cd2+, Ag+, Zn2+ and Pb2+) from aqueous solutions at different pH and temperature were investigated by FT-IR and atomic absorption spectroscopy. This research work provided simple and easily available methods to obtain high-performance and low-cost dithiocarbamates and allow them to be easily converted to practical applications.
2. Experimental
2.1. Chemicals and instrumentations
All chemicals were purchased from Sigma-Aldrich, Germany. Some of the starting materials were moisture sensitive and thus placed under an inert atmosphere (N2). The solvents used in this experimental work were ethanol, methanol, chloroform, diethyl ether, and carbon disulfide; purified and dried by the conventional and standard methods [Citation20]. All the apparatus were kept airtight like quick fit joints, cleaned, and dried at 105°C before using for the next experiment. The FTIR with ATR, a Bruker Tensor II spectrometer was used for recording the FTIR spectra in the range of 4000–250 cm−1. The NMR spectra for 1H, 13C 1H for all the ligands were recorded by using Bruker AV 400 spectrometer operating at 4000.23, and 100 MHz respectively. The value of chemical shift is described in ppm The multiplicities in signal in each spectrum for 1H NMR are mentioned as; singlet (S). For the determination of pH, the Hena pH meter, model 3510-USA was used. The stoichiometric ratio of metal to ligand was determined using a Shimadzu UV Visible spectrophotometer (model UV-1800). The concentration of heavy metals; Pb, Cd, Cr, Co, Ni, Ag, Zn, and Cu in the filtrate and precipitate were determined using a Perkin Elmer Flame Atomic Absorption Spectrophotometer (AAA-700).
2.2. Preparation of stock solution
First, stock solutions of 1000 ppm of lead, cadmium, chromium, cobalt, nickel, silver, and zinc were prepared. From the stock solutions, 10 ppm of each metal salt solution was prepared. A 4-ppm solution of cadmium was also prepared to avoid a negative deviation of its solution from Beer’s law. The standard reference solution for each was available with accurate concentration purchased from sigma Aldrich for the atomic absorption spectrophotometer.
2.3. Preparation of ligands
2.3.1. Synthesis of dipotassium 2, 2’-oxoxalylbis (hydrazine-1-carbodithioate; K2L1)
A dithiocarbamate (DTC) salt of dipotassium 2, 2’-oxoxalylbis (hydrazine-1-carbodithioate) was prepared under an inert atmosphere according to the reported method with slight modification [Citation21]. An oxalohydrazides (0.003 moles) were mixed in dry ethanol with KOH (0.006 moles) and stirred at least for 1 hr in an ice/acetone/salt bath. The dropwise addition of CS2 (0.006 moles) was continued in a stoichiometric amount until a pale yellow colour precipitate of ligands appeared and recrystallized from the ethanol as shown in Scheme . The mixture was then filtered, washed with diethyl ether, and dried in an inert atmosphere [Citation2] for future use.
2.3.2. Synthesis of dipotassium-2, 2’-malonylbis (hydrazine-1-carbodithioate) (K2L2)
A dithiocarbamate (DTC) salt of dipotassium-2, 2’-malonylbis (hydrazine-1-carbodithioate) was prepared under an inert atmosphere according to the reported method with slight modification [Citation21]. Malonohydrazide (0.003 moles) were mixed in dry ethanol with KOH (0.006 moles) and stirred at least for 1 hr in an ice/acetone/salt bath. The dropwise addition of CS2 (0.006 moles) was continued in a stoichiometric amount until a pale yellow colour precipitate of ligands appeared and recrystallized from the ethanol as shown in Scheme . The mixture was then filtered, washed with diethyl ether, and dried in an inert atmosphere [Citation2] for future use. Additionally, the formation of the both the ligands was confirmed from the NMR spectra (Figures –) and IR studies (Table ) which is similar to the reported one [Citation22].
Table 1. Comparison of infrared spectral details of ligands and their complexes with the literature.
2.3. Determination of the ligand-to-metal ratio
In this study, UV-Visible spectroscopy was used for the determination of the ligand-to-metal ratio via the molar ratio method. First, the concentration of each metal was fixed at 10 ppm (4 × 10−5 moles) in a solution at a pH with the highest efficiency for removing each metal as shown in Table , which was taken as a blank sample. The ligand was added to the metal sample at ascending concentrations (1–20 ppm). The absorption for each sample was measured, and a graph was plotted between ligands concentration and absorption. The plotted graph shows a direct correlation between the increase in the concentration of the ligand and the absorption until the absorption became stable when the ligand concentration reached 10 pm, which is equal to the number of moles of metal ion concentration. This confirms that the ligand-to-metal ratio is 1:1 as shown in Schemes and . This procedure was followed for each metal ion with both ligands. This step was repeated for all metals under investigation and with every ligand. Figures and are examples of the determination of nickle metal to ligand ratio in both the ligands.
Scheme 2. The ligand-to-metal ratio in the complex formation of Ligand K2L1 (M=Fe, Co, Ni, Cu, Cd, Ag, Zn, and Pb) at various temperatures and pH (1c).

Scheme 3. The ligand-to-metal ratio in the complex formation of Ligand K2L2 (M=Fe, Co, Ni, Cu, Cd, Ag, Zn, and Pb) at various temperatures and pH (2c).

Table 2. Metal removal efficiency of dithiocarbamate ligands (K2L1-2) at various pH.
