ABSTRACT
Introduction: Endogenous Cushing’s syndrome (CS) is a rare, life-threatening endocrine disorder that is caused by chronic exposure to cortisol overproduction. Levoketoconazole (Recorlev), a 2S, 4R stereoisomer of ketoconazole, is a steroidogenesis inhibitor under investigation for the treatment of CS.
Areas covered: This review covers the pharmacology, efficacy, and safety of levoketoconazole for the treatment of patients with endogenous CS.
Expert opinion: Based on the preclinical and clinical pharmacology findings, levoketoconazole appears to be the relevant enantiomer of ketoconazole for inhibition of steroidogenesis, with more potent inhibition of both cortisol and androgen synthesis relative to ketoconazole racemate and the 2R, 4S stereoisomer dextroketoconazole. Results from the phase III SONICS study showed that levoketoconazole was effective in normalizing cortisol levels and improving biomarkers of cardiovascular risk in a significant percentage of patients. In addition, treatment with levoketoconazole showed improvements in subjective clinical assessments of clinician-rated CS clinical signs and symptoms, patient-reported quality of life, and depression symptom severity. Testosterone levels decreased significantly in women. Levoketoconazole had an acceptable safety profile with no unexpected safety signals. The favorable pharmacology, efficacy, and safety profile of levoketoconazole supports its use as medical therapy for CS, if approved.
1. Introduction
1.1. Endogenous Cushing's syndrome
Endogenous Cushing’s syndrome (CS) is a rare, serious endocrine disorder that is caused by chronic exposure to overproduction of cortisol [Citation1]. While the incidence of overt endogenous CS is 2.4 to 3.2 people per million annually with a mortality risk, if not appropriately treated, of approximately 3.5 times greater than the general population [Citation2–5], it is likely that mild disease is much more prevalent [Citation6]. Cardiovascular events can, in part, explain the excess mortality in patients with active CS, which may persist even after cortisol normalization [Citation7,Citation8]. Because of the ubiquitous downstream effects of cortisol, chronic hypercortisolism leads to multisystem morbidities, including changes in physical appearance (central obesity, acne, hirsutism, skin fragility with striae), cardiovascular complications (hypertension, venous thromboembolism, peripheral edema), metabolic disturbances (insulin resistance with impaired glucose tolerance), skeletal damage (osteopenia, osteoporosis, skeletal fractures), impairment of reproductive and sexual function (hypogonadism, menstrual irregularities, decreased libido, infertility), and neuropsychiatric disorders (depression, sleep disorders, cognitive disturbance) [Citation9–11].
Endogenous CS results from chronic exposure to excess cortisol because of adrenocorticotrophic hormone (ACTH)–dependent or ACTH-independent etiologies [Citation12]. ACTH-dependent CS accounts for 80–85% of overt CS cases; approximately 80–90% of these are attributed to Cushing’s disease (CD; ACTH-producing pituitary adenoma), and the rest result from extrapituitary neuroendocrine tumors causing ectopic ACTH production [Citation12,Citation13]. ACTH-independent CS (15–20% of CS cases) results from autonomous adrenal overproduction of cortisol because of an adrenal adenoma, carcinoma, or bilateral hyperplasia. Mild adrenal cortisol excess or mild autonomous cortisol excess (MACE) describes a condition of ACTH-independent glucocorticoid overproduction from an adrenal mass, in the absence of overt clinical signs and symptoms of CS [Citation14]. In patients with adrenal incidentalomas, MACE has been associated with cardiovascular, bone, and metabolic comorbidities typical of cortisol excess, as well as increased mortality risk [Citation14,Citation15].
1.2. Treatment of Cushing's syndrome
The main goal of treatment of CS is to fully normalize cortisol production or its activity [Citation16]. Surgical removal of the underlying lesion (pituitary, adrenal, or ectopic neuroendocrine tumor resection) is typically the first-line treatment for CS. If surgical resection for ACTH-dependent CS is not an option or is unsuccessful, second-line treatment options include bilateral adrenalectomy, radiation therapy, and medical treatment; each of these treatment modalities has its advantages and drawbacks. CD may persist after surgery or recur after an initially successful surgery [Citation17,Citation18]. With radiation therapy, there is a considerable delay in therapeutic response, and radiation therapy for CD is associated with the risk of hypopituitarism, optic nerve damage, and the development of cerebrovascular disease or secondary tumors [Citation19]. Bilateral adrenalectomy causes life-long adrenal insufficiency and increases the risk for adrenal crisis and corticotroph tumor progression (Nelson’s syndrome; only in patients with CD) [Citation20]. Medical therapies that are currently approved or commonly used off-label for CS have limitations in some patients with regard to safety, efficacy, or addressing various manifestations of CS [Citation21,Citation22]. In a multicenter study in the United States, about 30% of patients with CD did not achieve cortisol normalization despite multiple treatments, underscoring the unmet need for more effective treatment options [Citation23].
Medical treatment can be used when surgery to remove the underlying lesion is delayed, contraindicated, declined, not feasible, or failed, or as a bridging treatment until radiation therapy becomes effective, though preoperative and primary medical therapy use are increasing [Citation16,Citation24]. Medical treatments for CS include pituitary-directed drugs (cabergoline, pasireotide), which are limited to patients with CD; adrenal steroidogenesis inhibitors (osilodrostat, ketoconazole, metyrapone, etomidate, mitotane); and glucocorticoid receptor antagonists (mifepristone) [Citation18,Citation22].
1.3. Levoketoconazole
Levoketoconazole (Recorlev), the 2S,4R stereoisomer of ketoconazole, is an orally administered adrenal steroidogenesis inhibitor currently in development for the treatment of endogenous CS [Citation18]; a New Drug Application (NDA) was submitted to the US Food and Drug Administration (FDA) for this purpose in March 2021. Ketoconazole is approved in the United States as an azole antifungal drug at 200–400 mg/day [Citation25] and used off-label for CS at higher doses (400–1200 mg/day) [Citation1]. In contrast to the US indication, ketoconazole is currently approved by the European Medicines Agency (EMA) only for the treatment of endogenous CS [Citation26]. The clinical evidence of ketoconazole use for the treatment of CS has been derived from retrospective and prospective observational studies, but due to safety concerns with respect to potential hepatotoxicity and prolongation of the QT interval, the US prescribing information includes a boxed warning [Citation25,Citation27–29].
Levoketoconazole, which is considered the eutomer of ketoconazole (i.e., the stereoisomer with desirable pharmacologic activity), was initially investigated as a potential treatment for diabetes mellitus type 2 (DM2) because it was hypothesized to reduce the cortisol-mediated contribution to hyperglycemia [Citation30,Citation31]. Levoketoconazole reduced hemoglobin A1c (HbA1c) and fasting blood glucose levels in patients with DM2 in a small, randomized, double-blind, placebo-controlled study [Citation30]. Levoketoconazole is currently being evaluated as a medical therapy for endogenous CS. Here, we review the pharmacology, efficacy, and safety of levoketoconazole for the treatment of patients with endogenous CS.
2. Pharmacologic profile of levoketoconazole
2.1. Chemistry
Levoketoconazole is the 2S,4R stereoisomer of ketoconazole, a racemic mixture () [Citation32]. Levoketoconazole is formulated as a 150 mg, immediate-release tablet.
