?Mathematical formulae have been encoded as MathML and are displayed in this HTML version using MathJax in order to improve their display. Uncheck the box to turn MathJax off. This feature requires Javascript. Click on a formula to zoom.
?Mathematical formulae have been encoded as MathML and are displayed in this HTML version using MathJax in order to improve their display. Uncheck the box to turn MathJax off. This feature requires Javascript. Click on a formula to zoom.ABSTRACT
Tuberculosis is a global health concern n impacting communities, health systems, and economies This study assessed the TB treatment outcomes among individuals aged 15+ at Chawama first level hospital in Lusaka, Zambia, using a retrospective design focussing on individuals notified in 2020. The sample was described using descriptive statistics. The Pearson Chi-square test and logistics regression were used to analyse the characteristics of the patients influencing the treatment outcomes at 5% significant level. Out of 404 participants, 83.4% of them had successful treatment outcomes. Varied outcomes were noted in sex, patient type, TB type, HIV status, and DOT plan, but lacked significance. Odds of success were lower by 72.4% for those aged 65+ compared to those aged 15–24 years (OR (95% CI): 0.276 (0.086–0.881), p = .030). Similarly, after adjusting for other variables, the odds of success were lower by 72.9% (AOR (95% CI): 0.271 (0.083–0.882), p = .030). This study yielded an encouraging 83.4% TB success rate highlighting the potential for improvement to meet WHO targets. Notably, individuals aged 65+ showed a distinct pattern with lower treatment success odds, suggesting a need for focussed interventions. Special attention to elderly patients and targeted TB program interventions are recommended.
Introduction
Tuberculosis (TB) is a serious worldwide health issue, wreaking havoc on communities, health systems, and economies across the world (Uplekar & Atre, Citation2021). According to the World Health Organization (WHO), tuberculosis (TB) was ranked as the thirteenth biggest cause of death and the world's second most infectious killer, trailing only corona virus illness (COVID-19) (WHO, Citation2023a). The World Health Organization reported that tuberculosis was present in all nations and age categories and estimated that in 2021, 10.6 million individuals were diagnosed with tuberculosis globally, with males accounting for the majority (6 million), followed by 3.4 million women and 1.2 million children. In the same year, estimated that, 1.6 million people died from tuberculosis in 2021, which included about 187,000 who were coinfected with HIV (WHO, Citation2023a). Despite substantial progress in diagnosing and treating TB, the disease continues to exerct a significant burden on individuals and societies. As the global health landscape evolves, understanding the intricate interplay of factors affecting TB treatment outcomes becomes essential for effective management and control strategies (Shete & Kahn, Citation2019).
The African continent is disproportionately affected with TB, having the highest burden of the disease (Obadire et al., Citation2022). In 2016, it was estimated 2.5 million people were diagnosed with TB in Africa and over 25% of TB deaths occur in Africa (WHO, Citation2023b). Being a country in Africa, particularly, the sub-Saharan region, Zambia stands as a microcosm of these challenges (Nanzaluka et al., Citation2019). In 2019, the country had a estimated burden of 59 thousand TB cases and only 61 percent of them were estimated to have been identified and treated (Lungu et al., Citation2022). In this case, with the help from partners, the government of the Republic of Zambia (GRZ) has implemented numerous interventions to prevent and combat TB, which include; TPT implementation, TB/HIV care delivery integration, TB public-private collaboration, TB infection control at the facility level, TB case management, supportive active TB case finding, strengthening TB data management and research, expanding access to more creative and sensitive diagnostic techniques such as GeneXpert, LAM, and Chest x-ray, prioritising supporting TB initiatives in correctional institutions (MOH, Citation2016).
Despite these steps to prevent tuberculosis, Zambia still has a big issue with the illness, since it has been a leading source of illness and mortality in the country, particularly among HIV-positive people (PLHIV) (Lungu et al., Citation2022). Hence, this study aims to assess the TB treatment outcomes and the associated factors among people aged 15 years and older who were treated at Chawama Level One Hospital in Lusaka District. As such, investigating the factors influencing TB treatment outcomes at a specific healthcare institution, such as Chawama Level One Hospital, offers insights that can inform targeted interventions and contribute to the advancement of TB control strategies both within Zambia and in similar contexts.
Methods and material
Study setting and period
This study was carried out at Chawama Level One Hospital in Lusaka District which is a public health institution that belong to the Government of the Republic of Zambia (GRZ). The study took place from January to September 2022 and the information gathered comprised demographics, clinical factors, and the treatment outcome for TB among people who were notified with TB in 2020.
Study design and population
This study used a retrospective cross-sectional design. This design was adopted for this study because it allowed the researcher(s) to collect and analyse data at a single point in time from past records and or events (Hutchinson et al., Citation2023). Therefore, this study design was appropriate to analyse the treatment outcomes of TB and the related characteristics in persons notified with TB in 2020 aged 15 and older who received treatment for TB at Chawama Level One Hospital. The study's population included patients who were notified with tuberculosis and commenced on treatment at Chawama Level One Hospital.