2.4. Removal of heavy metals from aqueous solution by (K2L1-2)
The metal salt solutions of required concentrations were prepared from a stock solution of 1000 ppm and its initial concentration as a blank was checked. Before the reaction, the pH of distilled water, metal salts, and dithiocarbamate was determined in the solution. The dithiocarbamate ligands and metal salts were mixed in the equivalent ratio (1: 1) in a 25 mL vial and stirred for 2–3 hrs. A robust reaction took place between the metal ion and the ligands resulting in a precipitate of different colours.
Similarly, the pH of the solution was also determined after complexation. The above process was repeated at different reaction conditions, using different pH and temperatures condition as shown in Tables and . The pH of the solution was adjusted by using HNO3, KOH, and NH4OH or by using acetate buffer (pH 3–5), phosphate buffer (pH 6–8), and carbonate buffer (pH 9–11). The precipitates were filtered, and the filtrates were analyzed for the decrease in heavy metal concentration in the solution and % recovery was calculated.
2.5. Metal selectivity by S, S donor ligand (K2L1-2)
The solution of different heavy metal ions like Fe2+, Co2+, Ni2+, Cu2+, Cd2+, Ag+, Zn2+ and Pb2+ was prepared in distilled water. The pH of the solution was adjusted by using HNO3, KOH, and NH4OH or by using acetate buffer (pH 3–5), phosphate buffer (pH 6–8), and carbonate buffer (pH 9–11). To the mixture of metal salts, a solution of dithiocarbamate was added which precipitated the metal ion from the solutions. This process was repeated till the extractions of all metal ions from the solutions. The colour of the precipitate obtained from this experiment was compared with the precipitate obtained for individual metal extraction for visual detection of respective heavy metals. For better selectivity, all the individual precipitates were dried and ground through a 300-mesh sieve. A small portion, of 0.01 g of finely ground precipitate of each metal was mixed with 1 mol/L hydrochloric acid (HCl), sulphuric acid (H2SO4), and nitric acid (HNO3) solution in a ratio of 1: 100 (solid to liquid). The reaction was refluxed for 30 min then the volume was reduced to 2 mL. Later, it was filtered; diluted up to 25 mL, and then subjected for analysis to atomic absorption spectrophotometer. A dilution factor was applied for concentrated solutions and the concentration of the sample in the extract (solid) in the solution was determined [Citation32].
3. Results and Discussion
Different types of analytical techniques have been used for the structural elucidation of dithiocarbamate ligands, their complexes, and heavy metal removal efficiency. In the 13C and 1H NMR spectra of K2L1 and K2L2 ligands (Figures –), a response for the CH2 protons was observed at around 2.15 ppm (Figure ), while the chemical shift for 13C1H at 165.26-166.3, 215.4 and 39.81 were observed for C=O, S= C=S and CH2 (Figures and ), respectively. The most important vibrational modes in infrared spectroscopy were recorded for the functional groups of the types; υ(NH), υ(NHC=O), υ(NHC-O), υ(N=C-SS), υ(C=S), υ(CSS), υ(N-N) and υ(M–S) [Citation33]. The absorption bands of dithiocarbamate ligands at; 3170–3401(b), 1620–1659 (vs), 1541–1598(s), 1425–1499. 1020–1100 (s), 952–1050 (m), 783–946 cm−1 are associated with υ(-NH), υ(-NH-C=O (ketonic form); amide II stretching), υ(NHC-O; enolic form), υ(-C=N; thiouride band; amide II 60% C–N stretching plus 40% NH in-plane bend), υ(-CSS; sym), υ(-C=S; asym) and υ(-N-N; hydrazinic band) respectively. Sometimes another band is also attributed to the CSK or CSH at 2750 cm−1 which always disappears in the complexes. These values agree well with the values reported in the literature [Citation34,Citation35] indicating that the ligands exist as thione in the solid-state [Citation36].
The broadband/overlapping in the spectra of the ligands is due to the hydrogen bonding of NH and the C=O group due to slight keto–enol tautomerism (delocalization) in the amido group and water [Citation37]. The C=O stretching frequency due to this keto–enol tautomerism appears between 1620 and 1598 cm−1 [Citation38] and thus ruled out the coordination of ligands with metals through the carbonyl group. The presence of the NH band in complexes shows that the nitrogen of NH is a poor electron donor as compared to thiol groups and the frequency remains unaltered in complexes indicating the non-involvement of nitrogen in coordination. Sometimes the NH band shifts to a higher frequency in the complexes.
Usually, the values for the thyroid, υ(-CN) for aliphatic dithiocarbamates (∼1500 cm−1) are different from the aromatic dithiocarbamates (∼1350 cm−1) due to structural differences [Citation39]. In the case of K2L2 and its complexes, the bands at 2886–2975 cm−1 are associated with the -CH2- moiety in the ligands and complexes which is absent in the ligand K2L1. The confirmation of the complex can be inferred from the difference between the IR spectra of ligands and their complexes as shown in Figures and and Table .
