Abstract
The effects of Dermatocarpon miniatum and Parmelia saxatilis lichens on growth, total phenolic, total flavonoid contents and antioxidant potentials of in vitro medicinal plant Bacopa monnieri were investigated. B. monnieri was treated with different concentrations of methanolic and aqueous extracts of D. miniatum and P. saxatilis lichens. Total phenolic, total flavonoid contents and antioxidant activities of B. monnieri that showed the best growth with lichen application and control plant were investigated. Methanolic extract of allelopathic plants showed strongest DPPH scavenging (IC50: 27.60 mg/L) and metal chelating activities (IC50: 120.29 mg/mL) compared to the control. Also the total phenolic and flavonoid contents of allelopathic samples for both extracts had significantly (p < 0.05) higher compared to the control group. Among the whole experimental group, the highest total phenol (93.71 µg gallic acid equivalent/mg extract) and flavonoid (68.34 µg quercetin equivalent/mg extract) contents were detected in methanolic extract of allelopathic plant.
1. Introduction
Secondary metabolites secreted by different living species, such as plants, fungi, and lichens, exhibit diversity in terms of biological activity [Citation1–3]. These compounds, many of which are used by species for defense, are called allelochemicals. Allelochemicals can directly or indirectly affect living organisms in the environment, either positively or negatively. The interactions between living organisms, influenced by these biochemicals, are defined as allelopathy [Citation4,Citation5].
Allelopathic substances, which are normally present in cultured and wild plants, are released into the soil as water-soluble phototoxic substances from various parts of plants, including roots, stems, leaves, rhizomes, flowers, fruits, and seeds [Citation6]. Consequently, other living organisms absorb the necessary metabolites from the soil into their own structures. In recent years, allelopathy studies have increased with the aim of enhancing yield in plants produced through plant tissue culture. Studies have shown that allelochemicals can have both inhibitory and stimulatory effects, affecting seed germination, seedling growth, and development [Citation7–9].
Bacopa monnieri (L.) Wettst. is an important perennial plant commonly utilized in plant tissue culture studies. B. monnieri, a plant rich in saponins, exhibits sedative, memory-enhancing, antipyretic, pain-relieving, anti-inflammatory, and antiepileptic effects [Citation10,Citation11]. It possesses unique compounds such as bacopasaponin E, bacopasaponin F, bacopaside N1, bacopaside III, bacopaside IV, and bacopaside V [Citation12]. The medicinal importance of B. monnieri, which has been extensively studied in recent years for its analgesic, anti-inflammatory, antidiabetic [Citation13], anticancer [Citation14], antioxidant, and antianxiety [Citation15] properties, continues to increase.
Because of their unique allelochemicals, lichens have very different biological activity. They are symbiotic livings formed by coming together in environments where it is not possible for algae and fungi to live separately. They can compete thanks to the secondary metabolites synthesized by the fungus in their structure [Citation16,Citation17]. These metabolites have been found to possess various allelopathic effects, such as antioxidant, cytotoxic [Citation18], antigenotoxic [Citation19], antibacterial [Citation20], antiviral, antifungal, anti-inflammatory, antiparasitic [Citation21], and insecticide [Citation22]. However, to the best of our knowledge, the allelopathic effects of lichens on plants produced under in vitro conditions have not yet been studied. Therefore, the present study aimed to examine the effects of Dermatocarpon miniatum (L.) W.Mann and Parmelia saxatilis (L.) Ach. lichens on the growth of in vitro propagated B. monnieri plants. Additionally, the effect of P. saxatilis on the changes in total phenolic and total flavonoid contents, as well as antioxidant activities (2,2-diphenyl-1-picrylhydrazyl (DPPH) and metal chelating), of B. monnieri was investigated.
2. Materials and methods
2.1. Extraction of lichens
D. miniatum and P. saxatilis lichen samples (15 g) were pulverized with an ultra-centrifuge grinder (Retsch ZM 200, Germany) and then extracted with methanol and water solvent (250 mL) in a Soxhlet (Thermal, Turkey) device. The extracts were then filtered through a Whatman No. 1 filter paper. The resulting filtrates were concentrated with a rotary evaporator (IKA, Germany) and then lyophilized. At the end of this process, crude extracts were obtained.
2.2. Herbal materials and explant isolation
B. monnieri is a herb that grows naturally in India. The sterile plants used in the study were obtained from Karamanoğlu Mehmetbey University, Kamil Özdağ Faculty of Science, Department of Biology and were identified according to Sosa et al. [Citation23] and Khan et al. [Citation24]. Node explants from sterile and stock plants were isolated in a sterile cabinet and these explants were placed in culture dishes with a nutrient medium.
2.3. Preparation of nutrient media and culture conditions
Murashige and Skoog (MS) mineral salts and vitamins were used as nutrient media in the studies. 0.5 mg/L Benzyl Amino Purine (BAP) and 30 g/L sucrose were added as plant growth regulator in all trials. The studies were carried out by adding 7 g/L agar. After adjusting the pH of the nutrient medium to 5.6-5.8 using 1 N NaOH or 1 N HCl, it was sterilized by keeping it at 121°C under 1.2 atmospheres for 20 min.