Eligibility criteria
Inclusion criteria
This study included individuals diagnosed with active tuberculosis and registered in the TB treatment register at Chawama Level One Hospital in Lusaka. Furthermore, the study included the participants who were notified in 2020 only and were aged 15 years and above. Participants coinfected with HIV were also included in the study as it is reported in the TB treatment registers.
Exclusion criteria
The participants who were not notified at Chawama Level One Hospital, aged less than 15 years were excluded from the study. Secondly, participants with missing or partial data in the TB treatment register were excluded from the study. Patients with Multi Drug Resistant (MDR) TB were also excluded from the research. This study did not incorporate additional comorbidities due to their absence in the TB treatment registers.
Sampling procedure and sample size determination
Sampling procedure
The subjects in this study were randomly selected as a subset of patients from the entire population of TB patients treated at Chawama Level One Hospital in 2020. This study's sample comprised tuberculosis patients (all forms) which included clinically diagnosed pulmonary tuberculosis (PTB), pulmonary bacteriologically confirmed tuberculosis (BTB), and extrapulmonary, bacteriologically confirmed or clinically diagnosed tuberculosis (EPTB).
Sample size determination
The minimum sample for this study was estimated using Cochran's sample size formula (Uakarn et al., Citation2021) as seen in Equation (1).
where;
Equation (1). The Cochrane formula used to calculate sample size.
In this regard, the minimal sample size for this study was established based on a limited population of 2,025 TB notifications (all forms) (Data produced from the national HMIS) reported in 2020 by Chawama Level One Hospital.
Equation (2). Calculated minimum sample size
Variables of the study
The characteristics needed for this investigation were gathered from TB treatment register(s). The explanatory variables included social demographics (which included; sex and age) and clinical characteristics (which included; patient type, TB type, HIV status and DOT plan). The dependant variable was labelled as a binary variable and had treatment success (which included; treatment completed and cured) and unsuccessful treatment outcomes (which included death, LTFU, failure and not evaluated).
Definition of terms
In this study, the key terms were defined in accordance to the World Health Organization definitions and reporting framework for tuberculosis (Meseret Tadele et al., Citation2022; Tok et al., Citation2020; WHO, Citation2013) as shown in .
Table 1. Definition of key terms.
TB case classifications were also defined according to WHO (Citation2013). Thus, new patients were those who had never been treated for tuberculosis or had taken anti-TB drugs for less than one month, whereas previously treated patients were defined as relapsed depending on the outcome of their most recent course of therapy. Those who had been cured or had completed therapy were classified as recurrence patients, those who did not respond to treatment were labelled as treatment after failure patients., and those who were lost to follow-up were classified as treatment after LTFU (WHO, Citation2013).
Data sources and sampling frame
The data source for this study was the tuberculosis treatment registers which is used at the health facility to track the patients on treatment throughout their treatment period. In this regard the sampling frame will be the list of all TB patients that were started on TB treatment in 2020.
Data analysis
Using frequency tables and contingency tables, all of the data were categorised and variables were summarised. In a contingency table, the Pearson Chi-square test was used to examine the relationship between the response variables and the independent factors. Logistics regression was used to explore the relationship between patients’ demographic and clinical factors and TB treatment outcomes. All analyses were performed with a 95% confidence level, and a p-value of ≤ 0.05 was considered statistically significant.
Data management and quality
Microsoft Excel was used to store and handle the obtained data. The personal computer used to gather, handle, and analyse data was password protected, and a backup file was stored on the researchers’ Google Drive account which was password protected. The researcher was the only one who had access to the data. In order to ascertain quality in the data, the following aspects were checked: correctness, the collected data was verified reviewed for correctness and completeness across all variables, respectively. The data was also checked for consistency on all variables according to the requirements specified on the TB treatment register.
Ethical consideration
Ethical clearance was obtained from the University of Lusaka Ethics Committee; reference number: IORG0010092-2022/1036 and authorisation to conduct the study was obtained the National Health Research Authority (NHRA); reference number: NHRA0000003/06/09/2022. Because the data was collected from the patient treatment register(s) at the health institution, informed consent from the participants was waived.