In addition, there is also a strong band at 1212 cm−1 in some complexes like AgL2 which is associated with the νC-N single bond that arises due to the splitting of C=N and, thus, confirming the introduction of the – NCSS group to the complexes via ligand as monodentate. An amide (NHCO) III stretch due to NH also appears as a sharp band at around 1232–1241 cm−1. The C–N stretching vibration, in most of the dithiocarbamates ligands usually appears as a strong band at around 1500 cm−1 [Citation24,Citation40]. In the literature, the C=N also appears at a slightly higher frequency (high energy or high wave number or lower wavelength) due to the double bond character in the complex (approximately 12–30 cm−1 shift) than the ligands showing an increase in bond order (shortening of C–N bond) between carbon and nitrogen [Citation41] which is attributed to the releasing of electrons by the amine nitrogen and accumulating high electron density on the sulfur atoms, via the thiouride π system. This results in a partial double bond and polar character between C–N. The value for thiouride is intermediate (combination of C–N and C=N) between a single C–N bond (1250–1350 cm−1) and double bond C=N (1649–1690 cm−1), suggesting the partial double bond character due to the three possible resonating structure, thus delocalization of pi electrons flow from nitrogen over the CSS− (dithiocarbamate group) via planar delocalized dπ-orbital in a Sulphur atom, and the dithiocarbamate group/sulfur atoms mesomerically drift the electron towards the metal ion and increase the electron density on metal [Citation42]. Apart from it, the sulfur atom also possesses the σ donor and π back donation capability.
The position of the C–N band (thiouride band) and their splitting also indicate the attachment of sulfur atoms to the metal in complexes. If two peak appears in this region, it shows the presence of unidentate dithiocarbamates and the ligand behaves as monodentate and sometimes as both monodentate and bidentate. It also depends on the size of the R group attached to the nitrogen atom and the electronic property of the NR2 group. However, the presence of a single strong band in complexes at high wavenumber as compared to the ligand at this region (C=N) also indicates the bidentate or anisobidentate character of the ligand and has been identified in the above ligands as a strong thiouride bands ν(C=N) at 1425–1456 cm−1 with slightly at higher frequency (12–43 cm−1) in the complexes (1446–1499) showing a pronounce partial double bond character of C–N (N-N=C) and thus, confirming the coordination of S, S atoms to the metal centre. The infrared active ν(N-N=C) mode is also sensitive to chain length in ligands as reported in the literature [Citation2] but in our case, the chain length (-CH2-) was not effective. Similarly, the second region of interest is the appearance of a broad double peak (i.e. shoulder C–S and sharp C=S peaks) or two separate peaks in the region 1000 ± 50 [Citation43], and in some cases high or lower than the mentioned region, depending upon the types of ligands [Citation44]. It may be attributed to the sharp νas(CSS) and medium νs(CSS) bands, demonstrating the presence of dithiocarbamate moiety in the ligands [Citation29]. Mostly in DTC complexes a single sharp CS peak appears in the same region (950–1100 cm−1) indicating the bidentate nature of the ligands. Sometimes the splitting (C–S) bands (950–1000 cm−1) appear as two CS bands; a shoulder and a sharp band with a separation of less than 20 cm−1 (< 20 cm−1) also indicate the bidentate nature of the ligands in the complex. However, in some cases, a separation of the two bands (splitting of C–S) is more than 20 cm−1 (>20 cm−1) in a complex shows that one group (C–S) is involved in coordination with the metal and not the second group (C=S) and thus showing the monodentate nature of the ligands. So, the two bands, the thiouride and thiol bands are important parameters in a complex to decide whether the ligand is monodentate or bidentate. If the ν(CS) bonds with non-equivalent stretching show two bands in the complex, it means that the ligand in the complex is monodentate but if the ligand in a complex shows only one ν(CS) band, it suggests that the ligand is bidentate in the complex with equivalent stretching [Citation45].
According to the FTIR spectra (Figures and ), the νas(C–S) and νs(C–S) modes for the ligands in this study appeared as two peaks at 1112 and 994 cm−1 for K2L1, and 1042 and 980 cm−1 for K2L2, respectively. While in the complexes, only single sharp peaks ν(C–S) were observed at 1008−952 cm−1 and 1010–954 cm−1 for M2(L1)2 complexes and M2 (L2)2, respectively indicating the band shifting to lower wavenumber (weakening of vibration) with respect to its position in the free ligands and thus, rendering both the characteristics, ν(CS2) band in dithiocarbamate ligands almost equivalent [Citation46] suggesting the bidentate nature of the dithiocarbamate ligands via the thiolate sulfur [Citation47]. However, the appearance of two peaks for ν(CS2) suggests the monodentate binding mode of these ligands as can be seen in compound Ag2(L1-2)2 where the splitting of the two peaks is wider than 20 cm−1. It was suggested by Bonati and Ugo that in such cases only one C–S is coordinated with the metal cation but not the other C=S group. whereas a single band is a sign of the bidenticity of the ligand through CS2 [Citation48]. The synthesis of the complex can also be inferred from the disappearance of S-H/or S-K peaks at 2750 cm−1 and the appearance of M-S at 250–430 cm−1 [Citation25,Citation45,Citation49] which is absent in both the ligands. Additionally, the IR spectral observations strongly support the proposed metal complex structure (Schemes and ).
The effect of heavy metal removal at different pH is an important factor. Increasing or decreasing the pH affect the removal efficiency [Citation2]. The stability of dithiocarbamates in solution is strongly dependent on the pH. and is considered unstable at pH < 4 [Citation31]. Other dithiocarbamates such as pyrrolidinedithiocarbamate and dibenzyldithiocarbamate, are far more stable at low pH [Citation50]. The removal efficiency for each metal from its solution (i.e. 10 ppm) using the two dithiocarbamate ligands with acyl (amido) functional groups and -CH2- linker effect was studied using a ligand to metal ratio of 1:1 at six pH values of 1, 2, 4, 7, 10, 13 and corresponding initial pH (self pH) as shown in Table . The removal efficiency increases by increasing the pH value from 2 to 7 in some cases whereas it decreases at higher pH (pH > 10). Since we succeeded in removing heavy metals from the aqueous solution and excellent removal efficiency was observed at pH 2, 4, 7, and 10, and the corresponding pH for iron (Fe) with a maximum value of 100% (pH-10) and minimum value at pH 4. For cobalt (Co) the highest removal efficiency was found at pH 4 (≈ 99.6%) and pH 7 (99.98%).