2.4. Extraction of B. monnieri
B. monnieri treated with different concentrations of methanolic and aqueous extracts of D. miniatum and P. saxatilis lichens showed its best growth in the application of aqueous extract of P. saxatilis with a concentration of 80 mg/L. Considering these obtained data, B. monnieri samples (15 g) that showed the best growth with lichen application (P. saxatilis with a concentration of 80 mg/L – allelopathic plant) and were not treated with lichen (control plant) (15 g) were pulverized with an ultra-centrifuge grinder (Retsch ZM 200, Germany) and extracted with methanol and water solvents (250 mL) in a Soxhlet device. The extracts were then filtered through a Whatman No. 1 filter paper. The obtained filtrates were concentrated by rotary evaporator (IKA, Germany) and crude extracts were obtained.
2.5. Addition of lichen extracts at different concentrations to medium
Lichen extracts were obtained by using methanol and water solvents. For the addition of lichen extract to the media, lichen extract stock solution was prepared. These extracts were added to the medium at different concentrations. Lichen extracts were added to the medium after sterilization with a filter. It was also established in trials that did not include lichen extracts as a control group. Sterile nutrient media containing lichen extracts at different concentrations were poured into tissue culture containers and explant cultivation was started.
2.6. Acquisition of culture conditions and regeneration data
Cultures were kept in the plant growth cabinet or plant growth room for 16 h in light and 8 h in darkness. The temperature was adjusted to be 24 ± 1°C. Regeneration data were obtained for shoots emerging from explants treated with lichen extracts for certain periods. These data are especially; percentage of regeneration, number of shoots per explant, and average shoot length were determined.
2.7. DPPH scavenging activity
In the measurement of 2,2-diphenyl-1-picrylhydrazil (DPPH) scavenging activity of methanolic and aqueous extracts obtained from allelopathic and control plants, applications were carried out with the final concentrations of the extracts in the plate wells of 12.5, 25, 50, 100, 200, and 400 mg/L (The methanolic and aqueous extracts were dissolved in methanol and water, respectively). Gallic acid was used as the standard antioxidant molecule. According to the method, 20 µL of the extracts were placed in each microplate well, and 180 µL of DPPH (0.06 mM in methanol) was added. Reduction of DPPH free radical was determined by measuring the absorbance values at 517 nm after 60 min in the dark. The free radical scavenging activities of the extracts were calculated as a percentage using the following formula: Radical scavenging activity = [(Control absorbance – Extract absorbance) / (Control absorbance)] × 100.
2.8. Metal chelating activity
In the measurement of the metal chelating activity of methanolic and aqueous extracts obtained from allelopathic and control plants, applications were carried out with the final concentrations of the extracts in the plate wells of 12.5, 25, 50, 100, 200, and 400 mg/L (The methanolic and aqueous extracts were dissolved in methanol and water, respectively). Ethylenediaminetetraacetic acid (EDTA) was used as standard chelating agent. According to the method, 50 µL of the extracts were added to each microplate well. 185 µL of water, 5 µL of FeCl2 (2 mM) and 10 µL of ferrozine (5 mM) were added to them, respectively, and kept at room temperature for 10 min. Spectrophotometric measurements were performed at 562 nm. The metal chelating activities of the extracts were calculated in percent with the following formula: Metal chelating activity = [(Control absorbance – Extract absorbance) / (Control absorbance)] × 100.
2.9. Determination of total phenol content
In determining the total phenol content of methanolic and aqueous extracts obtained from allelopathic and control plants, gallic acid was used as a standard. 20 µL of methanolic and aqueous extracts (400 mg/L) and standard were placed in the microplate wells. 20 µL of Folin reagent (2N) was added and the mixture was incubated in the dark for 3 min. Then, 20 µL of 35% (w/v) sodium carbonate and 140 µL of dH2O were added to them and kept in the dark for 10 min. Spectrophotometric reading was performed at 725 nm. Calculation in gallic acid equivalent (GAE) was performed using the standard calibration curve created with gallic acid. The graph was given as supplementary material (Figure S1a).
2.10. Determination of total flavonoid content
In determining the total flavonoid content of methanolic and aqueous extracts obtained from allelopathic and control plants, quercetin was used as a standard. 50 µL of methanolic and aqueous extracts (400 mg/L) and standard were placed in the microplate wells. Then, 215 μL of ethyl alcohol (80%, v/v), 5 μL of aluminum nitrate (10%, w/v) and 5 μL potassium acetate (1 M) were added in microtiter plates and incubated for 40 min at room temperature. Spectrophotometric reading was performed at 415 nm. Calculation in quercetin equivalent (QE) was performed using the standard calibration curve created with quercetin. The graph was given as supplementary material (Figure S1b).
2.11. Statistical analyses
Plant tissue culture and biochemical activities were analysed one-way ANOVA followed by Duncan test. Probit regression analysis was used to calculate the median inhibitor concentration (IC50) values. All analyses were done using SPSS (version 21.0, IBM Corporation, Armonk, NY, USA).