Results
Frequency distribution of the study participants characteristics
. shows the frequency distribution of the participants characteristics and the Chi- square test for association results. This study included 404 TB patients of which majority of them were male (68.56%, n = 337). Out of 337 participants with successful TB treatment, most of them were male (68.25%, n = 230). Out of 67 participants with unsuccessful TB treatment, most of them were male (70.15%, n = 47). The age distribution showed that the majority of the participants were between the ages of 25 and 34. (30.94%, n = 125) while the minority were aged 65 years plus (4.46%, 18). Among the participants with successful TB treatment, most of them were in the age groups 25–34 (30.86%, n = 104) and 35–44 (30.86%, n = 104). On the other hand, the minority were in the age group 65 years and above (3.26%). Among the participants with unsuccessful TB treatment, most of them were in the age group 25–34 (25.37%, n = 21) while the minority were in the age group 45–55 (7.46%, n = 5). Most of the TB patients were new cases (81.19%). Among the TB patients with successful TB treatment, most of them were new cases (81.01%, n = 273). Consequently, among those with unsuccessful TB treatment, most of them were also new cases (82.09%, n = 55). Clinically diagnosed TB was the most prevalent kind of TB among the patients (PTB) (51.24%). The majority of people who had effective TB therapy were clinically diagnosed (50.74%, n = 50.74%). Similarly, slightly over half of the participants with unsuccessful TB treatment were clinically diagnosed with TB (53.73%, n = 36). Most of the participants were HIV negative (52.72%). Consequently, among the participants with successful TB treatment, most of them were HIV negative (52.52%, n = 177). Similarly, among those with unsuccessful TB treatment, most of them were HIV negative (53.73%, n = 36). Most of the participants were observed daily by relative (55.20%). Among those with successful Tb treatment, most of the participants were observed by a relative (55.49%, n = 187). Similarly, among those with unsuccessful TB treatment, most of them were observed by a relative (53.73%, n = 36). In addition, a Chi-square test was conducted to examine whether there were statistically significant differences within the groups. The findings revealed that the differences in all the groups were not statistically significant, as indicated by p-values greater than 0.05.
Table 2. Frequency distribution of the participants.
TB treatment outcomes
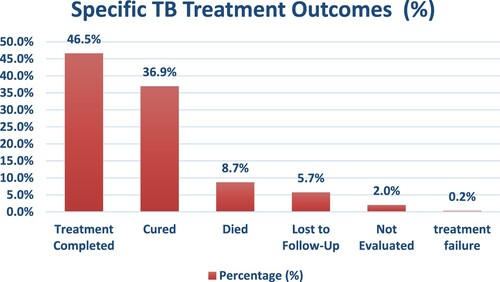
This research recruited 404 people who had been diagnosed with tuberculosis (all forms) in 2020 and were commenced on a standard TB regimen with a four-drug combination; Rifamin (R), Isonizid (H), Pyrazinamide (Z), Ethambutol (E) for 2 months followed by RH for 4 months (2RHZE/4RH). As shown in . the treatment outcomes showed that 337 participants (83.42%) had a successful TB treatment outcome which included 188 (46.5%) who completed treatment and 149 (36.9%) who were cured. On the other hand, 67 participants experienced unsuccessful treatment in TB which included 35 (8.7%) who died, 23 (5.7%) who were lost to follow up, 8 (2%) who were not evaluated and 1 who experienced treatment failure (0.2%).
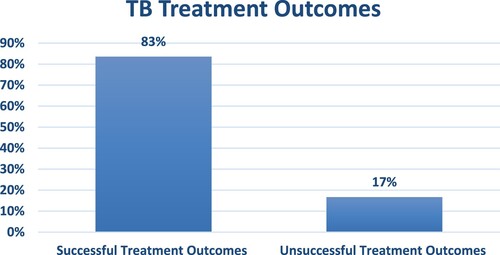
shows the TB treatment outcomes of the participants and the results showed that 83% of them had successful while 17% had unsuccessful treatment outcomes.
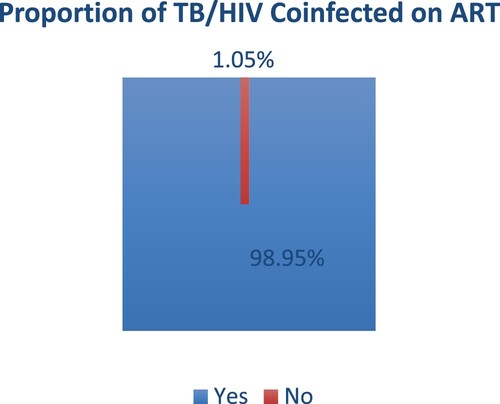
shows the proportion of the TB/HIV coinfected participants who were on ART. Out of the 404 participants, a total number of 191 (47.28%) were TB/HIV coinfected of which 98.95% were on ART. The 2 (1.05%) TB/HIV coinfected patients were lost to follow up before being initiated on ART. In revisiting the historical intricacies of TB-HIV/AIDS comorbidity, the present-day landscape reveals significant strides in treatment modalities. Integrating antiretroviral therapy (ART) with TB treatment stands out as a cornerstone in managing dual infections, promising improved patient outcomes (Water et al., Citation2022). A more modern approach recognises mental health, diet, and community involvement in addition to medicine and provides holistic care (Ikram et al., Citation2022). To address the comorbidity of TB and HIV/AIDS, however, requires persistent international cooperation; governments, non-governmental organisations, and the commercial sector should work together to remove obstacles and make investments in research and education. A dedication to growth and compassion becomes essential as we traverse this unstable terrain in order to steer clear of dual infections and towards a stronger, healthier global society.