The removal efficiency of other metals like nickel was found excellent at pH 4, 7 and corresponding pH and the lowest removing efficiency at pH 10 as shown in Table . Almost 100% efficiency was observed for copper (Cu), cadmium (Cd), silver (Ag), zinc, and lead (Pb) at pH 4, 7, and 10 higher than found in the literature. For Cu(II), the best working conditions were 2–7 because, at pH ∼ 9, the blue ammonium Cu(II) hydroxide complexes interfere with the spectral measurement as reported [Citation27]. The removal rate of Ni2+ increases by increasing the pH from 1 to 6.78 (Corresponding pH; pH without adding any acid, base, or buffer) with maximum removal efficiency (99.6%), which is almost similar to reported in the literature [Citation31] and with residual concentration less than 1 mg/L (at pH 2, 4, 7 and 6.78) which is within the permissible limit suggested by the National Integrated Wastewater Discharge Standard of China and Environmental Quality Standard for surface water [Citation31,Citation34]. But when the pH value increases from pH 4 onward except pH 6.78 the removal rate of Ni2+ decreases which could be compared with the reported value, but the residual concentration does not meet the recommended value.
Mostly, in dithiocarbamates at lower pH, the H+ ions compete with the metal ions to occupy the site of attachment and convert the CSS− group into the CSSH group, resulting in decreased chelation between the metal ion and the CSS− group [Citation31] and thus reduces the chelation ability of the ligands with the metal cations. However, we got a good result at lower pH probably due to different functional groups (Acyl) besides the thiolate groups (S2−) that can accommodate the extra protons (+H) in solutions, thus minimizing the effect of the acidic medium. At higher pH (alkaline medium) the removal efficiency would be decreased as part of the precipitated complex would either decompose or be suspended as fine particles [Citation51]. The other reason for less removal of heavy from aqueous solution by K2L1 and K2L2 might be due to the reason that at higher pH the resonating protons (NH) convert from amid form (ketonic form; HN C=O) to iminol form (enolic form; N=C-OH) [Citation38]. Thus, hindering the -C=N partial double bond and activating the acyl group for bonding (M-O-C=N) may cause the solubility of the precipitate and consequently decreases the removal efficiency. Thus, the chelation property of the CSS− group decreases for metal cations [Citation52].
The effect of pH 4 and 7 is more pronounced than other pH values as most of the heavy metals precipitated at this pH and could be found in the literature, and this pH has been used for further studies [Citation34,Citation53]. The effect of ligands dosage on the extraction and binding of heavy metals were investigated at 25°C with varying amount of ligands for Fe(II), Co(II), Ni(II), Cu(II), Cd(II), Ag(I), Zn(II) and Pb(II) at 10 ppm and pH 7 (the exception of Cd by reducing 10 ppm to 4 ppm and later on dilution factor was applied to calculate it in 10 ppm). The removal efficiency of ligands mostly increases with increasing the dosage of the ligands [Citation34] but in our case, the precipitate becomes soluble when a high amount of ligands was added. The results depicted that the value of pH and concentration of metal ions in solution plays a vital role in heavy metal removal efficiency. So, we can say that changing the pH and concentration of the heavy metal solutions even for the same ligands has different removal efficiency.
The effect of contact time on the removal of Fe(II), Co(II), Ni(II), Cu(II), Cd(II), Ag(I), Zn(II), and Pb(II) are also helpful. The removal of these heavy metal ions by the dithiocarbamate ligands was very fast due to the active trapping site on the ligands and took less than one minute at its normal pH but at different pH, the removal efficiency varied. The effect of the initial concentration of metal ions; Fe(II), Co(II), Ni(II), Cu(II), Cd(II), Ag(I), Zn(II), and Pb(II) was also investigated. Increasing the initial concentration of metal ions also enhanced the removal efficiency, suggesting that the removal process is highly concentration dependent.
Generally, the coordination ability and stability of the ligands are strongly influenced by their periodic position, Cr, Fe, Co, Ni, Cu, Zn, etc. The relative selectivity and predicted affinity sequence of heavy metals are based on some metallic properties like ionic radii, atomic weight, electronegativity, hydrolysis constant, softness and hardness of the metal, and ligand centre of attachment. The metal selectivity is shown in Table . The reactions of soft acids like Ag+ and Cd2+, borderlines acids like Fe2+, Co2+, Ni2+, Cu2+, Zn2+, and Pb2+ were carried out with the dithiolate ligands (-S2−) which showed excellent removing efficiency from aqueous solutions. The reactions of the dithiocarbamate (soft ligands S2−) were also tried with hard acids like Cr3+ and Cr6+, Mn2+, Fe3+, Co3+, and As3+ but almost no interaction was observed with these ligands [Citation54].