3. Results
3.1. Yields of the extracts
The yields of primary and secondary extracts were given in Table . The yields were in the ascending order of aqueous extract of D. miniatum < methanolic extract of allelopathic plant < methanolic extract of control plant.
Table 1. Yield (%) of obtained extracts.
3.2. Effect of methanolic and aqueous extracts of D. miniatum on in vitro shoot regeneration of B. monnieri
In this experiment, the impacts of methanolic and aqueous extracts of D. miniatum on shoot regeneration of B. monnieri was investigated (Figure ). In general, 100.0% regenerations were obtained at all lichen concentrations (Figure a).
Figure 1. Effect of methanolic and aqueous extracts of D. miniatum on in vitro shoot regeneration of B. monnieri. The effects of applications of different lichen extracts on (a) shoot regeneration percentage, (b) average number of shoots per explant and (c) shoot length (mean ± standard deviation, n = 3) (Values indicated by different letters differ from each other at the level of p < 0.05).
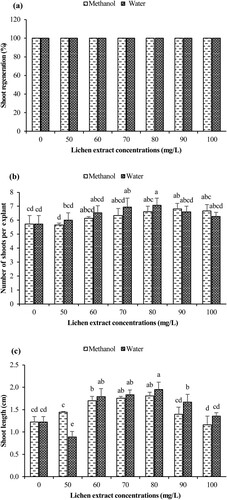
It was determined that methanolic and aqueous extracts of D. miniatum showed significant differences on shoot numbers (p < 0.05; Figure b). In methanolic extract applications, it was observed that the highest shoot number was obtained at 90 mg/L lichen concentration as 6.80 shoots/explant (Figure a), followed by 100 mg/L lichen concentration as 6.67 shoots/explant. When the different aqueous extracts of D. miniatum were examined, the maximum number of shoots (7.07 shoots/explant) was obtained in 80 mg/L lichen extract application (Figure b). The lowest number of shoots in methanolic and aqueous extracts were determined in the control group plants. While aqueous extract applications gave better results up to 80 mg/L, methanolic extracts gave higher results in lichen extract applications higher than 80 mg/L.
Figure 2. Multiple shoot regeneration from nodal explants of B. monnieri treated with methanolic and aqueous extracts of D. miniatum. Regenerated shoots in culture medium supplemented with (a) 90 mg/L methanol extract and (b) 80 mg/L aqueous extract, after eight weeks of culture.

The methanolic and aqueous extracts of D. miniatum added to the culture medium significantly affected shoot length values of B. monnieri (p < 0.05; Figure c). In methanolic extract applications, the highest length value was obtained at 80 mg/L lichen concentration (1.81 cm), while the shortest shoots were recorded at 100 mg/L lichen concentration (1.17 cm). On the other hand, in aqueous extract applications, the longest shoot was obtained as 1.95 cm in 80 mg/L lichen concentration and the shortest shoot was obtained as 0.89 cm in 50 mg/L lichen concentration. Aqueous extracts showed better results than methanolic extracts in all other applications except 50 mg/L lichen concentration.
3.3. Effect of methanolic and aqueous extracts of P. saxatilis on in vitro shoot regeneration of B. monnieri
In this experiment, the in vitro propagation of B. monnieri in culture medium containing different methanolic and aqueous extracts of P. saxatilis was investigated (Figure ). In both lichen extracts, shoot regeneration percentages were generally high and the values were statistically significant (p < 0.05; Figure a). In methanolic extract applications, 100.0% shoot regenerations were obtained at 60-90 mg/L extract concentrations. In addition, 100.0% regeneration values were recorded in the control group. Regeneration value of 86.7% was determined at 100 mg/L extract concentrations. In the aqueous extract of P. saxatilis, 100.0% regeneration was achieved at all concentrations except the 60 and 100 mg/L concentrations. The lowest shoot regeneration value (86.7%) was determined in 60 mg/L extract application.
Figure 3. Effect of methanolic and aqueous extracts of P. saxatilis on in vitro shoot regeneration of B. monnieri. The effects of applications of different lichen extracts on (a) shoot regeneration percentage, (b) average number of shoots per explant and (c) shoot length (mean ± standard deviation, n = 3) (Values indicated by different letters differ from each other at the level of p < 0.05).
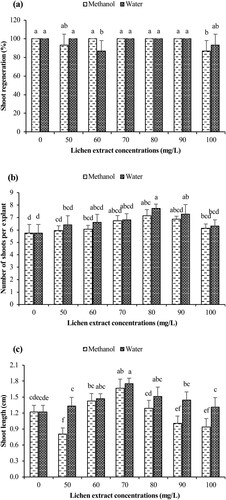
The effects of methanolic and aqueous extracts of P. saxatilis on shoot numbers of B. monnieri were investigated (Figure b). In methanolic extract application, the highest number of shoots per explant (7.13 shoots/explant) was obtained in 80 mg/L extract application (Figure a) and showed statistically significant differences compared to the control group (p < 0.05). In general, fewer shoots were obtained at low concentrations of methanolic extracts. The least number of shoots was obtained in the control group with 5.73 shoots/explant, followed by 50 mg/L methanolic extract application with 5.95 shoots/explant.