Figure 1. Chemical structure of levoketoconazole [Citation32]
![Figure 1. Chemical structure of levoketoconazole [Citation32]](/cms/asset/ddf3e543-feff-4bcd-a381-58b1a9e0ac1a/iere_a_1945440_f0001_b.gif)
2.2. Preclinical pharmacology
Levoketoconazole inhibits several enzymes in the steroidogenesis pathway in the adrenal cortex, including cytochrome P450 (CYP) enzymes: CYP11A1, CYP17A1, CYP11B1, and CYP11B2 [Citation32–35] (). The enantiomers of ketoconazole differ in their inhibitory potency toward these CYP enzymes [Citation32,Citation33]. In a study using human embryonic kidney (HEK) 293 cells or Chinese hamster lung cells (V79) engineered to stably express human CYP enzymes, levoketoconazole was a more potent inhibitor than dextroketoconazole (the 2R, 4S stereoisomer) and racemic ketoconazole [Citation33] (, ). These data are consistent with levoketoconazole being the active component of ketoconazole for inhibition of cortisol biosynthesis [Citation33]. In addition, the binding profile of levoketoconazole () suggests that its effects on inhibition of steroidogenesis extend beyond its affinities for the canonical ligand-binding pockets of these enzymes [Citation33]. The greater potency of levoketoconazole in inhibiting cortisol synthesis, compared with ketoconazole, was also reported in a study with human adrenocortical carcinoma cells (HAC15) [Citation36]. In preclinical animal studies, levoketoconazole was more potent in reducing serum corticosterone levels than dextroketoconazole or ketoconazole [Citation34]. A greater potency of levoketoconazole in inhibiting adrenal cortisol synthesis compared with ketoconazole may allow for a lower levoketoconazole therapeutic dose in the treatment of CS. Levoketoconazole has a potential for reduced liver toxicity compared with ketoconazole [Citation32]. Compared with dextroketoconazole, levoketoconazole is 12 times less potent in inhibiting CYP7A1, which is a rate-limiting enzyme for bile acid synthesis [Citation32,Citation37] and a key pathway for removal of cholesterol from the body [Citation38]. Furthermore, ketoconazole is known to be primarily eliminated via biliary excretion; therefore, a lower inhibition of bile acid synthesis by levoketoconazole compared with dextroketoconazole may lead to less interference in biliary elimination of the drug and potentially other medications used concomitantly, which may impact the safety profile [Citation28]. In addition to increased cholesterol degradation via inhibition of the CYP7A1 enzyme by levoketoconazole compared with ketoconazole, ketoconazole and its enantiomers also inhibit cholesterol synthesis by inhibiting CYP51A1 (lanosterol 14α-demethylase) enzyme, which is also a critical enzyme for fungal survival. Levoketoconazole inhibits CYP51A1 with greater than 2-fold more potency than dextroketoconazole [Citation32].
Table 1. Calculated IC50 values and spectral binding constants for ketoconazole enantiomers and its racemate toward different human steroidogenic P450 enzymes [Citation33]
Figure 2. Levoketoconazole inhibition of cortisol synthesis. Gray boxes depict the relative potency of levoketoconazole versus ketoconazole based on half maximal inhibitory concentration (IC50) values from an in vitro study using HEK-293 or V79 cell lines stably expressing human steroidogenic P450 enzymes [Citation33]
![Figure 2. Levoketoconazole inhibition of cortisol synthesis. Gray boxes depict the relative potency of levoketoconazole versus ketoconazole based on half maximal inhibitory concentration (IC50) values from an in vitro study using HEK-293 or V79 cell lines stably expressing human steroidogenic P450 enzymes [Citation33]](/cms/asset/ce46f854-3e8c-4446-b633-a89ee12fa85b/iere_a_1945440_f0002_b.gif)
Similar to ketoconazole, levoketoconazole is a substrate and a potent inhibitor of a major drug-metabolizing enzyme, CYP3A4 [Citation28,Citation39,Citation40]. Levoketoconazole is about 2-fold more potent than dextroketoconazole in inhibiting CYP3A4-mediated metabolism of substrates [Citation39,Citation40], which might be relevant for drug–drug interactions.
Ketoconazole and its enantiomers inhibit testosterone production via the CYP17A1 enzyme [Citation32]. In vitro, levoketoconazole showed more potent inhibition of testosterone production via the CYP17A1 enzyme compared with dextroketoconazole. However, as testosterone is a CYP3A4 substrate, levoketoconazole is approximately 2-fold more potent in inhibiting CYP3A4 enzyme-mediated testosterone metabolism than dextroketoconazole [Citation40]. This combination of opposing effects indicates that clinical evaluation is needed to understand the net effect of levoketoconazole treatment on testosterone levels in men and women.
Finally, levoketoconazole has been shown to inhibit ACTH secretion in mouse pituitary tumor cells (AtT20) and corticotroph growth in a primary human pituitary adenoma culture [Citation36]. These findings are consistent with the direct antisecretory effects on ACTH and growth inhibitory effects on neuroendocrine cells observed with ketoconazole [Citation36,Citation41–43].
2.3. Clinical pharmacology
The pharmacokinetics of levoketoconazole in humans were evaluated in five clinical studies; four in healthy volunteers and one in patients with type 2 diabetes mellitus () [Citation34,Citation44,Citation45]. The enantiomers in the ketoconazole racemic mixture are present in equal concentrations; however, a ketoconazole pharmacokinetic study showed that, following oral administration of ketoconazole (400 mg twice daily), the area under the curve (AUC) and the maximum serum concentration (Cmax) values of levoketoconazole exceeded those of dextroketoconazole by approximately 3-fold [Citation46]. Consistently, in levoketoconazole pharmacokinetic studies in healthy volunteers and patients with type 2 diabetes mellitus, the Cmax of levoketoconazole was approximately 3-fold higher compared with the dextroketoconazole enantiomer after single or multiple dosing with racemic ketoconazole 400 mg twice daily [Citation34]. This differential bioavailability of the enantiomers may indicate lower hepatic or intestinal metabolism of levoketoconazole. It is notable that there is no evidence of enantiomer interconversion in vivo; the serum concentration difference between levoketoconazole and dextroketoconazole, which was evident immediately after ketoconazole administration, remained constant during the course of drug elimination. Like ketoconazole, levoketoconazole is a weak base and therefore requires acidic pH conditions to dissolve in aqueous solution. As a result, absorption of oral levoketoconazole is dependent on the presence of stomach acid, such that sufficient inhibition of stomach acid (with H2 receptor antagonists or proton pump inhibitors) can substantially diminish bioavailability, as has been observed for ketoconazole intestinal absorption [Citation47,Citation48]. In a food-effect study, a high-fat meal did not influence the Cmax of a single oral dose of levoketoconazole 600 mg in healthy volunteers, but AUC values increased by approximately 30% (unpublished data).
Table 2. Levoketoconazole pharmacokinetic studies
Studies in healthy volunteers () confirm findings from in vitro studies that levoketoconazole has the potential for drug-drug interactions [Citation39,Citation40,Citation44,Citation45]. Levoketoconazole at steady state increased exposure to CYP3A4 substrates: felodipine by approximately 10-fold and atorvastatin by approximately 30% (unpublished data). Steady-state levels of levoketoconazole also increased metformin exposure by approximately 2-fold and reduced systemic clearance of metformin by approximately 62% (unpublished data), presumably due to reduction in renal elimination of metformin attributed to inhibition of OCT2 and MATE1 transporters by levoketoconazole [Citation49].
A pharmacodynamics assessment demonstrated a significant decrease in serum cortisol with levoketoconazole versus placebo (p < 0.005) or ketoconazole (p < 0.05) administration in a study with healthy volunteers [Citation34].
In summary, preclinical and clinical pharmacology findings suggest that levoketoconazole is a more potent inhibitor of cortisol synthesis compared with dextroketoconazole and may potentially have less hepatic exposure and less interference with metabolite elimination [Citation32–34]. Using a higher-potency cortisol synthesis inhibitor rather than a lower-potency one (ie, levoketoconazole vs. ketoconazole) is preferable in CS, as it would expose the patient to a lower risk of drug exposure–related toxicities, including QT prolongation and, perhaps, hepatic toxicity. Thus, levoketoconazole may have an improved therapeutic index relative to ketoconazole.
3. Clinical efficacy
The clinical development program for levoketoconazole for the treatment of endogenous CS includes three phase 3 multicenter studies: SONICS (Study Of levoketocoNazole In CS), a completed single-arm, open-label study (NCT01838551) [Citation50]; LOGICS (LevOketoconazole to fill a Gap In CS), a recently completed double-blind, placebo-controlled, randomized withdrawal study (NCT03277690); and OPTICS (OPen-label Treatment In Cushing’s Syndrome), an ongoing open-label, long-term safety study (NCT03621280).
3.1. SONICS study
3.1.1. Study design
The SONICS study was designed based on experience from treating patients with CS with ketoconazole [Citation51]. SONICS enrolled adults with confirmed CS and mean 24-hour urinary free cortisol (mUFC) ≥1.5 times the upper limit of normal (ULN). The study population consisted of patients with a moderate-to-severe CS phenotype. Enrolled patients were treated with oral levoketoconazole titrated to an individualized therapeutic dose in a dose titration phase (2–21 weeks) and then entered a 6-month maintenance phase followed by a 6-month extended evaluation phase. The levoketoconazole dose was adjusted in the dose titration phase based on mUFC response and tolerability from 150 mg twice daily to a maximum of 600 mg twice daily. This individualized dosing regimen of levoketoconazole was designed because the therapeutic dose needed for response with ketoconazole varies considerably among patients with CS [Citation51]. The levoketoconazole dose remained unchanged during the maintenance phase of SONICS unless adjustment was needed to maintain control of cortisol or because of safety and tolerability issues. The maintenance phase was designed to demonstrate durability of the therapeutic response.