According to the literature the different properties of metal ions which effect the selectivity are Atomic weight, Pb (207.2) > Cd (112.41) > Zn (65.38) > Cu (63.54) > Ni (58.70) [Citation54]; ionic radii and metal affinity sequence in Å, Pb2+ (1.33) > Ag1+ (1.29) > Cd2+ (1.09) > Fe2+ (0.92) > Co2+ (0.885) > Zn2+(0.88) > Cu2+ (0.87) > Ni2+ (0.83) [Citation55]; Electronegativity, Au +1 (2.54) > Cu (2.0) > Ag1+ (1.93) > Ni (1.91) > Co2+ (1.88) = Pb (1.87) > Fe2+ (1.83) > Cd (1.69) > Zn (1.6) [Citation55–57]; First Hydrolysis constant, (M2++ H2O = M(OH)2 + H+), Pb (7.8) ≥ Cu (8.0) > Zn (9.0) > Ni (9.9) > Cd (10.1) [Citation55]; Softness behaviour, Hg2+ (4.24) > Ag1+ (3.99) > Pb2+ (3.58) > Fe2+ (3.09) > Cd2+ (3.04) > Mn2+ (3.03) > Co2+ (2.96) > Cu2+ (2.89) > Ni2+ (2.82) > Zn2+ (2.34) [Citation55,Citation56,Citation59].
Keeping in view the above discussion we can say that for every “soft metal” cation the affinity is higher with the thiols group (S2−) [Citation60]. However, as Lewis’s borderline soft acids, Pb2+ and Cu2+ and soft acid Ag+ have nearly the same value but had precedence over Cd2+ (soft acid) and Fe2+, Co2+, Ni2+ and Zn2+ regarded as borderline soft acids, the interaction takes place with dithiocarbamates ligands which could be considered as a Lewis soft base. Therefore, dithiocarbamate ligands (K2L1-2) have a stronger affinity to Pb2+ even with moderate cationic softness followed by Cu2+ (moderate softness) and Ag1+ (highest softness) [Citation60,Citation61], comparatively weaker to Co2+, Ni2+ and Zn2+, more or less following the softness order or we assumed a noncompetitive removal order as shown Pb2+ ∼ Cu2+ ∼ Ag1+ > Cd2+ (4d10) > Co2+ (3d7) > Ni2+ (3d8) > Zn2+ (3d10) > Fe2+ (d6) which do not show agreement with the literature: Cd2+ > Cu2+ > Pb2+> Ni2+ > Zn2; Cu2+ > Cd2+ > Co2+ > Zn2+ > Ni2+ [Citation62].
The highest selectivity in our study was observed for Pb2+ followed by more or less equal for Ag1+ and Cu2+ which is according to the ionic radii pattern as compared to the other divalent ions. The selectivity order might be followed due to their different properties like ionic radius, ionic potential, and hardness-softness behaviour [Citation63]. Additionally, there is an enolic and ketonic tautomeric form of the ligands where the carbonyl oxygen and nitrogen can also interact with the metal through non-covalent interaction. Some ligands with increased length of carbon chain also influence the removal efficiency of metal cation than the ligands with short carbon chain [Citation2] but in our case, the effect of the chain length (linker effect) could not be observed.
However, the two ligands, K2L1-2 almost showed similar removal behaviour as compared to that reported in the literature. The dithiocarbamate ligands with extended carbon chains worked best in theory and practice for wastewater treatment containing heavy metal cations [Citation64]. The residual concentration of metal ions for the ligands K2L1-2 was less than suggested by WHO for most metals. The complexation equilibrium time was less than one minute for most of the complexes which is less than that reported in the literature.
The effect of temperature can never be ignored as most industries are discharging hot water from their boiler that may also contain heavy metals. The effect of temperature was evaluated at 60 and 80°C as shown in Table . The removal efficiency decreases at high temperatures due to the solubility of the precipitates. Similar results reported in the literature at different reaction conditions for the extraction of heavy metals Fe(II), Co(II), Ni(II), Cu(II), Cd(II), Zn(II), and Pb(II) [Citation27–29,Citation34,Citation65,Citation66] could be compared with the present study as reported. Finally, it was shown that there is not a significant difference in the heavy metal removal ability of both ligands (K2L1 and K2L2) and does not depend on the linker effect (-CH2- spacer), which may be due to the small flexible linker and rigidity in the structure of the ligands. However, the extraction ability for the heavy metals at various reaction conditions showed that the highest removal ability was observed for most of the metals and particularly 100% Pb(II) followed by 99.9% for Cu(II), and Ag(I). This, therefore, gives a viable approach for the treatment of polluted water containing heavy metal ions.
Table 3. Metal removal efficiency of dithiocarbamate ligands (K2L1-2) at various temp (°C).
4. Conclusion
The study showed that dithiocarbamate ligands with bifunctional groups were successfully synthesized and effectively removed single and multiple metal ions from an aqueous solution. The maximum removal efficiency of heavy metals by DTC from a single ions solution increase by increasing the pH value from 1- 7 in some cases, whereas at higher pH (pH 10) the trend is irregular. The effect of pH 4 and 7 is more pronounced than other pH values as most of the heavy metals precipitated at this pH and could be found in the literature. The extraction ability for the heavy metals at various reaction conditions showed that the highest removal ability of 100% was observed for Pb(II) followed by 99.9% for Cu(II), and Ag(I). The highest selectivity of DTC in multiple ions solutions was more or less based on the softness order or we assumed a noncompetitive removal order: Pb2+ ∼ Cu2+ ∼ Ag1+ > Cd2+ > Co2+ > Ni2+ > Zn2+ > Fe2+. The removal efficiency decreased by increasing the temperature as by increasing temperature the solubility of precipitates increases. The final comparison between the results showed that – neither the CH2- linker nor the competing acyl groups effect was observed in these two ligands. This is because the two ligands do not have the required flexibility and the S2− functional groups have a greater affinity for soft metal ions than acyl functional groups(NH=C=O). However, our observation showed that though the extraction efficiency was not dependent on the spacer effect and acyl functional groups of the two types of ligands, the developed DTC were effective and selective for the removal of heavy metal ions from aqueous solutions that provided a possible approach for the treatment of wastewater contaminated by multiples metal ions. we can also postulate that dithiocarbamate ligands could also be used for the removal of other thiophilic metals that are water-soluble.