Figure 4. Multiple shoot regeneration from nodal explants of B. monnieri treated with methanolic and aqueous extracts of P. saxatilis. Regenerated shoots in culture medium supplemented with (a) 80 mg/L methanolic extract and (b) 80 mg/L aqueous extract, after eight weeks of culture.

On the other hand, the best results in aqueous extract applications were determined in MS nutrient medium treated with 80 mg/L extract concentration (7.73 shoots/explant) (Figure b), and showed significant differences at p < 0.05 level compared to the results of the control group (Figure b). In applications containing aqueous extract, the least number of shoots was determined in plants in MS nutrient medium containing 100 mg/L aqueous extract (6.32 shoots/explant). In general, when both extracts were compared, aqueous extract application gave better results than methanolic extracts in terms of shoot numbers.
The effects of methanolic and aqueous extracts of P. saxatilis on shoot lengths of B. monnieri were evaluated and the results were found to be statistically significant (p < 0.05; Figure c). Maximum shoot lengths in methanolic extract were obtained in 70 mg/L extract application (1.67 cm), followed by 60 mg/L extract application (1.43 cm). The shortest shoot length was determined at 50 mg/L extract concentration (0.80 cm). In aqueous extract applications, the longest shoot was determined in 70 mg/L extract application (1.75 cm), followed by 70 mg/L extract application (1.51 cm). The shortest shoot was determined in 100 mg/L extract application (1.31 cm). When both lichen extracts were examined, the height of the regenerated plants in the nutrient medium treated with the aqueous extract was longer than the methanolic extract.
3.4. DPPH scavenging activities of the extracts
In DPPH scavenging activities of methanolic and aqueous extracts obtained from allelopathic and control plants, all applications of both extracts showed concentration-dependent activity. As the concentration increased the DPPH scavenging activity of the extracts increased. Considering the trials with methanolic extract, allelopathic plants showed stronger activities compared to the control groups. Applications of allelopathic samples with a concentration of 100-400 mg/L showed DPPH scavenging activity in the range of 85.5-86.0%. There was no statistically (p > 0.05) difference between these mentioned data. In addition, all concentrations of allelopathic plants had a significantly (p < 0.05) higher DPPH scavenging activity potential compared to the control group. When the trials with aqueous extract were examined, the allelopathic plant had higher activity compared to the control group. The experiment with 400 mg/L concentration significantly increased the DPPH scavenging activity (p < 0.05) compared to all other treatments with a rate of 89.8% (Figure a). Considering the IC50 values revealed to determine the effectiveness of different concentrations of the applications, the methanolic extract of the allelopathic plant emerged as the most effective application with a value of 27.60 mg/L (Table ).
Figure 5. (a) DPPH radical scavenging and (b) metal chelating activities of different extracts from allelopathic and control plants (mean ± standard deviation, n = 3).
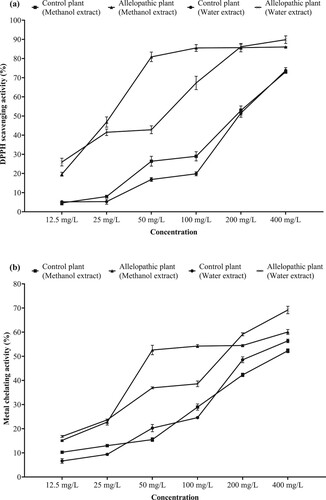
Table 2. IC50 values (mg/L) of gallic acid and extracts obtained from control and allelopathic plants for scavenging on DPPH radicals.
3.5. Ferrous chelating activities of the extracts
The ferrous chelating activities of methanolic and aqueous extracts obtained from allelopathic and control plants, all applications of both extracts showed concentration-dependent activity. Considering the trials with methanolic extract, it was determined that allelopathic plants had higher activities compared to the control groups. Among allelopathic samples, the application with 400 mg/L concentration showed significantly (p < 0.05) higher activity in comparison to the control and other applications in its group with 60.0%. Again, in allelopathic samples, while 100 and 200 mg/L trials showed approximately 54.0% metal chelating activity, the difference between these data was statistically indifferent (p > 0.05). Also, all concentrations of allelopathic samples had a significantly (p < 0.05) higher metal chelating activity potential compared to the control group. When the trials with aqueous extract were examined, compared to the control group, the allelopathic plant had higher activity. In the allelopathic sample, the experiment with 400 mg/L concentration significantly (p < 0.05) increased the metal chelating activity compared to all other treatments, including methanolic extracts, at a rate of 69.2% (Figure b). Considering the calculated IC50 values, the lowest value (120.29 mg/L) belonged to methanolic extract of the allelopathic plant (Table ).
Table 3. IC50 values (mg/L) of EDTA and of extracts obtained from control and allelopathic plants for chelating on ferrous ions.
3.6. Total phenolic and flavonoid contents
When the total phenol and flavonoid contents were examined, the allelopathic samples had significantly (p < 0.05) higher rates for both extracts compared to the control groups. In the whole experimental group, while the highest total phenol (93.71 µg GAE/mg extract) and flavonoid (68.34 µg QE/mg extract) contents were in the methanolic extract of the allelopathic plant, the lowest values (total phenol: 22.92 µg GAE/mg extract, total flavonoid: 2.51 µg QE/mg extract) were determined in the aqueous extract of the control plant (Table ).