3.1.2. Cortisol normalization
Ninety-four patients enrolled and received ≥1 dose of levoketoconazole in the SONICS study, of whom 77 patients entered the maintenance phase [Citation50]. Most patients had a diagnosis of CD (85%), and concurrent comorbidities of diabetes, hypercholesterolemia, and hypertension were reported in 38%, 36%, and 71% of the patients, respectively. Most patients had moderate-to-severe hypercortisolism at baseline (75% had baseline mUFC ≥2.0 ULN). During treatment with levoketoconazole, mean mUFC decreased to approximately the ULN at month 1, and improvement was sustained until month 6 of the maintenance phase () [Citation50]. At the end of the maintenance phase, mUFC was normalized in 30% of patients without a dose increase during the maintenance phase (study primary endpoint; 95% CI = 21–40%; p = 0.0154 vs. null hypothesis of ≤20%). The primary efficacy finding is strengthened by key elements of the study design, such as exclusion of patients with cyclic CS, no upper limit of mUFC levels at study entry, exclusion of inadequate urine samples from mUFC calculations, and assessments that were performed monthly and analyzed using a repeated-measures model. Further evidence of a conservative primary efficacy analysis in this study comes from the sensitivity and secondary analyses, which showed a greater efficacy than the primary analysis. When patients who needed a dose increase during the maintenance phase were included, 34 patients (36%) achieved normalization of mUFC, and 43 patients (46%) had ≥50% mUFC decrease or normalization. Therefore, among 55 patients completing the maintenance phase, 34 patients (62%) had normalized mUFC and 43 patients (78%) had ≥50% mUFC decrease or normalization, irrespective of dose increase. An indirect efficacy comparison between ketoconazole and levoketoconazole is complicated, due to the lack of comparably designed studies evaluating the efficacy of ketoconazole in CS. In retrospective studies in patients with CS receiving long-term treatment with ketoconazole, UFC normalization was reported in 45–49% of patients, whereas treatment was discontinued due to adverse events in 13–26% of patients and for lack of efficacy in up to 27% [Citation51,Citation52]. However, these retrospective studies have a number of methodological limitations, such as nonstandardized methods to establish UFC normalization, lack of standardized follow-up, and difficulty in recovering all data.
Figure 3. Mean urinary free cortisol (mUFC) concentration from baseline of the dose titration phase through the end of the maintenance phase in SONICS (maintenance population)
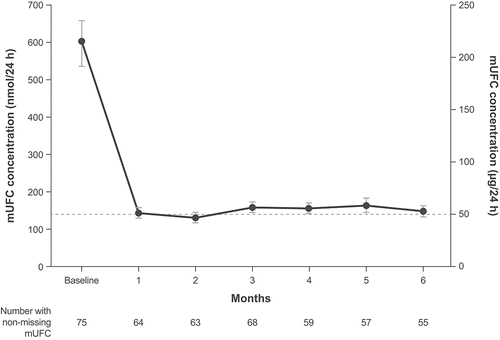
In the SONICS study, although a clear dose-response relationship was not observed with initial normalization and sustained maintenance of mUFC levels, the response rate (as assessed by mUFC normalization at month 6) was lower at the higher end of the levoketoconazole dose range [Citation50]. Patients uptitrated to higher doses of the drug had higher average baseline mUFC levels. Therefore, therapeutic response appears to be dependent, at least in part, on baseline mUFC levels. Nonetheless, 9 (56%) of 16 patients with mUFC ≥5 ULN at baseline and a median dose of 750 mg/day had mUFC normalized at the end of the maintenance phase.
Measuring late-night salivary cortisol (LNSC) levels can help assess loss in the diurnal physiological rhythm of cortisol secretion [Citation53,Citation54]. Restoring the diurnal cortisol rhythm is of clinical importance in patients with CS, as the disruption of this rhythm is thought to result in comorbidities such as hypertension and impaired glucose metabolism [Citation55,Citation56]. In the SONICS study, a significant decrease in mean LNSC concentration was observed from baseline to the end of month 1 (p < 0.01) and remained significantly lower than baseline through month 6 (p < 0.05) [Citation57]. However, LNSC was less commonly normalized (ranged from 4% to 19% of patients over time) than mUFC. As the cortisol rhythm is also affected by the dose and timing of therapy administration, salivary cortisol measurement at a single time point (late night for LNSC, a measure of nadir cortisol levels) during medical treatment may not adequately reveal improvements in diurnal cortisol rhythm [Citation55,Citation58]. Multiple measurements of salivary cortisol throughout the day, similar to the serum cortisol day curve used for metyrapone in some countries [Citation59], may provide a better marker for visualizing the change in diurnal cortisol rhythm; however, this information was not collected in the SONICS study.
3.1.3. Cardiovascular and metabolic comorbidities
Significant mean improvements from baseline to end of the maintenance phase were observed in cardiovascular and metabolic comorbidity biomarkers, such as fasting blood glucose, HbA1c, total and low-density lipoprotein cholesterol, and body weight (p < 0.0001) [Citation50]. These improvements did not appear to be associated with changes in concomitant medication use. Improvements in these risk biomarkers are important, as cardiovascular disease is the major cause of mortality and substantial morbidity in CS [Citation7,Citation8]. Also observed were a small but significant (p < 0.0001) mean decrease in HDL cholesterol and mean increase in triglycerides from baseline to end of maintenance [Citation50]. No significant changes in blood pressure were noted in this study.
3.1.4. Subjective clinical assessments
The SONICS study evaluated subjective clinical assessments of therapy on symptoms associated with excess testosterone secretion, fluid retention, depression, and quality of life [Citation50]. Despite generally mild symptom severity at baseline, significant mean improvements from baseline to end of the maintenance phase were observed in clinician-rated clinical signs and symptoms of CS (acne, hirsutism [in women], and peripheral edema; p < 0.05) [Citation60]. These improvements were noted early during levoketoconazole treatment for hirsutism (day 1 of maintenance; p < 0.0001) and acne (end of month 1 of maintenance; p < 0.05). Improvements in peripheral edema were observed at month 4 of maintenance (p < 0.01). In contrast, improvement on a 7-item composite score of Cushingoid appearance (moon facies, facial plethora, striae, bruising, and supraclavicular fat) and menstruation status in women (irregular menstruation or dysmenorrhea) did not reach statistical significance. With respect to patient-reported outcomes, significant improvements in quality of life (CushingQoL score; p < 0.0001) and depression severity (Beck Depression Inventory II; p < 0.01) were noted from baseline to end of maintenance with levoketoconazole treatment. Symptoms of depression improved with therapy even though they were mild in severity at baseline, and quality of life, which was moderately impaired at baseline, improved substantially. The moderate impairment of health-related quality of life reported by patients at study baseline exceeded the mild severity of CS signs and symptoms that was recorded in investigator assessments.
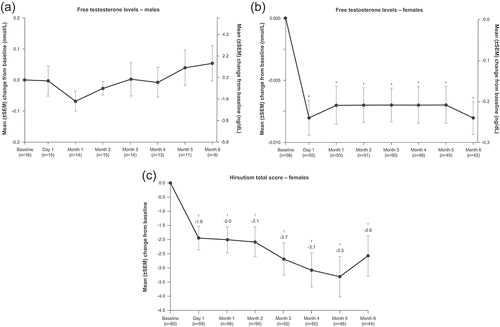
3.1.5. Testosterone
Consistent with clinician-rated improvements in acne and hirsutism, significant mean reductions in testosterone levels were noted in women from baseline to month 6 (p < 0.001) [Citation50] () [Citation60]; this is similar to findings of reduction in testosterone with ketoconazole treatment in women with CS [Citation61,Citation62]. In contrast, mean testosterone level tended to increase in males with levoketoconazole therapy, although the increase was not statistically significant and the number of patients was small [Citation50]. Ketoconazole is known to decrease testosterone production transiently in males with a normal hypothalamus-pituitary-gonadal axis, and a few cases of hypogonadism and gynecomastia have been reported when the drug has been used as an antifungal [Citation63–65]. However, case studies suggest that ketoconazole may function differently in male patients with CS (improvement or no change in testosterone levels) [Citation66,Citation67], as hypercortisolemia is known to suppress the hypothalamus-pituitary-gonadal axis [Citation9]. Based on the findings from SONICS and the known effects of ketoconazole, interindividual differences in testosterone response are likely in men treated with levoketoconazole.