Factors associated with TB treatment outcomes
displays the logistic regression findings, which demonstrate that males were 8.5 percent less likely to have a good outcome than females. Similar odds were observed after adjusting for other variables where males were 9.0% less likely to experience a successful treatment outcome compared to the female TB patients. With regards to age groups, the TB patients in the age group 25–34 were 13.1% less likely to have a successful treatment outcome compared to those in the group 15–24. Slightly lower odds were observed after adjusting for other variables where the participants in the age group 25–34 were 11.2% less likely to experience a successful treatment outcome compared to those in the age group 15 - 24. The TB patients in the age group 35–44 were 7.3% more likely to have a successful compared to those in the age group 15–24. Slightly higher odds were observed as the participants in the age group 35–44 were 10.1% more likely to have a Successful treatment outcome compared to those in the age group 15–24. Participants who were in the age group 45–54 were 36.8% more likely to have a successful treatment outcome compared to those who were in the age group 15–24. After adjusting for other variables, slightly higher odds were observed among the participants in the age group 45–54 who were 40.7% more likely to have as successful treatment outcome compared to those in the age group 15–24. The participants in the age group 55–64 were 44.9% less likely to have a successful treatment outcome compared to those in the age group 15–24. After adjusting for other variables, the participants in the age group 55–64 were 46.7% less likely to have a successful treatment outcome compared to those in the age group 15–24. The TB patients in the age group 65 + were 72.4% less likely to have a successful TB treatment outcome compared to those in the age group 15–24. This was statistically significant at 95% confidence level (AOR (95% CI): 0.276 (0.086–0.881), p = 0.030). After adjusting for other variables, The TB patients in the age group 65 + were 72.9% less likely to have a successful TB treatment outcome compared to those in the age group 15–24 which was statistically significant at 95% confidence level (AOR (95% CI): 0.271 (0.083–0.882), p = 0.030).
Table 3. Factors Associated with TB treatment outcomes among TB patients aged 15 years and older.
Patients with relapsed tuberculosis were 7.4% less likely to have a successful TB treatment outcome compared to the TB patients who were newly diagnosed. Slightly higher odds were observed after adjusting for other variables where the participants with relapse TB were 11.2% more likely to have a successful TB treatment outcome compared to the TB patients who were newly diagnosed. Participants with PTB patients were 11.3% less likely to have a successful treatment outcome compared to those with BTB patients. After adjusting for other variables, the participants with PTB patients were 30.5% less likely to have a successful treatment outcome compared to those with BTB patients. The participants who were coinfected with HIV were 5.0% more likely to have a successful treatment outcome compared to those who were not co-infected. After adjusting for other variables, the participants who were coinfected with HIV were 8.3% less likely to have a successful treatment outcome compared to those who were not co-infected. The TB patients who were observed daily by a relative were 7.4% more likely to have a successful TB treatment outcome compared to those who were observed daily at the clinic. Higher odds were observed after adjusting for other variables as the participants who were observed daily by a relative were 52.8% more likely to have a successful TB treatment outcome compared to those who were observed daily at the clinic.
Discussion
This study aimed to assess the TB treatment outcomes and the associated factors among TB patients aged 15 years and older who accessed TB treatment from Chawama first level hospital in Lusaka district. This study found a treatment success rate of 83.42% among the participants. This finding indicated a lower treatment success rate from the recommended WHO target of 90% (Izudi et al., Citation2020). The TB treatment outcomes may have been affected by the COVID-19 pandemic. COVID-19's interruption has had a significant impact on health services, especially national TB programmes and TB services (Tiberi et al., Citation2021). Furthermore, the symptoms of tuberculosis and COVID-19 might be identical, leading to diagnostic misunderstanding and subsequent stigmatisation of tuberculosis patients (Garg & Lee, Citation2020). The study also found that 94% of the TB/HIV coinfected patients were on ART. Despite policy recommendations that, in order to optimise treatment results for individuals who are coinfected with TB and HIV (CDCTB, Citation2023), various studies have indicated that TB patients may not be initiated on ART due to fear, perception of being in good health, lack of medicines, high CD4 count and other reasons (Egelund & Bucciarelli, Citation2022; Goovaerts et al., Citation2020; Kebogile & Elias, Citation2014). Majority of the participants were male which was a common finding among other studies. That is, various studies have had a similar finding where the TB disease was more prevalent among males compared to males (Chidambaram et al., Citation2021; Nwene, Citation2016). Despite not being statistically significant, our findings showed that females were more likely to have successful TB treatment outcomes compared to men. Other studies indicated similar findings. According to a study done in Nigeria, it was concluded that females had better treatment outcomes and lower default rates (Nwene, Citation2016). Similarly another study stated that males have a higher infection and mortality rate in TB treatment (Chidambaram et al., Citation2021). Furthermore, Murphy et al. (Citation2018) affirms that gender differences in TB treatment outcomes may be explained by poor outcomes in men. To this effect, studies have indicated that the factors contributing to this include men's prioritisation of work and adherence to masculine norms, challenges in coordinating clinic hours (Daniels et al., Citation2021; Kintu et al., Citation2023; Medina-Marino et al., Citation2022).