Table 4. Total phenol and total flavonoid contents (µg/mg - mean ± standard deviation, n = 3) of extracts obtained from control and allelopathic plants.
4. Discussion
Plant tissue culture has immense importance in plant science, agriculture, and biotechnology. It offers the ability to propagate plants on a large scale, modify plant traits through genetic transformation, conserve plant diversity, produce valuable compounds, study plant diseases, and advance our understanding of plant biology. These applications contribute significantly to crop improvement, sustainable agriculture, and the development of novel plant-based products [Citation25,Citation26].
Multiproduction of important medicinal plants, such as B. monnieri, through tissue culture techniques, is crucial in terms of time and efficiency. B. monnieri has long been used as a nootropic drug, facilitating learning, improving memory and motivation, and reducing anxiety and epilepsy. It is often referred to as a brain tonic. Moreover, it finds application in common conditions like insomnia, stress, and asthma. B. monnieri contains a rich array of biochemical compounds, including saponins, polyphenols, sulfhydryl-derived compounds, bacoside A and B, bacopasides II, IV, and V, betulic acid, and bacosaponin A, B, C, D, E, and F [Citation27]. Numerous studies have explored its antioxidant capacity, ability to prevent DNA damage, anticancer activities, and its effect in reducing edema. The bioactivities of the phytochemicals found in the B. monnieri plant cover a wide range of research topics in human health studies [Citation28]. B. monnieri is a medicinal plant that serves as a valuable source for secondary metabolite production. Therefore, a study was conducted to enhance its production through somatic embryogenesis and subsequent plant regeneration. The highest callus development from leaf explants was achieved using naphthalene acetic acid, while the highest callus formation in nodal explants was obtained through 2,4-dichlorophenoxyacetic acid hormone [Citation29].
While most studies have traditionally relied on serial and multiple production procedures using synthetic hormones, the current study presents a different perspective by exploring natural alternatives with lichens that contain natural components. The lichens D. miniatum and P. saxatilis, utilized in the present study, also possess specific bioactive components [Citation30]. Both aqueous and methanolic extracts of D. miniatum and P. saxatilis lichens exhibited a positive effect on the overall growth of B. monnieri. Consequently, these lichens have the potential to serve as additional stimulating components that support tissue culture studies. Biostimulants refer to substances, microorganisms, or materials that promote plant growth [Citation31]. Many researchers have reported the use of various extracts to enhance plant growth and development [Citation32,Citation33]. In this study, we investigated the effects of aqueous and methanolic extracts of D. miniatum and P. saxatilis on in vitro shoot regeneration of B. monnieri. Overall, the best results in terms of shoot number and shoot length were obtained with the aqueous extracts for both lichen species. These findings indicate that the aqueous extracts contain compounds or substances that are more conducive to the in vitro growth of B. monnieri, which may include macronutrients, micronutrients, and growth regulators. Similarly, it has been reported that seaweed extracts can serve as valuable additives to improve tissue culture studies due to their high levels of macronutrients, micronutrients [Citation34] and growth regulators [Citation33–35].
Lichens and plants share the same environment, and as a result, allelopathic effects can be observed between them. Epiphytic lichens decompose cellulose and pectin materials, which comprise the plant cell wall, in order to attach themselves to the plant as host species. This process causes damage to the surrounding plant tissues, creating opportunities for plant-damaging insects to lay eggs and exert negative allelopathic effects [Citation36]. The interactions between lichens and plants have primarily been studied in vitro. For instance, extracts obtained from the lichen Cladonia foliacea have been found to induce changes in the growth of mosses. While most aqueous extractions from Peltigera canina and a few Cladonia species had a negative effect on the growth of gymnosperm plant species, a few had a positive effect on their growth [Citation37]. Lichens contain aliphatic and aromatic compounds as well as polysaccharides, all of which possess wide allelopathic potential. Some of these effects include cytotoxicity [Citation18,Citation38], genotoxicity [Citation39,Citation40], antimicrobial activity [Citation41,Citation42], anti-inflammatory properties [Citation43,Citation44] and antiviral activity [Citation43,Citation45].
Lichens, known for containing phenolic compounds in their structure, exhibit varying antioxidant capacities. It is well-known that the P. saxatilis used in our research contains different phenolic compounds, which have been found to enhance the antioxidant capacity of human lymphocytes. In related studies, when comparing extracts obtained from this species, the methanolic extract exhibited a higher proportion of phenolic compounds compared to the aqueous extract [Citation30]. Similarly, in our study, the methanolic extract demonstrated higher total phenol and flavonoid contents compared to the aqueous extract. Additionally, the methanolic extract exhibited a higher allelopathic potential among the plant extracts. In another study, acetone extracts of P. saxatilis were eluted using high-performance liquid chromatography (HPLC), and the highest peaks were attributed to the depsidone salazinic acid. The predominant phenolic compounds in these extracts exhibited activities such as free radical scavenging, superoxide anion radical scavenging, and reducing power [Citation46]. A study comparing three species of the genus Parmelia found that the acetone extract of P. saxatilis had the highest total phenol and flavonoid contents, as well as the strongest DPPH scavenging activity [Citation47]. Another study using methanolic and aqueous extracts of P. saxatilis found that the methanolic extract exhibited a higher inhibition of linoleic acid peroxidation and greater reducing power activity [Citation48]. These studies in the literature on P. saxatilis support the results of our present study. In other words, although both extracts demonstrated antioxidant potential, the methanolic extract exhibited stronger antioxidant activity compared to the aqueous extract. Furthermore, the in vitro propagated B. monnieri plant treated with the methanolic extract of P. saxatilis lichen exhibited an increased antioxidant capacity compared to the control plant.