Figure 4. Mean free testosterone levels in males (A) and females (B) and hirsutism scores in females (C), from baseline through the end of the maintenance phase in SONICS (maintenance population). *p < 0.0001. †p < 0.001. SEM: standard error of the mean
3.1.6. Efficacy in a subgroup of patients with type 2 diabetes mellitus
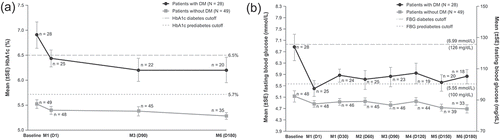
Diabetes mellitus is one of the comorbidities associated with CS, and management of these patients must address both excess cortisol and hyperglycemia [Citation68,Citation69]. Normalization of cortisol is sufficient, in some cases, to improve glycemic control and may also reverse diabetes [Citation2,Citation70]. In SONICS, efficacy and safety of levoketoconazole in patients with CS and diabetes mellitus (36 patients) were evaluated as exploratory analyses [Citation71]. At the end of the maintenance phase, normalization of mUFC was observed in 34% and 25% of patients with and without diabetes mellitus, respectively, without a dose increase during the maintenance phase. In addition, the measures of glycemic control, HbA1c and fasting blood glucose levels, were improved in both subgroups, but change was more pronounced in patients with diabetes mellitus () [Citation71].
Figure 5. Measures of glycemic control over time: HbA1c (A) and fasting blood glucose (B) in SONICS (maintenance population). D: day; DM: diabetes mellitus; FBG: fasting blood glucose; HbA1c: hemoglobin A1c; M: month; SE: standard error
4. Clinical safety
The levoketoconazole safety profile observed in the SONICS study was largely as expected based on safety information from studies in patients with type 2 diabetes and healthy volunteers, and from prior published clinical experience with ketoconazole [Citation30,Citation34,Citation50]. Most of the adverse events reported were mild to moderate in intensity. The most commonly reported adverse events (≥15% of patients in the dose titration and maintenance phases combined) were nausea (32%), headache (28%), peripheral edema (19%), hypertension (17%), fatigue (16%), diarrhea (15%), and increased alanine aminotransferase (ALT) levels (15%) [Citation50].
Adverse events of special interest that were anticipated a priori and predefined were reported in 14 patients during dose titration or maintenance treatment: liver-related adverse events (n = 7), QT interval prolongation (n = 5), and adrenal insufficiency (n = 3) [Citation50]. Serious adverse events were reported in 14 patients, of which 4 were considered to be probably or definitely related to levoketoconazole: elevated liver function test results (1 patient), prolonged QT interval (2 patients), and adrenal insufficiency (1 patient). One patient died of colon cancer, which was considered unrelated to levoketoconazole treatment. Twelve patients discontinued from the dose titration or maintenance phase because of adverse events, most commonly liver-related events (six patients).
Routine liver test abnormalities reported in the SONICS study suggest idiosyncratic, drug-related effects that were uncommon, mild to moderate in severity, and fully reversible without any clinical sequelae [Citation50]. In all, 11% of patients had ALT more than 3 times the ULN (including 3% with ALT >5x ULN), with the highest ALT increases occurring by month 2 of maintenance treatment. This incidence of liver test abnormalities is lower than observed in a prospective observational compassionate registry study of ketoconazole in CS in France, the most relevant comparison data [Citation50,Citation72]. The incidence of ALT ≥5 times ULN assessed over 6 months of treatment with ketoconazole in patients who were ketoconazole treatment-naïve was 13% compared with 3% with levoketoconazole in the SONICS study () [Citation25,Citation28,Citation29,Citation51,Citation52,Citation57,Citation61–63,Citation65–67,Citation72–76]. Ketoconazole is known to prolong the QT interval and increase the risk of torsades de pointes, either by direct effect through the KCNH2 IKr channel or through drug–drug interaction with QT prolonging drugs [Citation28,Citation29,Citation75]. Similar to ketoconazole, levoketoconazole was also shown to affect the KCNH2 IKr channel, but with less potency (unpublished data). The incidence of the adverse event of adrenal insufficiency ranged from 0% to 18.5% with ketoconazole treatment in patients with CS [Citation51,Citation52,Citation62,Citation72,Citation76]. The low incidence of adrenal insufficiency observed with levoketoconazole in the SONICS study may result from instructions to investigators to titrate levoketoconazole doses slowly (no more than 150 mg increments in dose every 2 weeks) and to advance patients to the maintenance phase immediately after first mUFC normalization in the dose titration phase [Citation50].
Table 3. Safety profiles of levoketoconazole and ketoconazole
As adrenal steroidogenesis is regulated by the hypothalamus-pituitary-adrenal (HPA) axis, a reduction in the cortisol level is expected to result in a compensatory increase in ACTH through the negative feedback loop, as seen with other steroidogenesis inhibitors [Citation53,Citation77,Citation78]. However, mean ACTH levels in the subset of patients with CD (85% of patients) increased modestly during dose-titration and remained elevated through the maintenance phase in the SONICS study [Citation57]. Mean ACTH levels observed at the end of month 6 were less than 2-fold higher than those reported at baseline. Furthermore, there were no discontinuations from SONICS ascribed to tumor growth or pituitary enlargement, although longer follow-up is needed.
5. Conclusion
Levoketoconazole, a 2S, 4R enantiomer of the ketoconazole racemate, is a steroidogenesis inhibitor in development for the treatment of CS. Based on preclinical and clinical pharmacology findings, levoketoconazole may be a more potent inhibitor of cortisol synthesis when compared with ketoconazole or the other enantiomer of ketoconazole, dextroketoconazole. Furthermore, findings from pharmacokinetics studies suggest a preferred hepatic extraction of dextroketoconazole compared with levoketoconazole, which may lower the risk of hepatotoxicity with levoketoconazole. Therefore, these pharmacologic findings suggest that levoketoconazole may have an improved therapeutic index compared with ketoconazole. Results from the phase III SONICS study in patients with CS demonstrated that levoketoconazole treatment was effective in improving biochemical disease markers, such as normalizing mUFC levels and improving LNSC, testosterone levels, and biomarkers of cardiovascular risk, in a significant percentage of patients. In addition, levoketoconazole treatment was associated with improvements in subjective clinical assessments; that is, clinician-rated CS clinical signs and symptoms and patient-reported quality of life and depression symptom severity. Levoketoconazole was generally well tolerated, with no unexpected safety signals reported during the SONICS study. The pharmacology, efficacy, and safety data suggest that levoketoconazole may improve overall outcomes of CS. The data support use of levoketoconazole as medical therapy for patients with CS, if approved.
6. Expert opinion
Patients with CS benefit from a tailored clinical management approach. Individual factors to consider include disease characteristics, comorbidities, and prior treatments. Medical therapy is an important part of CS management in some patients. Levoketoconazole is a steroidogenesis inhibitor in phase III development for the treatment of patients with CS. As of this writing, CS medications approved by both the FDA and the EMA are pasireotide, a somatostatin receptor ligand indicated for the treatment of adult patients with CD for whom pituitary surgery is not an option or has not been curative [Citation79,Citation80], and osilodrostat, a cortisol synthesis inhibitor with the same indication in the US [Citation81] and approval in Europe for the treatment of endogenous CS in adults [Citation82]. In the US, the glucocorticoid-receptor antagonist mifepristone is FDA-approved to control hyperglycemia secondary to hypercortisolism in adults with endogenous CS [Citation83], and in Europe, ketoconazole [Citation26] and metyrapone [Citation84] are approved as CS treatments. Regulatory approval of levoketoconazole would add another option to the treatment armamentarium for CS and further optimize pharmacotherapy.