Disclosure statement
No potential conflict of interest was reported by the author(s).
Additional information
Funding
References
- Chai, WS, Tan, WG, Munawaroh, HSH, et al. Multifaceted roles of microalgae in the application of wastewater biotreatment: a review. Environ Pollut. 2021;269:116236. doi:10.1016/j.envpol.2020.116236.
- Abu-El-Halawa R, Zabin SA. Removal efficiency of Pb, Cd, Cu and Zn from polluted water using dithiocarbamate ligands. J Taibah Univ Sci. 2017;11:57–65. doi:10.1016/j.jtusci.2015.07.002.
- Niaz AS, Shah Z, Hussain M, et al. Hazardous effects of titanium dioxide nanoparticles in ecosystem. Bioinorg Chem Appl. 2017;2017:1–12. doi:10.1155/2017/4101735.
- Ullah H, Noreen S, Rehman A, et al. Comparative study of heavy metals content in cosmetic products of different countries marketed in Khyber Pakhtunkhwa, Pakistan. Arab J Chem. 2017;10:10–18. doi:10.1016/j.arabjc.2013.09.021.
- Din AU, Abdel-Reheem M, Ullah H, et al. Assessment of heavy metals in onion and potato in the imported and local variety of Pakistan and Afghanistan. Life Sci. 2013;10:198–204.
- Engwa GA, Ferdinand PU, Nwalo FN, et al. Mechanism and health effects of heavy metal toxicity in humans. In: Poisoning in the modern world-New Tricks for an Old Dog? London, UK: Intechopen; 2019.
- Waseem A, Ullah H, Rauf MK, et al. Distribution of natural uranium in surface and groundwater resources: a review. Crit Rev Environ Sci Technol. 2015;45:2391–2423. doi:10.1080/10643389.2015.1025642.
- Ullah H, Rehman A, Ahmad I, et al. Estimation of uranium concentration in drinking water sources of Tehsil Takht-e-Nasrati, District Karak, Khyber Pakhtunkhwa, Pakistan using fission-track technique. J Chem Soc Pak. 2013;35:999–1003.
- Fu F, Wang Q. Removal of heavy metal ions from wastewaters: a review. J Environ Manag. 2011;92:407–418. doi:10.1016/j.jenvman.2010.11.011.
- Pohl A. Removal of heavy metal ions from water and wastewaters by sulfur-containing precipitation agents. Wat Air Soil Poll. 2020;231:1–17. doi:10.1007/s11270-020-04863-w.
- Hadian M, Hadian M. Comparison of Spiegler-Kedem combined with film theory model and original SK model. Desalin Water Treat. 2022;272:1–5.
- Shahid M, Pinelli E, Dumat C. Review of Pb availability and toxicity to plants in relation with metal speciation; role of synthetic and natural organic ligands. J Hazard Mater. 2012;219-220:1–12. doi:10.1016/j.jhazmat.2012.01.060.
- Hayat F, Niaz AS, Bélanger-Gariepy F, et al. Antimony(III) dithiocarbamates: structural studies and exploration of the rare Sb···Sb interaction. Inorg Chem Commun. 2022;146:110148. doi:10.1016/j.inoche.2022.110148.
- Ul Ain N, Aamir A, Khan Y, et al. Catalytic and photocatalytic efficacy of hexagonal CuS nanoplates derived from copper(II) dithiocarbamate. Mater Chem Phys. 2020;242:122408. doi:10.1016/j.matchemphys.2019.122408.
- Imran M, Kondratyuk T, Bélanger-Gariepy F. New ternary platinum(II) dithiocarbamates: synthesis, characterization, anticancer, DNA binding and DNA denaturing studies. Inorg Chem Commun. 2019;103:12–20. doi:10.1016/j.inoche.2019.02.007.
- Imran M, Rehman Z, Hogarth G, et al. Two new monofunctional platinum(II) dithiocarbamate complexes: phenanthriplatin-type axial protection, equatorial-axial conformational isomerism, and anticancer and DNA binding studies. Dalton trans. 2020;49:15385–15396. doi:10.1039/D0DT03018J.
- Bai L, Hu H, Fu W, et al. Synthesis of a novel silica-supported dithiocarbamate adsorbent and its properties for the removal of heavy metal ions. J Hazard Mater. 2011;195:261–275. doi:10.1016/j.jhazmat.2011.08.038.
- Fu H, Lv X, Yang Y, et al. Removal of micro complex copper in aqueous solution with a dithiocarbamate compound. Desalin Water Treat. 2012;39:103–111. doi:10.1080/19443994.2012.669165.
- Li Z. Synthesis of a carbamide-based dithiocarbamate chelator for the removal of heavy metal ions from aqueous solutions. J Ind Eng Chem. 2014;20:586–590. doi:10.1016/j.jiec.2013.05.018.
- Armarego W, Chai C. Purification of laboratory chemicals, butterworth. 5th ed. Oxford: Oxford University Press; 2003.
- Hayat F, Khan MH, Zia-ur-Rehman, Two new heteroleptic ruthenium (II) dithiocarbamates: synthesis, characterization, DFT calculation and DNA binding. J Coord Chem. 2017;70:279–295. doi:10.1080/00958972.2016.1255328.