The age distribution analysis revealed that the highest proportion of participants fell within the age range of 25–34 while those aged 65 years and above constituted a smaller percentage. In the group of participants who experienced successful TB treatment, the most common age groups were 25–34 and 35–44. Conversely, the age group of 65 years and above was the least represented in this successful treatment cohort. For those with unsuccessful TB treatment, the majority were in the age range of 25–34, while a smaller portion belonged to the 45–55 age group. In this study, the age group 65 + was associated with higher odds of unsuccessful TB treatment outcome. This finding was similar to a study that was conducted in Ethiopia which found that found higher odds of unsuccessful treatment among patients aged 65 + with lower odds in the age group 15–24 (Taye et al., Citation2018). Furthermore, another study conducted in Asia, found that advanced age particularly among those aged 75 + was a determinant of mortality during the treatment for TB. In other age groups treatment outcomes were not significantly different, thus, treatment outcomes in TB may vary in various settings.
In this study we observed that the majority of tuberculosis (TB) patients were identified as new cases. This finding was in line with a study done by (Afshar et al., Citation2019). This trend was consistent among patients who achieved successful TB treatment, where the highest percentage also constituted new cases. Similarly, among individuals with unsuccessful TB treatment, the majority were categorised as new cases. Thus, we found no difference However, a similar study found that in new cases, the presence of large cavities swollen and lymph nodes due to TB may be an indicator for poor treatment outcomes (Rosenfeld et al., Citation2021). In another study, it was found that high risk of unfavourable TB treatment outcomes were associated with relapse cases which included loss to follow up and deaths (Vaghela & Mangal, Citation2020).
Clinically diagnosed pulmonary tuberculosis (PTB) was the prevailing TB type among patients, accounting for the highest proportion. This finding was consistent with a study done by Yusmaniar and Kurniawan (Citation2020) which found a high prevalence of clinically diagnosed pulmonary TB compared to other forms. This study further found that among those who achieved successful TB treatment, a significant portion were clinically diagnosed mirroring this trend. Likewise, among individuals with unsuccessful TB treatment, slightly over half were clinically diagnosed. In contrast with our findings, a study done in Nigeria found that the majority of patients who had effective TB treatment were clinically diagnosed with pulmonary tuberculosis (Alao et al., Citation2020).
In terms of HIV status, the majority of individuals in our research tested negative for HIV, which was consistent with earlier studies. (Akanbi et al., Citation2019; Baluku et al., Citation2020; Ganiger et al., Citation2019). Furthermore, our study found no association between TB treatment outcome and HIV status. On the contrary, a study done in Ghana showed that TB patients who were HIV coinfected had higher chances of adversity in the treatment outcome compared to those who were not coinfected with HIV (Hayibor et al., Citation2020). Another study found that TB/HIV integration was linked to decreased mortality during TB treatment but had no effect on other TB treatment outcomes (Burnett et al., Citation2018). Thus, these findings signify the importance of adherence in treatment among people who are on TB treatment.
Most of the participants were observed daily by relative. Similarly, among those with successful Tb treatment, most of the participants were observed by a relative. Our findings did not find an association between the DOT plan and treatment outcomes. On the other hand, a study done by found that When compared to clinic-based DOT, community-based DOT (CB-DOT) was found to enhance TB treatment results (Zhang et al., Citation2016). Furthermore, a study done in Armenia discovered that self-administered drug intake accompanied by a family member, as well as educational/counselling sessions and medication reminders, resulted in non-inferior treatment success compared to in-clinic DOT (Khachadourian et al., Citation2020).
Limitations
Despite the fact that this research was a success, it was not without limitations. The scope of the study is limited to a single hospital and may not fully represent the diversity of TB cases and treatment experiences in the broader region. Furthermore, characteristics not investigated in this study, such as socioeconomic situation and education level, may impact therapy outcomes.
Conclusion
This study yielded an encouraging 83.4% TB success rate which has potential to increase in order to achieve the 90% WHO target. This emphasises the need for continuous efforts to improve TB treatment outcomes at the health facility. Despite no significant difference in the sex distribution, the results in this study still it suggested that males were less likely to have successful TB treatment outcomes compared to females. In this regard, attention is required to eradicated the gender disparities regarding TB treatment outcomes. Most notably, a distinctive pattern emerged when focusing on age groups, particularly among individuals aged 65 and above as they had lower odds of TB treatment success. Therefore, we recommend special attention to patients who are aged 65 years and above. The clinical characteristics of the patients, although no significant association was identified in this particular context, there is a notable prevalence of new TB cases, with clinically diagnosed pulmonary cases being the most common among participants. The HIV status of the patients emerges as a crucial factor to consider, given its potential to complicate treatment regimens and impact overall health outcomes. It is worth noting that alternative DOT strategies showed promise in enhancing treatment outcomes. Thus, recommendation is made that the TB programme develops tailored interventions to improve the TB treatment success rate among TB patients.