6.1. Place of levoketoconazole in the treatment algorithm for Cushing's syndrome
For CS caused by pituitary, adrenal, or ectopic neuroendocrine tumors, surgery is the first-line treatment in most patients [Citation16]. Options for second-line treatment of CD include medical therapy, repeat surgery, or radiotherapy (with a medical bridge until radiation takes effect). Treatment selection is guided by individual patient characteristics and treatment risk-benefit profiles [Citation16,Citation17]. As a cortisol synthesis inhibitor that affects multiple steps in the steroidogenesis pathway (), levoketoconazole is appropriate for patients with endogenous CS of both pituitary (i.e., CD) or non-pituitary etiologies. More details are needed about the effects of levoketoconazole on the pituitary and adrenal glands. Mean ACTH levels increased less than 2-fold from baseline at the end of month 6 with levoketoconazole treatment in the SONICS study. This is in contrast with increases from baseline in mean ACTH levels observed with other adrenal steroidogenesis inhibitors such as osilodrostat (~3- to 4-fold) [Citation85,Citation86] metyrapone [Citation77], and mitotane [Citation78], and also with glucocorticoid receptor antagonist mifepristone [Citation87]. The modest rise in ACTH in patients with CD receiving levoketoconazole [Citation57], and the preclinical findings of inhibitory effects on ACTH secretion and corticotroph cell growth [Citation36], suggest the possibility of direct antisecretory effects on ACTH and/or a direct effect of tumor inhibition in the pituitary gland, similar to what has been reported with ketoconazole [Citation42,Citation62]. In addition, there is no evidence that short- or long-term treatment with ketoconazole has adverse effects on pituitary function in patients with CD or in healthy individuals or patients with other endocrine disorders [Citation88–95].
Findings from the SONICS study indicate that levoketoconazole may be effective in a wide range of patients across the spectrum of disease severity, disease duration, and previous treatments [Citation50]. In light of the beneficial effects on glycemic control, levoketoconazole may be particularly useful in patients with comorbidities such as diabetes or prediabetes.
Each medical therapy for CS has a unique safety profile, but comparisons across medications are challenging in the absence of head-to-head studies. Nonetheless, it appears that levoketoconazole has an advantageous antidiabetic profile compared with pasireotide, which is known to increase fasting glucose, HbA1c, and incidence of diabetes in treated patients [Citation96,Citation97]. Patients receiving levoketoconazole may have a lower risk of adrenal insufficiency, and women on this therapy may have lower testosterone levels compared with those receiving osilodrostat [Citation81,Citation86]. Furthermore, osilodrostat increases circulating levels of aldosterone precursors, which activate mineralocorticoid receptors and may cause hypokalemia, edema, and hypertension in some patients [Citation81,Citation98]. As with ketoconazole, levoketoconazole has the potential for drug–drug interactions [Citation39,Citation40]. The efficacy and safety data for levoketoconazole support its use as a first-choice medical therapy for some patients with CS. However, clinical utilization of levoketoconazole, if it is approved by the regulatory agencies, will depend not only on the efficacy and safety profile but also on cost and insurance coverage.
6.2. Management of Cushing's syndrome with levoketoconazole
Before initiating treatment with levoketoconazole, patients with CS should be assessed for potential contraindications. Specific concerns include women who are pregnant or planning to become pregnant and patients with QTc prolongation or moderate-to-severe hepatic impairment. Pretreatment assessment should include a hepatic panel, basic metabolic panel, and electrocardiogram (ECG). Consideration of drug–drug interactions and careful examination of the patient’s medication list are essential when prescribing levoketoconazole, as this drug is a potent inhibitor and substrate of CYP3A4 [Citation39,Citation40]. Drugs with narrow therapeutic windows that are major CYP3A4 substrates should not be administered with levoketoconazole. Medications that are weak or moderate CYP3A4 inhibitors or inducers should be avoided if alternative therapy is available or should be used with caution. Similarly, drugs that can cause QT prolongation should be avoided unless no acceptable alternative is available; in such cases, ECG monitoring will be important. It has been suggested that combinations of medications may be beneficial for patients with CS who need additional therapy [Citation22,Citation24,Citation99]. Among the other medications for CS, there is a notable potential drug–drug interaction between mifepristone (a CYP3A4 inhibitor) and levoketoconazole. Based on its long half-life [Citation83], we suggest discontinuing mifepristone at least 2 weeks before starting treatment with levoketoconazole, unless combined use is medically necessary. If levoketoconazole is approved for treatment of CS in the US, more specific recommendations about contraindicated medications and those that should be avoided, if possible, should be included in the prescribing information.
Another drug interaction issue that requires consideration is gastric acid inhibition. Because sufficient gastric acidity is required for absorption of oral levoketoconazole [Citation47,Citation48], agents that increase gastric pH (proton pump inhibitors, H2-receptor antagonists) should not be administered concomitantly. In patients who are treated with inhibitors of gastric acid secretion, substitution with short-acting antacids at least 1 hour after levoketoconazole doses is suggested.
Although clinical trial data indicated that tumor size did not change in most patients during treatment with levoketoconazole for up to 12 months [Citation100], we recommend performing a baseline pituitary MRI for subsequent comparison in patients with CD before initiating levoketoconazole, if a recent study is not available.
As with other therapies for CS (except for the glucocorticoid receptor antagonists) [Citation16], the goal of levoketoconazole treatment is to normalize urinary free cortisol levels to ≤ULN and to further titrate the dose to treat comorbidities and eliminate clinical signs and symptoms associated with CS. To optimize efficacy and tolerability, the dose of levoketoconazole should be titrated based on individual patient response. We suggest a titration regimen consistent with the one used in SONICS, starting at a dose of 150 mg twice daily with incremental adjustment every 2–3 weeks if UFC remains high, to a maximum dose of 600 mg twice daily. Patients should be clinically evaluated before each dose adjustment to assess therapeutic response, including 24-hour UFC measurement and assessment of LNSC. Dose increases in increments of 150 mg/d are suggested, although larger increments could be envisioned in some patients with very high baseline UFC and many comorbidities requiring rapid control.
Monitoring for tolerability should include periodic liver function tests and ECG. In addition to monitoring of cortisol levels, patients should be evaluated clinically for adrenal insufficiency. Patients should be educated about the cardinal symptoms of adrenal insufficiency (nausea, vomiting, abdominal pain, fatigue, headache, dizziness) and instructed to immediately report any such changes to their physician. Temporary medication interruption or dose reduction may be sufficient to address mild-to-moderate elevations in liver enzymes, prolongation of the QT interval, or symptoms of adrenal insufficiency, while more severe reactions may warrant drug discontinuation. Additionally, blood glucose and lipid levels should be monitored in patients taking levoketoconazole who are also being treated with medications for hyperglycemia and/or hyperlipidemia. Improvements in glycemic control and cholesterol levels observed in SONICS suggest that dose reduction of antidiabetic or cholesterol-lowering medications may be appropriate in some patients treated with levoketoconazole. For men, testosterone should be monitored and replaced, if needed, as appropriate for each individual patient.
6.3. Future developments in research and practice
The results of the SONICS study, summarized above, and the recently completed LOGICS study is the basis of an NDA to the FDA for levoketoconazole. LOGICS was a phase III, double-blind, placebo-controlled, randomized withdrawal study that evaluated the efficacy and safety of levoketoconazole in patients who completed SONICS and patients with CS who were levoketoconazole treatment–naïve; 44 patients were enrolled in the randomized withdrawal phase [Citation101].
Furthermore, we expect that other advances in surgery, radiotherapy, and medical therapy will also improve the treatment of CS. Advances in surgical methods may improve remission rates, while new radiation techniques may improve the safety profile. Earlier diagnosis of disease recurrence could provide an overall reduction in disease duration and severity, with better potential response to medical therapy. With the FDA approval of osilodrostat and the possible approval of levoketoconazole, increased use of adrenal steroidogenesis inhibitors, including increased preoperative use, may occur if surgery is contraindicated or no tumor is seen on MRI. It will be important to determine whether preoperative use is beneficial for long-term outcomes, complications including hypercoagulability, or overall perioperative risks. To optimize the use of medical therapy, there is a need for improved parameters related to dosing and monitoring, for both efficacy and side effects. Specific issues to be addressed include decision-making regarding dose adjustment if UFC is normalized but LNSC remains elevated, and whether these medications should be dosed to clinical effect regardless of laboratory test values. Other forthcoming changes in the medical treatment of CS may include the increased use of combination medical therapies that target different components of the HPA axis and the clinical development of additional medications, including novel pituitary-directed therapies and a next-generation glucocorticoid receptor antagonist (relacorilant) [Citation18,Citation24]. The increasing availability of effective medical therapies, such as levoketoconazole, will enhance treatment options and improve overall outcomes in patients with CS.