In various studies in the literature, results regarding the DPPH scavenging activities and antioxidant compounds of B. monnieri have been reported. The DPPH scavenging activities of B. monnieri plants collected from different regions were examined, and the lowest IC50 value found was 130.6 mg/L [Citation49]. With the methanolic extract obtained from our allelopathic B. monnieri, we were able to significantly reduce the IC50 value to 27.60 mg/L. Similarly, in the same study conducted by Martínez-García et al. [Citation49], the highest total phenolic content of B. monnieri was reported to be 70.3 µg GAE/mg extract, whereas we determined a total phenolic content of 93.71 µg GAE/mg extract in our allelopathic plant with the methanolic extract. In another study by Largia et al. [Citation50]. working with germplasm lines of B. monnieri, the highest total phenolic content was detected as 56.62 µg GAE/mg extract, while we determined these ratios as 56.70 and 93.71 µg GAE/mg extract in the methanolic and aqueous extracts of our allelopathic plants, respectively. Similarly, Phulara et al. [Citation51]. reported a total phenolic content of 57.54 µg GAE/mg extract in the aqueous extract of B. monnieri and an IC50 value for DPPH scavenging activity of 336 mg/L. In a study by Mathew et al. [Citation52], the IC50 value of the methanolic B. monnieri extract for DPPH scavenging activity was 115 mg/L, and they reported a total phenolic content of 8.8 µg GAE/mg extract. Another research using ethanol extract of B. monnieri showed a lower IC50 value (79.84 mg/L) [Citation53]. Nevertheless, the results of our study with allelopathic plants appear to be more effective. In two previous studies, the total phenolic contents of methanolic B. monnieri extracts were estimated to be 261.16 mg/g of ascorbic acid equivalent [Citation54] and 27 µg GAE/mg extract [Citation55], with IC50 values of 456.07 mg/L [Citation54] and 104.82 mg/L [Citation55] in the DPPH method.
5. Conclusions
During the in vitro propagation of B. monnieri, which has medical importance, lichens D. miniatum and P. saxatilis, especially P. saxatilis, showed a high growth regulatory effect on the plant, contributing to an increase in yield. Furthermore, the stronger antioxidant capacity of allelopathic B. monnieri samples compared to the control group revealed the allelopathic capacity of P. saxatilis lichen, possibly due to certain compounds in its aqueous extracts having plant growth and development-promoting properties. As a result, this study will contribute to further research in the field of plant growth regulators and the increase of bioactive component yields of plants, particularly through the use of P. saxatilis lichen.
Supplemental Material
Download MS Word (20.2 KB)Disclosure statement
No potential conflict of interest was reported by the author(s).
Additional information
Funding
References
- Mambe FT, Voukeng IK, Beng VP, et al. Antibacterial activities of methanol extracts from Alchornea cordifolia and four other Cameroonian plants against MDR phenotypes. J Taibah Univ Med Sci. 2016;11(2):121–127.
- Tripathi AH, Negi N, Gahtori R, et al. A review of anti-cancer and related properties of lichen-extracts and metabolites. Anticancer Agents Med Chem. 2022;22(1):115–142.
- Huang Q, Wang Y, Wu H, et al. Xanthone glucosides: isolation, bioactivity and synthesis. Molecules. 2021;26(18):5575.
- Singh R, Upadhyay SK, Singh BJ, et al. Allelopathic effect of eucalyptus (Eucalyptus camaldulensis Dehnh) on the growth of Aloe vera. Plant Cell Biotechnol Mol Biol. 2021;22(21–22):94–100.
- Xia Z-C, Kong C-H, Chen L-C, et al. A broadleaf species enhances an autotoxic conifers growth through belowground chemical interactions. Ecology. 2016;97(9):2283–2292.
- Shi H, Sun S, Liu X, et al. Allelopathic potential and mechanism of rosebay willowherb [Chamaenerion angustifolium (L.) Scop.] demonstrated on model plant lettuce. Phyton (B Aires). 2021;90(1):159–170.
- Wang R, Miao Y, Kang C, et al. Degradation of phenolic acids and relief of consecutive monoculture obstacle of Rehmannia glutinosa by the combination of Bacillu ssp. and Pichia pastoris. Pakistan J Bot. 2017;49(5):1965–1969.