- Arora A, Arora C. Synthesis of transition metal diethyldithiocarbamates and their effect on nodulation and other growth characters in mungbean, vigna radiata. Asian J Chem. 2003;15:144–150.
- Al-Obaidy GS, Ibraheem KR, Mesher MF. Metal complexes derived from dithiocarbamate ligand: formation, spectral characterization and biological activity. Sys Rev Pharm. 2020;11:360–368. doi:10.31838/srp.2020.6.57.
- Pages A, Casaş J, Sanchez A, et al. J Inorg Biochem. 1985;25:35–42. doi:10.1016/0162-0134(85)83005-1.
- Kane S, Lazo P, Ylli F, et al. Separation of heavy metal from water samples, the study of the synthesis of complex compounds of heavy metal with dithiocarbamates. J Environ Sci Health B, Part A. 2016;51:335–340. doi:10.1080/10934529.2015.1109408.
- Ahmed, S. A. Synthesis and characterization of new ligand of Dithiocarbamate derived from “2-Aminopyridine” with some metal ions. Karbala J Pharm Sci. 2017;12:84–98.
- Liu LK, Cheng TH, Young DS, et al. Trace analysis of heavy metals with two new disodium bisdithiocarbamates. J Chin Chem Soc. 1995;42:773–782. doi:10.1002/jccs.199500104.
- Ayalew ZM, Zhang X, Guo X, et al. Removal of cu, ni and Zn directly from acidic electroplating wastewater by oligo-ethyleneamine dithiocarbamate (OEDTC). Sep Purif Technol. 2020;248:117114. doi:10.1016/j.seppur.2020.117114.
- Zheng Hl, Sun Xp, He Q, et al. Synthesis and trapping properties of dithiocarbamate macromolecule heavy-metal flocculants. J Appl Polym Sci. 2008;110:2461–2466. doi:10.1002/app.28526.
- Nzeneri, JU, Ndukwe, G I, Abayeh, OJ. Synthesis and metal removal efficiency of sodium phenyldithiocarbamate and sodium cyclohexyldithiocarbamate ligands. J. Appl. Chem. 2018;11:72–82. doi:10.9790/5736-1101017282.
- Liu L, Wu J, Li X, Ling Y. Synthesis of poly (dimethyldiallylammonium chloride-co-acrylamide)-graft-triethylenetetramine–dithiocarbamate and its removal performance and mechanism of action towards heavy metal ions. Sep Purif Technol. 2013;103:92–100. doi:10.1016/j.seppur.2012.10.028.
- Kalra, Y. Handbook of reference methods for plant analysis. Boca Raton, FL: Taylor & Francis Group; 1998. p. 85–88.
- Okawara R, Webster DE, Rochow EG. The infrared spectra of the methylacetoxysilanes and some methyltin carboxylates. The configuration of the trimethyltin and the dimethyltin cations. J Am Chem Soc. 1960;82:3287–3290. doi:10.1021/ja01498a013.
- Qin L, Ge Y, Beng B. Poly (ethylene imine) anchored lignin composite for heavy metals capturing in water. J Taiwan Inst Chem Eng. 2017;71:84–90. doi:10.1016/j.jtice.2016.11.012.
- Ali S, Rehman Z, Zia-ur-Rehman, et al. New homobimetallic organotin(IV) dithiocarbamates as potent antileishmanial agents. J Coord Chem. 2014;67:3414–3430. doi:10.1016/j.jece.2018.03.029.
- Houari B, Louhibi S, Tizaoui K, et al. New synthetic material removing heavy metals from aqueous solutions and wastewater. Arab J Chem. 2019;12:5040–5048. doi:10.1016/j.arabjc.2016.11.010.
- Ullah H, Previtali V, Mihigo HB, et al. Structureactivity relationships of new Organotin (IV) anticancer agents and their cytotoxicity profile on HL-60, MCF-7 and HeLa human cancer cell lines. Eur J Med Chem. 2019;181:111544. doi:10.1016/j.ejmech.2019.07.047.
- Ullah H, Twamley B, Waseem A, et al. Tin···Oxygen Tetrel Bonding: A Combined Structural, Spectroscopic, and Computational Study. Cryst Growth Des. 2017;17:4021–4027. doi:10.1021/acs.cgd.7b00678.
- Coucouvanis D The chemistry of the dithioacid and 1,1-dithiolate complexes,1968–1977. Prog Inorg Chem. 1979;26:301–449.
- Amir MK, Hayat F, Khan SZ, et al. Monofunctional platinum (II) dithiocarbamate complexes: synthesis, characterization and anticancer activity. RSC Adv. 2016;6: 110517–110524. doi:10.1039/C6RA19469A.
- Angeloski A, Baker AT, Bhadbhade M, et al. Bis (κ2s, s-di (isopropyl) dithiocarbamato) Nickel (II): Anagostic C–H···Ni interactions and physical properties. J Mol Struct. 2016;1113:127–132. doi:10.1016/j.molstruc.2016.02.028.
- Angeloski A, Gentle AR, Scott JA, et al. From lead (II) dithiocarbamate precursors to a fast response PbS positive temperature coefficient thermistor. Inorg Chem. 2018;57:2132–40. doi:10.1021/acs.inorgchem.7b03009.
- Sainorudin, MH, Sidek, NM, Ismail, N, et al. Synthesis, Characterization and Biological Activity of Organotin (IV) Complexes featuring di-2-ethylhexyldithiocarbamate and N-methylbutyldithiocarbamate as Ligands. J Chem Sci. 2015;2:1–9. doi:10.7603/s40837-015-0002-3.