Article highlights
Levoketoconazole, the 2S,4R stereoisomer of ketoconazole, is in development for the treatment of Cushing’s syndrome (CS)
Preclinical and clinical pharmacologic findings suggest that levoketoconazole is a more potent inhibitor of cortisol synthesis, with potentially lower hepatic exposure and interference with metabolite elimination compared with the dextroketoconazole stereoisomer and the ketoconazole racemate; these attributes may improve its therapeutic index relative to ketoconazole
Findings from the phase III SONICS study showed sustained clinical benefits of levoketoconazole in cortisol normalization and improvements in cardiovascular risk biomarkers
Results from the SONICS study also showed subjective clinical benefits in clinician-rated clinical signs and symptoms of CS and patient-reported quality of life and depression symptom severity
Treatment with levoketoconazole in SONICS led to sustained normalization of mUFC and improvement in glycemic control that was more pronounced in patients with diabetes mellitus type 2
Levoketoconazole has an acceptable safety and tolerability profile, with no new safety signals reported during the SONICS study
Declaration of interest
M Fleseriu reports serving as an investigator with research grants to Oregon Health and Science University from Novartis, Recordati, and Strongbridge Biopharma; and serving as a consultant to Novartis, Recordati, and Strongbridge Biopharma.
RJ Auchus reports receiving research grants to the University of Michigan from Corcept Therapeutics, Novartis, and Strongbridge Biopharma; and serving as a consultant to Corcept Therapeutics, Novartis, and Strongbridge Biopharma.
R Pivonello reports serving as the principal investigator of research grants to Federico II University from Corcept Therapeutics, Novartis, and Strongbridge Biopharma; and receiving consulting honoraria from Novartis and Strongbridge Biopharma.
R Salvatori reports receiving an educational grant to Johns Hopkins University from Corcept and Pfizer; serving as an investigator with research grants to Johns Hopkins University from Novartis and Strongbridge Biopharma; and serving as a consultant for Strongbridge Biopharma, Sterotherapeutics, and HRA Pharma.
S Zacharieva reports receiving consulting honoraria from Novartis.
BMK Biller reports serving as the principal investigator of research grants to Massachusetts General Hospital from Millendo Therapeutics, Novartis, and Strongbridge Biopharma; and receiving consulting honoraria from Novartis, Recordati, and Strongbridge Biopharma.
Reviewer disclosures
Peer reviewers on this manuscript have no relevant financial or other relationships to disclose.
Acknowledgments
Editorial assistance was provided by Nancy Holland and Pratibha Hebbar of Synchrony Medical Communications.
Additional information
Funding
References
- Fleseriu M, Castinetti F. Updates on the role of adrenal steroidogenesis inhibitors in Cushing's syndrome: a focus on novel therapies. Pituitary. 2016 Dec; 19(6):643–653.
- Etxabe J, Vazquez JA. Morbidity and mortality in Cushing's disease: an epidemiological approach. Clin Endocrinol (Oxf). 1994 Apr; 40(4):479–484.
- Lindholm J, Juul S, Jørgensen JO, et al. Incidence and late prognosis of Cushing’s syndrome: a population-based study. J Clin Endocrinol Metab. 2001 Jan;86(1)117–123.
- Dekkers OM, Horváth-Puhó E, Jørgensen JO, et al. Multisystem morbidity and mortality in Cushing's syndrome: a cohort study. J Clin Endocrinol Metab. 2013 Jun;98(6)2277–2284.
- Wengander S, Trimpou P, Papakokkinou E, et al. The incidence of endogenous Cushing's syndrome in the modern era. Clin Endocrinol (Oxf). 2019 Aug;91(2)263–270.
- Guaraldi F, Salvatori R. Cushing syndrome: maybe not so uncommon of an endocrine disease. J Am Board Fam Med. 2012 Mar-Apr; 25(2):199–208.
- Clayton RN, Jones PW, Reulen RC, et al. Mortality in patients with Cushing's disease more than 10 years after remission: a multicentre, multinational, retrospective cohort study. Lancet Diabetes Endocrinol. 2016 Jul;4(7)569–576.
- Van Haalen FM, Broersen LH, Jorgensen JO, et al. Mortality remains increased in Cushing's disease despite biochemical remission: a systematic review and meta-analysis. Eur J Endocrinol. 2015 Apr;172(4)R143–R149.
- Pivonello R, Isidori AM, De Martino MC, et al. Complications of Cushing's syndrome: state of the art. Lancet Diabetes Endocrinol. 2016 Jul;4(7)611–629.
- Pivonello R, Simeoli C, De Martino MC, et al. Neuropsychiatric disorders in Cushing's syndrome. Front Neurosci. 2015;9:129.
- Santos A, Resmini E, Pascual JC, et al. Psychiatric symptoms in patients with Cushing's syndrome: prevalence, diagnosis and management. Drugs. 2017 May;77(8)829–842.
- Lacroix A, Feelders RA, Stratakis CA, et al. Cushing's syndrome. Lancet. 2015 Aug 29;386(9996)913–927.
- Sharma ST, Nieman LK, Feelders RA, et al. Cushing's syndrome: epidemiology and developments in disease management. Clin Epidemiol. 2015;7:281–293.
- Turcu AF, Auchus RJ. Mild adrenal cortsol excess. In: Geer EB, editor. The Hypothalamic-Pituitary-Adrenal Axis in Health and Disease: Cushing's Syndrome and Beyond. Cham, Switzerland: Springer Nature; 2017. p. 181–197.
- Fassnacht M, Arlt W, Bancos I, et al. Management of adrenal incidentalomas: european society of endocrinology clinical practice guideline in collaboration with the European network for the study of adrenal tumors. Eur J Endocrinol. 2016 Aug;175(2)G1–G34.
- Nieman LK, Biller BM, Findling JW, et al. Treatment of Cushing's syndrome: an endocrine society clinical practice guideline. J Clin Endocrinol Metab. 2015 Aug;100(8):2807–2831.
- Pivonello R, De Leo M, Cozzolino A, et al. The treatment of Cushing's disease. Endocr Rev. 2015 Aug;36(4):385–486.
- Hinojosa‑Amaya JM, Cuevas‑Ramos D, Fleseriu M, et al. Medical management of Cushing's syndrome: current and emerging treatments. Drugs. 2019;79(9):935–956.
- Castinetti F, Brue T, Ragnarsson O, et al. Radiotherapy as a tool for the treatment of Cushing's disease. Eur J Endocrinol. 2019 May 1;180(5)D9–D18.
- Reincke M, Ritzel K, Osswald A, et al. A critical reappraisal of bilateral adrenalectomy for ACTH-dependent Cushing's syndrome. Eur J Endocrinol. 2015 Oct;173(4)M23–M32.
- Rubinstein G, Osswald A, Zopp S, et al. Therapeutic options after surgical failure in Cushing's disease: a critical review. Best Pract Res Clin Endocrinol Metab. 2019 Apr;33(2)101270.
- Tritos NA, Biller BM. Medical therapy for Cushing's syndrome in the twenty-first century. Endocrinol Metab Clin North Am. 2018 Jun; 47(2):427–440.
- Geer EB, Shafiq I, Gordon MB, et al. Biochemical control during long-term follow-up of 230 adult patients with Cushing disease: a multicenter retrospective study. Endocr Pract. 2017 Aug;23(8)962–970.
- Feelders RA, Newell-Price J, Pivonello R, et al. Advances in the medical treatment of Cushing's syndrome. Lancet Diabetes Endocrinol. 2019 Apr;7(4)300–312.
- Ketoconazole tablets USP, 200 mg. Haifa Bay, Israel: Taro Pharmaceutical Industries Ltd.; 2017.
- European Medicines Agency. Ketoconazole HRA 200 mg tablets: summary of product characteristics. London. 2014.
- Newell-Price J. Ketoconazole as an adrenal steroidogenesis inhibitor: effectiveness and risks in the treatment of Cushing's disease. J Clin Endocrinol Metab. 2014 May; 99(5):1586–1588.