Disclosure statement
No potential conflict of interest was reported by the author(s).
References
- Afshar, B., Carless, J., Roche, A., Balasegaram, S., & Anderson, C. (2019). Surveillance of tuberculosis (TB) cases attributable to relapse or reinfection in London, 2002-2015. PLoS One, 14(2), e0211972. https://doi.org/10.1371/journal.pone.0211972
- Akanbi, K., Ajayi, I., Fayemiwo, S., Gidado, S., Oladimeji, A., & Nsubuga, P. (2019). Predictors of tuberculosis treatment success among HIV-TB co-infected patients attending major tuberculosis treatment sites in abeokuta, ogun state, Nigeria. Pan African Medical Journal, 32(Suppl 1), 7. https://doi.org/10.11604/pamj.supp.2019.32.1.13272
- Alao, M. A., Maroushek, S. R., Chan, Y. H., Asinobi, A. O., Slusher, T. M., & Gbadero, D. A. (2020). Treatment outcomes of Nigerian patients with tuberculosis: A retrospective 25-year review in a regional medical center. PLoS One, 15(10), e0239225. https://doi.org/10.1371/journal.pone.0239225
- Baluku, J. B., Mugabe, P., Mulwana, R., Nassozi, S., Katuramu, R., & Worodria, W. (2020). High prevalence of rifampicin resistance associated with rural residence and very low bacillary load among TB/HIV-coinfected patients at the national tuberculosis treatment center in Uganda. BioMed Research International, 2020, 1–7. https://doi.org/10.1155/2020/2508283
- Burnett, S. M., Zawedde-Muyanja, S., Hermans, S. M., Weaver, M. R., Colebunders, R., & Manabe, Y. C. (2018). Effect of TB/HIV integration on TB and HIV indicators in rural Ugandan health facilities. JAIDS Journal of Acquired Immune Deficiency Syndromes, 79(5), 605–611. https://doi.org/10.1097/QAI.0000000000001862
- CDCTB. (2023, October 20). Tuberculosis (TB)—Treatment of LTBI and TB for Persons with HIV. Centers for Disease Control and Prevention. https://www.cdc.gov/tb/topic/treatment/tbhiv.htm.
- Chidambaram, V., Tun, N. L., Majella, M. G., Castillo, J. R., Ayeh, S. K., Kumar, A., Neupane, P., Sivakumar, R. K., Win, E. P., Abbey, E. J., Wang, S., Zimmerman, A., Blanck, J., Gupte, A., Wang, J.-Y., & Karakousis, P. C. (2021). Sex differences in TB treatment outcomes: Retrospective cohort study and meta-analysis. medRxiv, 2021.04.26.21256155. https://doi.org/10.1101/2021.04.26.21256155
- Daniels, J., Medina-Marino, A., Glockner, K., Grew, E., Ngcelwane, N., & Kipp, A. (2021). Masculinity, resources, and retention in care: South African men’s behaviors and experiences while engaged in TB care and treatment. Social Science & Medicine, 270, 113639. https://doi.org/10.1016/j.socscimed.2020.113639
- Egelund, E. F., & Bucciarelli, A. L. (2022). Art and the fight against tuberculosis. JAMA, 328(6), 509–510. https://doi.org/10.1001/jama.2021.24029
- Ganiger, A., Patil, L., & Mrudula, N. (2019). Evaluation of serum electrolyte status among normal healthy individuals and newly diagnosed cases of pulmonary TB in tertiary care hospital in bidar: An observational study. Indian Journal of Medical Biochemistry, 23(3), 316–319. https://doi.org/10.5005/jp-journals-10054-0111
- Garg, N., & Lee, Y. I. (2020). Reactivation TB with severe COVID-19. CHEST, 158(4), A777. https://doi.org/10.1016/j.chest.2020.08.724
- Goovaerts, O., Massinga-Loembé, M., Ondoa, P., Ceulemans, A., Worodria, W., Mayanja-Kizza, H., Colebunders, R., Kestens, L. & Group, the T.-I. S. (2020). Lack of elevated pre-ART elastase-ANCA levels in patients developing TB-IRIS. PLoS One, 15(12), e0244800. https://doi.org/10.1371/journal.pone.0244800