- Barrales-Cureño HJ, Herrera-Cabrera BE, Montiel-Montoya J, et al. Metabolomics studies of allelopathy: a review. Rev Colomb Ciencias Quim. 2022;51(1):243–274.
- Popa VI. Natural polyphenols as compounds with biological activity. Stud Univ Babes-Bolyai Chem. 2021;66(3):89–96.
- Pandey SK, Jangra MK, Yadav AK. Evaluation of anticonvulsant activity of synthetic and herbal drug combination. Res J Pharm Biol Chem Sci. 2015;6(2):1101–1107.
- De K, Chandra S, Misra M. Assessment of the effect of Bacopa monnieri (L.) Wettst. extract on the labeling of blood elements with technetium-99 m and on the morphology of red blood cells. Rev Bras Farmacogn. 2009;19(3):664–671.
- Pawar SS, Jadhav MG. Determination and quantification of bacoside a from Bacopa monnieri L. by high performance thin layer chromatography. Int J Pharmacogn Phytochem Res. 2015;7(5):1060–1065.
- Faisal S, Jan H, Abdullah IA, et al. In vivo analgesic, anti-inflammatory, and anti-diabetic screening of Bacopa monnieri-synthesized copper oxide nanoparticles. ACS Omega. 2022;7(5):4071–4082.
- Ghosh S, Khanam R, Acharya Chowdhury A. The evolving roles of Bacopa monnieri as potential anti-cancer agent: a review. Nutr Cancer. 2021;73(11–12):2166–2176.
- Nancy KJ, Bansal Y, et al. WHO guidelines in practice in India: stability studies on selected CNS active herbal products through physicochemical, chromatographic and biological evaluations. Curr Tradit Med. 2020;6(4):360–379.
- Molnár K, Farkas E. Current results on biological activities of lichen secondary metabolites: a review. Zeitschrift fur Naturforsch - Sect C J Biosci. 2010;65(3–4):157–173.
- Kalra R, Conlan XA, Goel M. Lichen allelopathy: a new hope for limiting chemical herbicide and pesticide use. Biocontrol Sci Technol. 2021;31(8):773–796.
- Kocovic A, Jeremic J, Bradic J, et al. Phytochemical analysis, antioxidant, antimicrobial, and cytotoxic activity of different extracts of Xanthoparmelia stenophylla lichen from Stara Planina, Serbia. Plants. 2022;11(13):1624.
- Emsen B. The antioxidant and antigenotoxic potential of Peltigera canina and Umbilicaria nylanderiana based on their phenolic profile. Farmacia. 2019;67(5):912–921.
- Türkmenoğlu I, Toksöz O, Berber D, et al. Antibacterial properties of several lichen extracts against two moderately halophilic bacteria from salted sheepskins. J Am Leather Chem Assoc. 2022;117(4):153–163.
- Araújo HDA de, Silva HAMF, Silva Júnior JG da, et al. The natural compound hydrophobic usnic acid and hydrophilic potassium usnate derivative: applications and comparisons. Molecules. 2021;26(19):5995.
- Emsen B, Yildirim E, Aslan A, et al. Insecticidal effect of the extracts of Cladonia foliacea (Huds.) Willd. and Flavoparmelia caperata (L.) Hale against adults of the grain weevil, Sitophilus granarius (L.) (Coleoptera: Curculionidae). Egypt J Biol Pest Control. 2012;22(2):145–149.
- Sosa MM, Moroni P, O’Leary N. A taxonomic revision of the genus Bacopa (Gratioleae, Plantaginaceae) in Argentina. Phytotaxa. 2018;336(1):1–27.
- Khan NS, Chaurasia B, Dixit AK. Pharmacognostic characterization for taxonomic identification of Bacopa monnieri (L.). Wettst. for Quality Control. Int J Lifescience Pharma Res. 2021;11(1):L54–L62.
- Gairola S, Al Shaer KI, Al Harthi EK, et al. Strengthening desert plant biotechnology research in the United Arab Emirates: a viewpoint. Physiol Mol Biol Plants. 2018;24(4):521–533.
- Daldoul S, Boubakri H, Gargouri M, et al. Recent advances in biotechnological studies on wild grapevines as valuable resistance sources for smart viticulture. Mol Biol Rep. 2020;47(4):3141–3153.
- Bhandari P, Sendri N, Devidas SB. Dammarane triterpenoid glycosides in Bacopa monnieri: a review on chemical diversity and bioactivity. Phytochemistry. 2020;172:112276.
- Russo A, Borrelli F. Bacopa monniera, a reputed nootropic plant: an overview. Phytomedicine. 2005;12(4):305–317.
- Ali D, Alarifi S, Pandian A. Somatic embryogenesis and in vitro plant regeneration of Bacopa monnieri (Linn.) Wettst., a potential medicinal water hyssop plant. Saudi J Biol Sci. 2021;28(1):353–359.
- Emsen B, Kolukisa AL. Cytogenetic and oxidative effects of three lichen extracts on human peripheral lymphocytes. Zeitschrift fur Naturforsch Sect C-A J Biosci. 2021;76(7–8):291–299.
- Brown P, Saa S. Biostimulants in agriculture. Front Plant Sci. 2015;6:671.