- European Medicines Agency Committee for Medicinal Products for Human Use. Ketoconazole HRA: assessment report. London. 2014.
- Boyce MJ, Baisley KJ, Warrington SJ, et al. Pharmacokinetic interaction between domperidone and ketoconazole leads to QT prolongation in healthy volunteers: a randomized, placebo-controlled, double-blind, crossover study. Br J Clin Pharmacol. 2012 Mar;73(3)411–421.
- Schwartz SL, Rendell M, Ahmann AJ, et al. Safety profile and metabolic effects of 14 days of treatment with DIO-902: results of a phase iIa multicenter, randomized, double-blind, placebo-controlled, parallel-group trial in patients with type 2 diabetes mellitus. Clin Ther. 2008 Jun;30(6)1081–1088.
- Arakaki R, Welles B. Ketoconazole enantiomer for the treatment of diabetes mellitus. Expert Opin Investig Drugs. 2010 Feb; 19(2):185–194.
- Rotstein DM, Kertesz DJ, Walker KA, et al. Stereoisomers of ketoconazole: preparation and biological activity. J Med Chem. 1992 Jul 24;35(15):2818–2825.
- Auchus RJ, Wu Y, Liu J, et al. 2S,4R-ketoconazole is the relevant enantiomer of ketoconazole for cortisol synthesis inhibition: steroidogenic P450s inhibition involves multiple mechanisms [abstract]. Endocr Rev. 2018;39(2 suppl). https://www.endocrine.org/meetings-and-events/endo-annual-meetings?ID=46236
- Thieroff-Ekerdt R, Lavin P, Abou-Gharbia M, et al. Pharmacology of COR-003 (levoketoconazole), an investigational treatment for endogenous Cushing's syndrome. 98th Annual Meeting and Expo of the Endocrine Society (ENDO); 2016 Apr 1–4; Boston, MA.
- Blass BE, Iyer P, Abou-Gharbia M, et al. Design, synthesis, and evaluation of (2S,4R)-ketoconazole sulfonamide analogs as potential treatments for metabolic syndrome. Bioorg Med Chem Lett. 2016 Dec 1;26(23)5825–5829.
- Creemers SG, Feelders RA, de Jong FH, et al. Levoketoconazole, the 2S,4R enantiomer of ketoconazole, a new steroidogenesis inhibitor for Cushing's syndrome treatment [published online ahead of print January 5, 2021]. J Clin Endocrinol Metab. 2021;106(4):e1618–e1630.
- Pricen HM, Huijsmans CM, Kuipers F, et al. Ketoconazole blocks bile acid synthesis in hepatocyte monolayer cultures and in vivo in rat by inhibiting cholesterol 7a-hydroxylase. J Clin Invest. 1986;78(4):1064–1071.
- Chiang JY. Bile acids: regulation of synthesis. J Lipid Res. 2009 Oct; 50(10):1955–1966.
- Novotná A, Krasulová K, Bartoňková I, et al. Dual effects of ketoconazole cis-enantiomers on CYP3A4 in human hepatocytes and HepG2 cells. PLoS One. 2014;9(10):e111286.
- Dilmaghanian S, Gerber JG, Filler SG, et al. Enantioselectivity of inhibition of cytochrome P450 3A4 (CYP3A4) by ketoconazole: testosterone and methadone as substrates. Chirality. 2004;16(2):79–85.
- Stalla GK, Stalla J, Loeffler JP, et al. Pharmacological modulation of CRH-stimulated ACTH secretion by ketokonazole. Horm Metab Res Suppl. 1987;16:31–36.
- Herrera-Martinez AD, Feelders RA, De Herder WW, et al. Effects of ketoconazole on ACTH-producing and non-ACTH-producing neuroendocrine tumor cells. Horm Cancer. 2019 Jun;10(2-3)107–119.
- Patalano R, Pivonello C, Solari D, et al. Ketoconazole induces inhibition of cell viability and apoptosis in an ACTH-secreting tumour cell line model [abstract EP 927]. Endocr Abst. 2017;49:3.
- Boudriau S, Demnati R, Swearingen D, et al. Differential effects of co-administration of racemic ketoconazole and levdexketoconazole of the pharmacokinetic profile of atorvastatin. American Society of Clinical Pharmacology and Therapeutics (ASCPT); 2008 Apr 2–5; Orlando, FL.
- Thieroff-Ekerdt RI, Mould DR Differential pharmacokinetics of levoketoconazole (COR-003), the single 2S,4R-enantiomer of ketoconazole, a new investigational drug for the treatment of Cushing's syndrome. 18th European Congress of Endocrinology (ECE); 2016 May 28–31; Munich, Germany.
- Gerber JG, Dilaghanian S, Gal J, et al. Stereoselective pharmacokinetics (PK) of oral ketoconazole (K) in healthy subjects. 43rd Interscience Conference on Antimicrobial Agents (ICAAC); 2003 Sept 14–17; Chicago, IL.
- Chin TW, Loeb M, Fong IW, et al. Effects of an acidic beverage (Coca-Cola) on absorption of ketoconazole. Antimicrob Agents Chemother. 1995 Aug;39(8)1671–1675.
- Piscitelli SC, Goss TF, Wilton JH, et al. Effect of ranitidine and sucralfate on ketoconazole bioavailability. Antimicrob Agents Chemother. 1991;35(9):1765–1771.
- Liang X, Giacomini KM. Transporters involved in metformin pharmacokinetics and treatment response. J Pharm Sci. 2017 Sep; 106(9):2245–2250.
- Fleseriu M, Pivonello R, Elenkova A, et al., Efficacy and safety of levoketoconazole in the treatment of endogenous Cushing's syndrome (SONICS): a phase 3, multicentre, open-label, single-arm trial. Lancet Diabetes Endocrinol. 2019;7(211): 855–865.
- Castinetti F, Guignat L, Giraud P, et al. Ketoconazole in Cushing's disease: is it worth a try?. J Clin Endocrinol Metab. 2014 May;99(5):1623–1630.
- Castinetti F, Morange I, Jaquet P, et al. Ketoconazole revisited: a preoperative or postoperative treatment in Cushing's disease. Eur J Endocrinol. 2008 Jan;158(1)91–99.
- Chung S, Son GH, Kim K, et al. Circadian rhythm of adrenal glucocorticoid: its regulation and clinical implications. Biochim Biophys Acta. 2011 May;1812(5)581–591.
- Newell-Price J, Pivonello R, Tabarin A, et al. Use of late-night salivary cortisol to monitor response to medical treatment in Cushing's disease. Eur J Endocrinol. 2020 Feb;182(2)207–217.
- Yoshida K, Fukuoka H, Odake Y, et al. Multiple salivary cortisol measurements are a useful tool to optimize metyrapone treatment in patients with Cushing's syndromes treatment: case presentations. Front Endocrinol (Lausanne). 2017;8:375.
- Varlamov EV, Langlois F, Vila G, et al. Management of endocrine disease: cardiovascular risk assessment, thromboembolism, and infection prevention in Cushing's syndrome: a practical approach. Eur J Endocrinol. 2021 Apr 22;184(5)R207–R224.
- Fleseriu M, Pivonello R, Elenkova A, et al. Levoketoconazole in the treatment of endogenous Cushing's syndrome: secondary endpoint results from a phase 3 study (SONICS). 16th International Pituitary Congress; 2019 Mar 20–22; New Orleans, LA.
- Varlamov EV, Han AJ, Fleseriu M, et al. Updates in adrenal steroidogenesis inhibitors for Cushing's syndrome - a practical guide. Best Pract Res Clin Endocrinol Metab. 2021 Feb 6;(1):101490. DOI: 10.1016/j.beem.2021.101490.
- Daniel E, Aylwin S, Mustafa O, et al. Effectiveness of metyrapone in treating Cushing's syndrome: a retrospective multicenter study in 195 patients. J Clin Endocrinol Metab. 2015 Nov;100(11)4146–4154.
- Geer EB, Salvatori R, Elenkova A, et al., Levoketoconazole improves clinical signs and symptoms and patient-reported outcomes in patients with Cushing's syndrome. Pituitary. 2021;24(1): 104–115.
- Weber MM, Luppa P, Engelhardt D, et al. Inhibition of human adrenal androgen secretion by ketoconazole. Klin Wochenschr. 1989;67(14):707–712.