- Hayibor, K. M., Bandoh, D. A., Asante-Poku, A., & Kenu, E. (2020). Predictors of adverse TB treatment outcome among TB/HIV patients compared with non-HIV patients in the greater Accra regional hospital from 2008 to 2016. Tuberculosis Research and Treatment, 2020, 1–8. https://doi.org/10.1155/2020/1097581
- Hutchinson, J., Checkland, K., Gibson, J., Kontopantelis, E., & Sutton, M. (2023). Consequences of the closure of general practices: A retrospective cross-sectional study. British Journal of General Practice, 73(731), e399–e406. https://doi.org/10.3399/BJGP.2022.0501
- Ikram, M., Naeem, M., Zahoor, M., Hanafiah, M. M., Oyekanmi, A. A., Ullah, R., Farraj, D. A. A., Elshikh, M. S., Zekker, I., & Gulfam, N. (2022). Correction: Ikram et al. Biological degradation of the azo dye basic orange 2 by escherichia coli: A sustainable and ecofriendly approach for the treatment of textile wastewater. Water 2022, 14, 2063. Water, 14(19), 2969. https://doi.org/10.3390/w14192969
- Izudi, J., Tamwesigire, I. K., & Bajunirwe, F. (2020). Treatment success and mortality among adults with tuberculosis in rural eastern Uganda: A retrospective cohort study. BMC Public Health, 20(1), 501. https://doi.org/10.1186/s12889-020-08646-0
- Kebogile, M., & Elias, P. (2014). Why do patients refuse antiretroviral therapy before they complete tuberculosis treatment?: A qualitative enquiry Journal of AIDS and HIV Research, 6(2), 33–38. https://doi.org/10.5897/JAHR2013.0284
- Khachadourian, V., Truzyan, N., Harutyunyan, A., Petrosyan, V., Davtyan, H., Davtyan, K., van den Boom, M., & Thompson, M. E. (2020). People–centred care versus clinic–based DOT for continuation phase TB treatment in Armenia: A cluster randomized trial. BMC Pulmonary Medicine, 20(1), 105. https://doi.org/10.1186/s12890-020-1141-y
- Kintu, T. M., Mwanahamisi, B. S., Muwanguzi, M., Kyagambiddwa, T., Miiro, E., Tishekwa, N., Lodiong, L. J. D., Timbiine, A. K., Tumukunde, P., Baluku, J. B., & Nuwagira, E. (2023). Unfavorable treatment outcomes among patients with drug-resistant TB in Uganda. The International Journal of Tuberculosis and Lung Disease, 27(4), 291–297. https://doi.org/10.5588/ijtld.22.0638
- Lungu, P. S., Kabaso, M. E., Mihova, R., Silumesii, A., Chisenga, T., Kasapo, C., Mwaba, I., Kerkhoff, A. D., Muyoyeta, M., Chimzizi, R., & Malama, K. (2022). Undernotification and underreporting of tuberculosis in Zambia: A national data quality assessment. BMC Health Services Research, 22(1), 1074. https://doi.org/10.1186/s12913-022-08431-2
- Medina-Marino, A., Bezuidenhout, D., Ngcelwane, N., Cornell, M., Wainberg, M., Beyrer, C., Bekker, L.-G., & Daniels, J. (2022). Qualitative identification of intervention preferences to support men’s engagement and retention in TB care in South Africa. American Journal of Men's Health, 16(5), 155798832211293. https://doi.org/10.1177/15579883221129349
- Meseret Tadele, M., Tizazu, G., Temesgen Denekew, H., & Tesfa Gebeyehu, M. (2022). Successful tuberculosis treatment outcome in east gojjam zone, Ethiopia: Cross-sectional study design. Alexandria Journal of Medicine, 58(1), 60–68. https://doi.org/10.1080/20905068.2022.2090064
- MOH. (2016, November). ZMB-CH-43-01-PLAN-STRATEGY-2018-eng-TB-Zambia-National-TB-Strategic-Plan-2017-2021.pdf. https://platform.who.int/docs/default-source/mca-documents/policy-documents/plan-strategy/ZMB-CH-43-01-PLAN-STRATEGY-2018-eng-TB-Zambia-National-TB-Strategic-Plan-2017-2021.pdf.