- Yadav MK, Rajput G, Gupta AN, et al. Exploring the coordinative behaviour and molecular architecture of new PhHg(II)/Hg(II) dithiocarbamate complexes. Inorganica Chim Acta. 2014;421:210–217. doi:10.1016/j.ica.2014.05.031.
- Sharma C, Kumar N, Khandpal M, et al. Studies on the preparation and characterization of bis-dithiocarbamato derivatives of di-n-butyl-and di-n-hexyl Sn (IV). J inorg Nucl. 1981;43:923–930. doi:10.1016/0022-1902(81)80151-0.
- Nabipour H, Ghammamy S, Ashuri S, et al. Synthesis of a new dithiocarbamate compound and study of its biological properties. J Org Chem. 2010;2:75–80. doi:10.1039/C6RA19469A.
- Ramos LA, Cavalheiro ÉTG. Preparation, characterization and thermal decomposition of sodium and potassium salts of dithiocarbamate. Braz J Therm Anal. 2013;2:34–38. doi:10.1016/j.molstruc.2016.02.028.
- Oluwalana AE, Ajibade PA. Synthesis and crystal structures of Pb (II) dithiocarbamates complexes: Precursors for PbS nanophotocatalyst. J Sulphur Chem. 2020;41:182–199. doi:10.1080/17415993.2019.1703986.
- Hayat, F, Shah, SNA., Zia-ur-Rehman, et al. Antimony (III) dithiocarbamates: Crystal structures, supramolecular aggregations, DNA binding, antioxidant and antileishmanial activities. Polyhedron, 2021;194:114909. doi:10.1016/j.poly.2020.114909.
- Kanchi S, Singh P, Bisetty K. Dithiocarbamates as hazardous remediation agent: A critical review on progress in environmental chemistry for inorganic species studies of 20th century. Arab J Chem. 2014;7:11–25. doi:10.1016/j.arabjc.2013.04.026.
- Chen H, Zhao Y, Yang Q, et al. Preparation of poly-ammonium/sodium dithiocarbamate for the efficient removal of chelated heavy metal ions from aqueous environments. J Environ Chem Eng. 2018;6:2344–2354. doi:10.1016/j.jece.2018.03.029.
- Jeragh B, El-Asmy AA. Structure and spectroscopic studies of homo-and heterometallic complexes of adipic acid dihydrazide. Spectrochim Acta A: Mol Biomol Spectrosc. 2014;125:25–35. doi:10.1016/j.saa.2014.01.071.
- Ge Y, Li Z, Xiao D, et al. Sulfonated multi-walled carbon nanotubes for the removal of copper (II) from aqueous solutions. J Ind Eng Chem. 2014;20:1765–1771. doi:10.1016/j.jiec.2013.08.030.
- Ho TL. Hard and soft acids and bases principle in organic chemistry. New York: Academic Press; 2012.
- Usman ARA. The relative adsorption selectivities of Pb, Cu, Zn, Cd and Ni by soils developed on shale in a new valley, in Egypt. Geoderma. 2008;144:334–343. doi:10.1016/j.geoderma.2007.12.004.
- McBride M. Reactions controlling heavy metal solubility in soils. Advances in soil science. New York: Springer; 1989. p. 1–15.
- Huheey JE, Keiter EA, Keiter RL, et al. Inorganic chemistry: principles of structure and reactivity. New York: HarperCollins; 1993.
- Allen HE, Huang CP, Bailey GW, et al. Metal speciation and contamination of soil. Boca Raton, FL: Lewis Publishers; 1995.
- Förstner U. Land contamination by metals: global scope and magnitude of problem. In: Metal speciation and contamination of soil. New York: Lewis Publishers; 1995. p. 1–33.
- Mattigod SV, Parker K, Fryxell GE. Correlation of heavy metal binding capacity of thiol-samms using the misono softness parameter. Inorg Chem Commun. 2006;9:96–98.
- Kang T, Park Y, Yi J. Highly selective adsorption of Pt2+ and Pd2+ using thiol-functionalized mesoporous silica. Ind Eng Chem Res. 2004;43:1478–1484.
- Cheng X, Cheng R, Ou S, et al. Synthesis and adsorption performance of dithiocarbamate-modified glycidyl methacrylate starch. Carbohydr Polym. 2013;96:320–325.
- Wan MW, Kan CC, Ibarra-Buscano S, et al. Comparative adsorption of Cd2+, Cu2+, Ni2+, Pb2+ and Zn2+ in aqueous medium onto chitosan-montmorillionite composite beads. Conference: 5th Cross-Straits Drinking Water Symposium, At Macao, China. 2009.
- Yan P, Ye M, Sun S, et al. Removal performances and mechanisms of action towards ethylenediaminetetraacetic acid nickel (II) salt by dithiocarbamate compounds having different carbon chain lengths. J Clean Prod. 2016;122:308–314. doi:10.1016/j.jclepro.2016.02.037.
- Dai Y, Niu L, Zou J, et al. Preparation of core-shell magnetic Fe3O4@SiO2-dithiocarbamate nanoparticle and its application for the Ni2+, Cu2+ removal. Chin Chem Lett. 2018;29:887–891. doi:10.1016/j.cclet.2017.11.029.
- Maurya VK, Singh RP, Prasad LB. Comparative evaluation of trace heavy metal ions in water sample using complexes of dithioligands by flame atomic absorption spectrometry. Orient J Chem. 2018;34:100. doi:10.13005/ojc/340111.