- Vinoth S, Gurusaravanan P, Sivakumar S, et al. Influence of seaweed extracts and plant growth regulators on in vitro regeneration of Lycopersicon esculentum from leaf explant. J Appl Phycol. 2019;31(3):2039–2052.
- Faize M, Faize L, Burgos L, et al. Application of Ascophyllum nodosum-based soluble extract on micropropagation and regeneration of Nicotiana benthamiana and Prunus domestica. Plants. 2021;10(7):1354.
- Khan W, Rayirath UP, Subramanian S, et al. Seaweed extracts as biostimulants of plant growth and development. J Plant Growth Regul. 2009;28(4):386–399.
- Stirk WA, Arthur GD, Lourens AF, et al. Changes in cytokinin and auxin concentrations in seaweed concentrates when stored at an elevated temperature. J Appl Phycol. 2004;16(1):31–39.
- Robertson JA, Bradler S, Whiting MF. Evolution of oviposition techniques in stick and leaf insects (Phasmatodea). Front Ecol Evol. 2018;6:216.
- Favero-Longo SE, Piervittori R. Lichen-plant interactions. J Plant Interact. 2010;5(3):163–177.
- Šeklić DS, Jovanović MM. Platismatia glauca-lichen species with suppressive properties on migration and invasiveness of two different colorectal carcinoma cell lines. J Food Biochem. 2022;46(7):e14096.
- Emsen B, Togar B, Turkez H, et al. Effects of two lichen acids isolated from Pseudevernia furfuracea (L.) Zopf in cultured human lymphocytes. Zeitschrift fur Naturforsch Sect C-A J Biosci. 2018;73(7–8):303–312.
- Prokopiev I, Filippova G, Filippov E, et al. Genotoxicity of (+)-and (−)-usnic acid in mice. Mutat Res Toxicol Environ Mutagen. 2019;839:36–39.
- Zorrilla JG, D’Addabbo T, Roscetto E, et al. Antibiotic and nematocidal metabolites from two lichen species collected on the Island of Lampedusa (Sicily). Int J Mol Sci. 2022;23(15):8471.
- Ureña-Vacas I, González-Burgos E, Divakar PK, et al. Lichen depsidones with biological interest. Planta Med. 2022;88(11):855–880.
- Mendili M, Khadhri A, Mediouni-Ben Jemâa J, et al. Anti-inflammatory potential of compounds isolated from Tunisian lichens species. Chem Biodivers. 2022;19(8):e202200134.
- Tartouga MA, Elouar I, Zeghina I, et al. The evaluation of antioxidant and anti-inflammatory activities of Parmotrema hypotropa lichen extract. Egypt J Chem. 2022;65(9):109–118.
- Do T-H, Duong T-H, Nguyen HT, et al. Biological activities of lichen-derived monoaromatic compounds. Molecules. 2022;27(9):2871.
- Manojlović N, Ranković B, Kosanić M, et al. Chemical composition of three Parmelia lichens and antioxidant, antimicrobial and cytotoxic activities of some their major metabolites. Phytomedicine. 2012;19(13):1166–1172.
- Kosanić MM, Ranković BR, Stanojković TP. Antioxidant, antimicrobial and anticancer activities of three Parmelia species. J Sci Food Agric. 2012;92(9):1909–1916.
- Özen T, Kinalioğlu K. Determination of antioxidant activity of various extracts of Parmelia saxatilis. Biologia (Bratisl). 2008;63(2):211–216.
- Martínez-García M, Garduño-Solórzano G, Lopes G, et al. Antioxidant, anti-inflammatory and anti-obesity potential of extracts containing phenols, chlorophyll and carotenoids from Mexican wild populations of Bacopa monnieri (L.) Wettst. Biology (Basel). 2023;12(4):620.
- Largia MJV, Shilpha J, Pothiraj G, et al. Analysis of nuclear DNA content, genetic stability, bacoside a quantity and antioxidant potential of long term in vitro grown germplasm lines of Bacopa monnieri (L.). Plant Cell Tissue Organ Cult. 2015;120(1):399–406.
- Phulara SC, Shukla V, Tiwari S, et al. Bacopa monnieri promotes longevity in Caenorhabditis elegans under stress conditions. Pharmacogn Mag. 2015;11(42):410–416.
- Mathew M, Subramanian S. In vitro screening for anti-cholinesterase and antioxidant activity of methanolic extracts of ayurvedic medicinal plants used for cognitive disorders. PLoS One. 2014;9(1):e86804.
- Biswas SK, Das J, Chowdhury A, et al. Evaluation of antinociceptive and antioxidant activities of whole plant extract of Bacopa monniera. Res J Med Plant. 2012;6(8):607–614.
- Alam MN, Wahed TB, Sultana F, et al. In vitro antioxidant potential of the methanolic extract of Bacopa monnieri L. Turkish J Pharm Sci. 2012;9(3):285–292.
- Volluri SS, Bammidi SR, Chippada SC, et al. In-vitro antioxidant activity and estimation of total phenolic content in methanolic extract of Bacopa monniera. Rasayan J Chem. 2011;4(2):381–386.