- Murphy, M. E., Wills, G. H., Murthy, S., Louw, C., Bateson, A. L. C., Hunt, R. D., McHugh, T. D., Nunn, A. J., Meredith, S. K., Mendel, C. M., Spigelman, M., Crook, A. M., Gillespie, S. H., Diacon, A., Hanekom, M., Venter, A., Dawson, R., Narunsky, K., & Mtafya, B. & … for the REMoxTB consortium. (2018). Gender differences in tuberculosis treatment outcomes: A post hoc analysis of the REMoxTB study. BMC Medicine, 16(1), 189. https://doi.org/10.1186/s12916-018-1169-5
- Nanzaluka, F. H., Chibuye, S., Kasapo, C. C., Langa, N., Nyimbili, S., Moonga, G., Kapata, N., Kumar, R., & Chongwe, G. (2019). Factors associated with unfavourable tuberculosis treatment outcomes in Lusaka, Zambia, 2015: A secondary analysis of routine surveillance data. Pan African Medical Journal, 32, 159. https://doi.org/10.11604/pamj.2019.32.159.18472
- Nwene, E. K. (2016). Tuberculosis and gender in Nigeria, Sex differences in diagnosis and treatment outcome of TB and TB HIV infected patients. TEXILA INTERNATIONAL JOURNAL OF PUBLIC HEALTH, 4(4), 388–395. https://doi.org/10.21522/TIJPH.2013.04.04.Art035
- Obadire, S. O., Mitsan, O., Ige, I. P., Oke, O. C., & Odewusi, F. O. (2022). Current innovations in medical laboratory diagnosis of tuberculosis. Sokoto Journal of Medical Laboratory Science, 7(3), 60–70. https://doi.org/10.4314/sokjmls.v7i3.7
- Rosenfeld, G., Gabrielian, A., Wang, Q., Gu, J., Hurt, D. E., Long, A., & Rosenthal, A. (2021). Radiologist observations of computed tomography (CT) images predict treatment outcome in TB portals, a real-world database of tuberculosis (TB) cases. PLoS One, 16(3), e0247906. https://doi.org/10.1371/journal.pone.0247906
- Shete, P. B., & Kahn, J. G. (2019). Economic analyses to inform public health decision-making for tuberculosis: The role of understanding implementation. BMC Medicine, 17(1), 224. https://doi.org/10.1186/s12916-019-1468-5
- Taye, G., Defar, A., Taddele, T., Bekele, A., Getachew, T., Teklie, H., Gonfa, G., Getnet, M., Tadesse, M., & Demissie, M. (2018). Treatment outcome and associated factors among TB patients in Ethiopia: Hospital-based retrospective study. American Journal of Epidemiology and Infectious Disease, 6(1), 14–19. https://doi.org/10.12691/ajeid-6-1-3
- Tiberi, S., Vjecha, M. J., Zumla, A., Galvin, J., Migliori, G. B., & Zumla, A. (2021). Accelerating development of new shorter TB treatment regimens in anticipation of a resurgence of multi-drug resistant TB due to the COVID-19 pandemic. International Journal of Infectious Diseases, 113, S96–S99. https://doi.org/10.1016/j.ijid.2021.02.067
- Tok, P. S. K., Liew, S. M., Wong, L. P., Razali, A., Loganathan, T., Chinna, K., Ismail, N., & Kadir, N. A. (2020). Determinants of unsuccessful treatment outcomes and mortality among tuberculosis patients in Malaysia: A registry-based cohort study. PLoS One, 15(4), e0231986. https://doi.org/10.1371/journal.pone.0231986
- Uakarn, C., Chaokromthong, K., & Sintao, N. (2021). Sample Size Estimation using Yamane and Cochran and Krejcie and Morgan and Green Formulas and Cohen Statistical Power Analysis by G*Power and Comparisons.
- Uplekar, M., & Atre, S. (2021). Health systems and tuberculosis control. In G. B. Migliori, & M. C. Raviglione (Eds.), Essential tuberculosis (pp. 367–373). Springer International Publishing. https://doi.org/10.1007/978-3-030-66703-0_40.
- Vaghela, J. F., & Mangal, A. (2020). TB outcome of DOTS category I TB cases started on Re-treatment with category II regimen during the years 2002 to 2015, in a slum in Delhi. Journal of Communicable Diseases, 51(4), 62–68. (E-ISSN: 2581-351X & P-ISSN: 0019-5138). https://doi.org/10.24321/0019.5138.201938
- Water, B. J. v. d., Fulcher, I., Cilliers, S., Meyer, N., Wilson, M., Young, C., Gaunt, B., & Roux, K. l. (2022). Association of HIV infection and antiretroviral therapy with the occurrence of an unfavorable TB treatment outcome in a rural district hospital in eastern cape, South Africa: A retrospective cohort study. PLoS One, 17(4), e0266082. https://doi.org/10.1371/journal.pone.0266082
- WHO. (2013). Definitions and reporting framework for tuberculosis – 2013 revision: Updated December 2014 and January 2020. In Définitions et cadre de notification pour la tuberculose – révision 2013. World Health Organization. https://apps.who.int/iris/handle/10665/79199.
- WHO. (2023a, April 21). Tuberculosis (TB). https://www.who.int/news-room/fact-sheets/detail/tuberculosis.
- WHO. (2023b, August 17). Tuberculosis (TB). WHO | Regional Office for Africa. https://www.afro.who.int/health-topics/tuberculosis-tb.
- Yusmaniar, Y., & Kurniawan, A. H. (2020). Medication adherence to successful tuberculosis treatment outcome among TB/HIV patient at prof. Dr. Sulianti saroso infectious disease hospital. Pharmacology and Clinical Pharmacy Research, 5(3), 98. https://doi.org/10.15416/pcpr.v5i3.29166
- Zhang, H., Ehiri, J., Yang, H., Tang, S., & Li, Y. (2016). Impact of community-based DOT on tuberculosis treatment outcomes: A systematic review and meta-analysis. PLoS One, 11(2), e0147744. https://doi.org/10.1371/journal.pone.0147744