- Sonino N, Boscaro M, Paoletta A, et al. Ketoconazole treatment in Cushing's syndrome: experience in 34 patients. Clin Endocrinol (Oxf). 1991 Oct;35(4)347–352.
- Pont A, Williams PL, Azhar S, et al. Ketoconazole blocks testosterone synthesis. Arch Intern Med. 1982;142(12):2137–2140.
- Santen RJ, Van den Bossche H, Symoens J, et al. Site of action of low dose ketoconazole on androgen biosynthesis in men. J Clin Endocrinol Metab. 1983;57(4):732–736.
- DeFelice R, Johnson DG, Galgiani JN, et al. Gynecomastia with ketoconazole. Antimicrob Agents Chemother. 1981;19(6):1073–1074.
- Mortimer RH, Cannell GR, Thew CM, et al. Ketoconazole and plasma and urine steroid levels in Cushing's disease. Clin Exp Pharmacol Physiol. 1991;18(8):563–569.
- De Martin M, Toja PM, Goulene K, et al. No untoward effect of long-term ketoconazole administration on electrocardiographic QT interval in patients with Cushing's disease. Basic Clin Pharmacol Toxicol. 2016 Apr;118(4)279–283.
- Pivonello R, De Martino MC, Iacuaniello D, et al. Metabolic alterations and cardiovascular outcomes of cortisol excess. Front Horm Res. 2016;46:54–65.
- Mazziotti G, Formenti AM, Frara S, et al. Diabetes in Cushing disease. Curr Diab Rep. 2017 May;17(5)32.
- Barbot M, Ceccato F, Scaroni C, et al. Diabetes mellitus secondary to Cushing's disease. Front Endocrinol (Lausanne). 2018;9:284.
- Pivonello R, Elenkova A, Fleseriu M, et al. Levoketoconazole in the treatment of patients with Cushing's syndrome and diabetes mellitus: phase 3 SONICS results. Front Endocrinol. 2021;12:595894.
- Young J, Bertherat J, Vantyghem MC, et al. Hepatic safety of ketoconazole in Cushing's syndrome: results of a compassionate use programme in France. Eur J Endocrinol. 2018 May;178(5):447–458.
- Lewis JH, Zimmerman HJ, Benson GD, et al. Hepatic injury associated with ketoconazole therapy. Analysis of 33 cases. Gastroenterology. 1984;86(3):503–513.
- Lake-Bakaar G, Scheuer PJ, Sherlock S, et al. Hepatic reactions associated with ketoconazole in the United Kingdom. Br Med J (Clin Res Ed). 1987;294(11):419–422.
- Takemasa H, Nagatomo T, Abe H, et al. Coexistence of hERG current block and disruption of protein trafficking in ketoconazole-induced long QT syndrome. Br J Pharmacol. 2008 Feb;153(3)439–447.
- Moncet D, Morando DJ, Pitoia F, et al. Ketoconazole therapy: an efficacious alternative to achieve eucortisolism in patients with Cushing's syndrome. Medicina (B Aires). 2007;67(1):26–31.
- Verhelst JA, Trainer PJ, Howlett TA, et al. Short and long-term responses to metyrapone in the medical management of 91 patients with Cushing's syndrome. Clin Endocrinol (Oxf). 1991 Aug;35(2)169–178.
- Baudry C, Coste J, Bou Khalil R, et al. Efficiency and tolerance of mitotane in Cushing's disease in 76 patients from a single center. Eur J Endocrinol. 2012 Oct;167(4)473–481.
- Signifor (pasireotide) injection, for subcutaneous use. East Hanover, NJ: Novartis Pharmaceuticals. 2020.
- European Medicines Agency. Signifor (pasireotide) injection, for subcutaneous use: summary of product characteristics. Amsterdam. 2016.
- Isturisa (osilodrostat) tablets, for oral use. Lebanon, NJ: Recordati Rare Disease, Inc. 2020.
- European Medicines Agency. Isturisa (osilodrostat): summary of product characteristics. Amsterdam. 2020.
- Korlym (mifepristone) 300 mg tablets. Menlo Park, CA: Corcept Therapeutics Incorporated. 2019.
- Metopirone 250 mg: summary of product characateristics. London: HRA Pharma UK and Ireland Limited. 2017.
- Fleseriu M, Pivonello R, Young J, et al. Osilodrostat, a potent oral 11β-hydroxylase inhibitor: 22-week, prospective, phase II study in Cushing's disease. Pituitary. 2016 Apr;19(2)138–148.
- Pivonello R, Fleseriu M, Newell-Price J, et al. Efficacy and safety of osilodrostat in patients with Cushing's disease (LINC 3): a multicentre phase III study with a double-blind, randomised withdrawal phase. Lancet Diabetes Endocrinol. 2020;8(9): 748–761.
- Fleseriu M, Findling JW, Koch CA, et al. Changes in plasma ACTH levels and corticotroph tumor size in patients with Cushing's disease during long-term treatment with the glucocorticoid receptor antagonist mifepristone. J Clin Endocrinol Metab. 2014 Oct;99(10)3718–3727.
- Boscaro M, Sonino N, Rampazzo A, et al. ACTH response to corticotropin releasing hormone in Cushing's disease before and after ketoconazole: in vivo and in vitro studies. Horm Metab Res Suppl. 1987;16:60–62.
- Correa-Silva SR, Nascif SO, Silva MR, et al. Effect of one month ketoconazole treatment on GH, cortisol and ACTH release after ghrelin, GHRP-6 and GHRH administration in patients with Cushing's disease. Arq Bras Endocrinol Metab. 2007;51(7):1110–1117.
- Correa-Silva SR, Nascif SO, Molica P, et al. Adrenocorticotrophic hormone (ACTH) responsiveness to ghrelin increases after 6 months of ketoconazole use in patients with Cushing's disease: comparison with GH-releasing peptide-6 (GHRP-6). Clin Endocrinol (Oxf). 2010 Jan;72(1)70–75.
- De Pedrini P, Tommaselli A, Montemurro G, et al. No effect of ketoconazole on thyroid function of normal subjects and hypothyroid patients. Int J Clin Pharmacol Res. 1988;8(6):485–488.
- Britton H, Shehab Z, Lightner E, et al. Adrenal response in children receiving high doses of ketoconazole for systemic coccidioidomycosis. J Pediatr. 1988 Mar;112(3)488–492.
- Martikainen H, Heikkinen J, Ruokonen A, et al. Hormonal and clinical effects of ketoconazole in hirsute women. J Clin Endocrinol Metab. 1988;66(5):987–991.
- Vidal-Puig AJ, Munoz-Torres M, Jodar-Gimeno E, et al. Ketoconazole therapy: hormonal and clinical effects in non-tumoral hyperandrogenism. Eur J Endocrinol. 1994;130(4):333–338.
- Glass AR. Ketoconazole-induced stimulation of gonadotropin output in men: basis for a potential test of gonadotropin reserve. J Clin Endocrinol Metab. 1986;63(5):1121–1125.
- Colao A, Petersenn S, Newell-Price J, et al. A 12-month phase 3 study of pasireotide in Cushing's disease. N Engl J Med. 2012 Mar 8;366(10)914–924.
- Lacroix A, Gu F, Gallardo W, et al. Efficacy and safety of once-monthly pasireotide in Cushing's disease: a 12 month clinical trial. Lancet Diabetes Endocrinol. 2018;6(1):17–26.
- Tritos NA. Adrenally directed medical therapies for Cushing syndrome. J Clin Endocrinol Metab. 2021 Jan 1; 106(1):16–25.
- Feelders RA, De Bruin C, Pereira AM, et al. Pasireotide alone or with cabergoline and ketoconazole in Cushing's disease. N Engl J Med. 2010 May 13;362(19)1846–1848.
- Fleseriu M, Auchus RJ, Greenman Y, et al. Levoketoconazole in the treatment of endogenous Cushing's syndrome: extended evaluation phase results of the SONICS study. ECE 2020: 22nd European Congress of Endocrinology; Sept 5–9; virtual meeting. 2020.
- Zacharieva SZ, Pivonello R, Elenkova A, et al. Safety and efficacy of levoketoconazole in the treatment of endogenous Cushing's syndrome (LOGICS): a double-blind, placebo-controlled, withdrawal study. J Endocr Soc. 2020;4(Supplement_1):A572–A573.

